Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a common pediatric condition characterized by recurrent partial or complete cessation of airflow during sleep, typically due to inadequate upper airway patency. Continuous positive airway pressure (CPAP) is a therapeutic option that reduces morbidity. Despite efforts to promote use, CPAP adherence is poor in both pediatric and adult populations. We sought to determine whether demographics, insurance status, OSA severity, therapeutic pressure, or comorbid conditions were associated with pediatric CPAP adherence.
Methods:
A retrospective review of adherence download data was performed on all pediatric patients with initiation or adjustment of CPAP treatment over a one-year period with documented in-laboratory CPAP titration. Patients were grouped as CPAP adherent or non-adherent, where adherence was defined as > 70% nightly use and average usage ≥ 4 hours per night. Differences between the groups were analyzed by χ2 test.
Results:
Overall, nearly half of participants were CPAP adherent (49%, 69/140). Of the demographic data collected (age, ethnicity, sex, insurance status), only female sex was associated with better adherence (60.9% vs 39.5% of males adherent; odds ratio [OR] = 2.41, 95%CI = 1.20–4.85; p = 0.01). Severity of OSA (diagnostic apnea-hypopnea index [AHI] and degree of hypoxemia), therapeutic pressure, and residual AHI did not impact CPAP adherence (p > 0.05). Patients with developmental delay (DD) were more likely to be adherent with CPAP than those without a DD diagnosis (OR = 2.55, 95%CI = 1.27–5.13; p = 0.007). Female patients with trisomy 21 tended to be more adherent, but this did not reach significance or account for the overall increased adherence associated with female sex.
Conclusions:
Our study demonstrates that adherence to CPAP therapy is poor but suggests that female sex and developmental delay are associated with better adherence. These findings support efforts to understand the pathophysiology of and to develop adherence-promoting and alternative interventions for pediatric OSA.
Citation:
Hawkins SM, Jensen EL, Simon SL, Friedman NR. Correlates of pediatric CPAP adherence. J Clin Sleep Med 2016;12(6):879–884.
Keywords: obstructive sleep apnea, sleep-disordered breathing, CPAP, pediatrics, adherence
INTRODUCTION
Obstructive sleep apnea (OSA) affects about 2% of children and is characterized by recurrent partial or total cessation of airflow during sleep, typically due to inadequate upper airway patency.1 Adverse outcomes associated with pediatric OSA include neurobehavioral, cardiovascular, metabolic, and growth abnormalities, which contribute to decreased social functioning and quality of life; successful and consistent treatment is associated with reduction of morbidity, including improvement of daytime symptoms and neurobehavioral parameters.2–7 First-line therapy for children entails optimization of airway patency by adenotonsillectomy (AT).1 Overall surgical intervention is successful in 79% of patients, however those with severe OSA, obesity, and additional risk factors, such as genetic syndromes are less likely to be cured by AT alone.1,8,9 In these circumstances, or when AT is contraindicated, continuous positive airway pressure (CPAP) is a therapeutic option.1
CPAP splints the airway open by acting as a pneumatic stent, increasing the airway pressure above the critical closing pressure of the pharynx. The efficacy of CPAP depends upon an adequate interface, appropriate pressure settings, and adherence to use.10,11
Despite efforts to promote use, CPAP adherence in children is generally poor.3,12–14 Non-adherence or untreated OSA may result in development of the aforementioned comorbidi-ties, compromised daily functioning, increased health care utilization, and contribution to early mortality.6,15–23 OSA severity, pressure settings, and mask type, have not been found to affect use,14,24–30 whereas psychosocial factors and perception of OSA's importance do.14,23,31 One study suggested that higher maternal education correlated with higher adherence.13 The optimal duration and frequency of CPAP use remains unknown, though increased use correlates with improved outcomes in a dose-dependent nature.21,25,29 A minimum use of 70% of nights for an average of 4 hours per night was until recently utilized as a compliance threshold by equipment providers and payers.32
BRIEF SUMMARY
Current Knowledge/Study Rationale: Pediatric obstructive sleep apnea is plagued by poor adherence to CPAP therapy. We found that female sex and diagnosis of developmental delay were associated with improved adherence; adherence was not associated with age, medical complexity, OSA severity, CPAP factors, or other diagnoses.
Study Impact: This study reinforces the need to identify populations at risk for poor adherence to CPAP therapy, to bolster CPAP desensitization efforts, and to develop CPAP alternatives. It is also provides justification for tailoring pediatric OSA treatment to the individual, as opposed to utilizing a “one-size-fits-all” approach.
To our knowledge, the relationship between CPAP adherence, demographic factors, and medical comorbidities has not been fully examined in a pediatric population. We therefore aim to examine demographic and disease-specific characteristics in relation to CPAP adherence within the sleep program of our large, tertiary care facility. With pragmatic optimism, we hypothesized that the majority of our patients would be considered adherent to CPAP, but that public insurance status (as a surrogate of socioeconomic status) and greater medical complexity would be associated with poor adherence, while other demographic characteristics would not.
METHODS
After obtaining institutional review board approval, we performed a retrospective review of patients evaluated and treated for OSA through one of our pediatric sleep, pulmonary, or otorhinolaryngology clinics. We searched our electronic medical record to fulfill the inclusion/exclusion criteria outlined in Table 1. Patient data was compiled in a secure REDCap database.
Table 1.
Criteria for study inclusion and exclusion.

As standard practice in our sleep center, patients typically receive a mask fitting and air pressure trial in clinic with a respiratory therapist prior to initiating therapy, and regular CPAP follow-up clinic appointments with a medical provider are recommended. Adherence data is requested from durable medical equipment (DME) providers following initiation or adjustment to CPAP settings and routinely collected at all clinic encounters; the DME providers are asked to equip home CPAP devices with a wireless modem that may be accessed remotely to provide adherence and efficacy data. We do not routinely record mask leak, so we do not report it here. For this study, when a single patient had multiple downloads or CPAP titration studies, only the most recent download and earliest titration were utilized for our analyses.
Based on the adherence downloads, patients were classified as CPAP adherent if > 70% nightly use and an average usage ≥ 4 hours per night, or otherwise as CPAP non-adherent. At the time of data collection, this was the standard for adherence set by many payers, including Medicare, and recorded by DMEs for reimbursement purposes.32
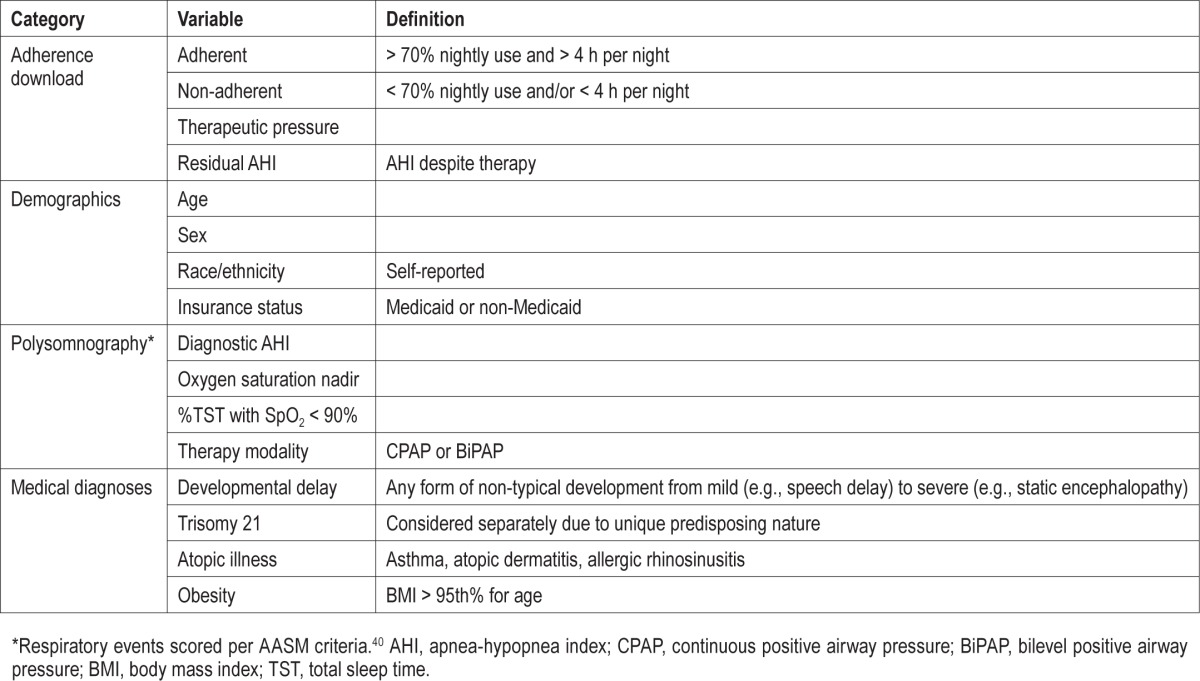
All variables included in analyses, including adherence, demographic, polysomnography, and medical diagnoses data are described in Table 2. Of note, as our EMR does not allow for reliable documentation or stratification of diagnoses based on severity, this includes a spectrum of each diagnostic category.
Table 2.
Variables collected from chart review and used for analyses.
All statistical analyses were performed using SAS software (Version 9.2; SAS Institute Inc., Cary, NC, USA). Descriptive statistics were obtained for patients classified as adherent versus non-adherent. Differences between the groups regarding demographics, comorbidities, and polysomnography results were analyzed by the χ2 test.
RESULTS
From our clinical practice, 329 pediatric OSA patients with initiation or adjustment of CPAP within the review period were identified, of whom 302 had a documented in-laboratory titration. Adherence data was available for 158 subjects, but of these 18 had incomplete datasets. Thus, 140 patients were examined for the current analyses. Of this population, 49% (69/140) demonstrated adherence per the previously defined criteria. Table 3 suggests that the adherent group had high rates of use with 7.4 ± 1.9 h average use per night with 94% ± 7.8% of nights used.
Table 3.
Study population CPAP use.
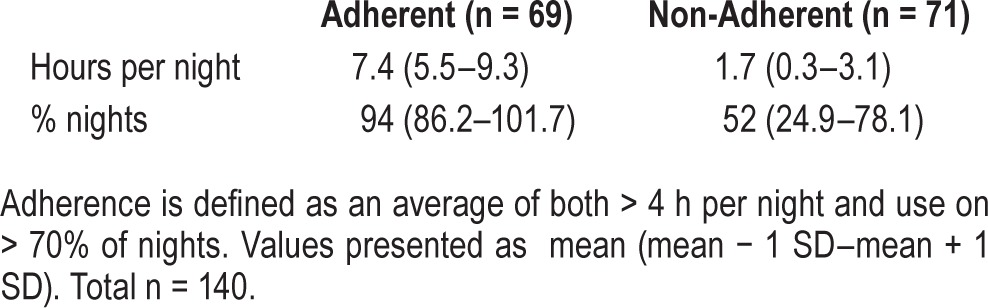
Table 4 outlines the demographic and medical characteristics of youth classified as CPAP adherent and non-adherent. Of the demographic data, only female sex was associated with adherence (60.9% vs 39.5% of males adherent, odds ratio = 2.41, 95%CI = 1.20–4.85; p = 0.01). No differences in adherence were found by patient age, ethnicity, or insurance status. Severity of OSA (represented by both diagnostic AHI and degree of hypoxemia), therapeutic CPAP setting, CPAP versus BiPAP modality, and residual AHI despite treatment were not related to adherence (Table 4; pressure modality data not shown; p > 0.05).
Table 4.
Demographic, OSA, and CPAP download characteristics of pediatric patients grouped by CPAP adherence status.
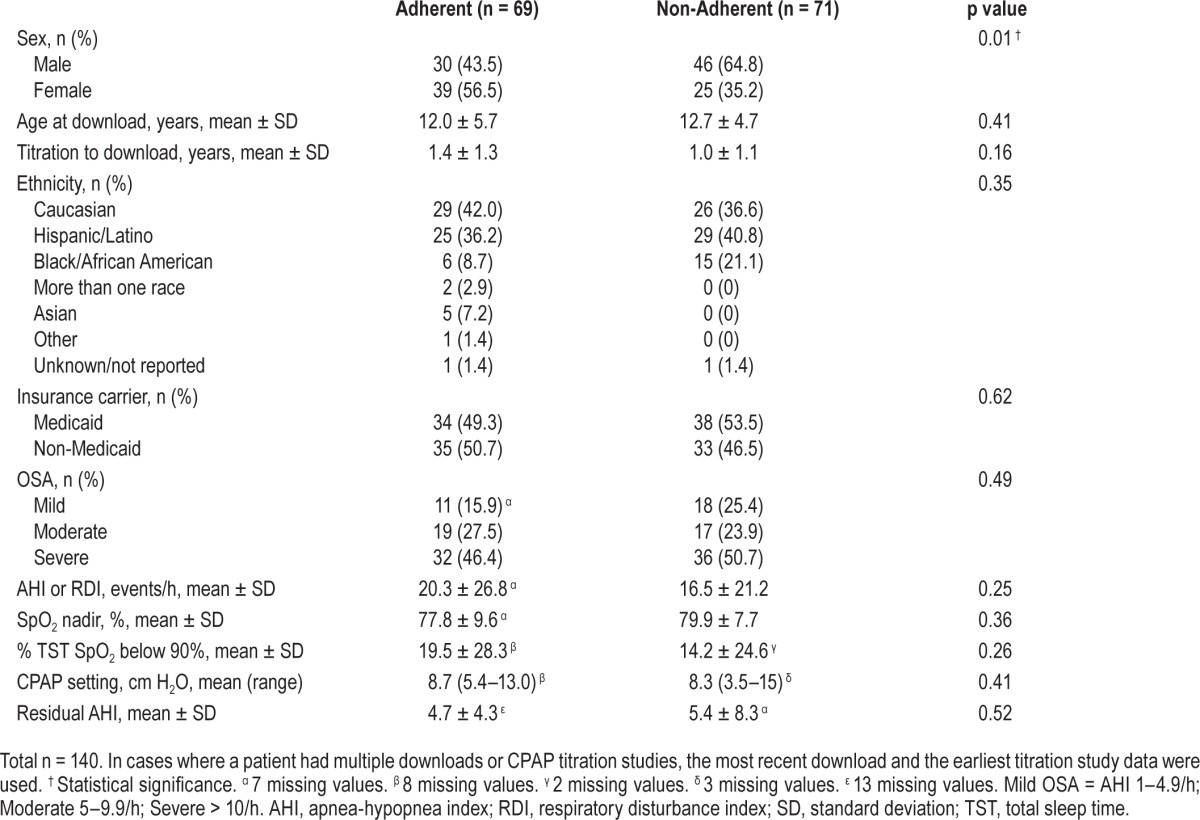
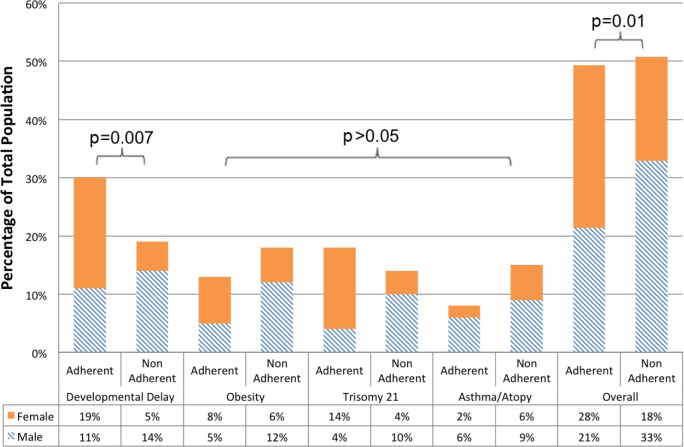
Figure 1 illustrates select comorbidities and adherence versus non-adherence in each population, further broken down by sex. A diagnosis of developmental delay (DD) was associated with CPAP adherence (OR = 2.55, 95%CI = 1.27–5.13; p = 0.007). Sex did not account for increased adherence in the DD population, nor did presence of a DD diagnosis account for better adherence of females following univariate analysis. Patients with obesity or atopic illness trended towards poor adherence, but these did not reach statistical significance. Trisomy 21 diagnosis was not associated with adherence. Medical complexity was further evaluated using the diversity of recorded diagnoses as a surrogate. We found that neither the number of diagnoses nor the number of organ systems involved was associated with adherence.
Figure 1. Medical comorbidities of pediatric patients with OSA grouped by sex and CPAP adherence status.
DISCUSSION
Our study suggests that in a large pediatric sleep medicine practice, approximately half of the patient population demonstrated poor adherence to CPAP therapy. These rates of adherence are similar to 5% to 55% estimated rate of non-adherence documented in other pediatric chronic illness regimens33,34 and are also similar to other reports of pediatric CPAP adherence in smaller samples.3,12–14 Alarmingly, the slight majority of our population failed to meet payer adherence criteria and CPAP coverage may be subject to denial. Focusing on the positive, nearly half of our patients had excellent adherence, averaging nearly 7.5 hours usage per night, for over 90% of nights.
Females were more likely to be classified as having good adherence. This is in contrast to findings of no sex differences in adult CPAP use,28 but similar to results of a meta-analysis of adherence to treatment in chronic illness that found greater adherence for girls compared to boys.34 Adult female patients with OSA are more likely to present with a predominance of hypopneas, as opposed to apneas, and are more likely to report daytime sleepiness.28,35 It is possible that there are sex-specific phenotypes of sleep-disordered breathing which are more tolerant of CPAP therapy. Alternatively, parental behavior management strategies, monitoring, and expectations may be different for boys and girls.
Despite previous findings of correlations of age and socioeconomic status with adherence in a pediatric population without developmental delay, these factors were not associated with adherence in the current population.13,34 Potentially, insurance status is not a suitable proxy for socioeconomic status in this population, as Medicaid-insured children may be medically complex but of variable socioeconomic standing.
Prior research has demonstrated increased OSA prevalence36 and lower CPAP adherence13,37 in African Americans (AA). Our data demonstrate 15.0% of the study population to be AA, which is disparate from the 4.4% of the Colorado state census identified as AA in 2013 (quickfacts.census.gov). This may reflect the population served by our institution rather than suggest higher OSA prevalence among AA. We did observe a trend towards lower adherence in AA patients but there were insufficient numbers to suggest significance.
Children with DD were more likely to be adherent to CPAP therapy than youth with normal development. Given the broad spectrum of DD severity (mild learning disability or speech delay to global developmental delay or profound intellectual disability), one must interpret this finding with caution. Potential reasons why those with DD would demonstrate higher adherence may include increased dependence upon caregiver support and decreased ability to remove mask due to either physical or intellectual disability. Parental perception of CPAP necessity for a child with neurodevelopmental disabilities may be increased. Alternatively, children without disabilities may be less adherent due to increased independence or less parental supervision. Regardless, children with developmental delays in addition to OSA are an at-risk population and are expected to benefit from therapeutic intervention.
There was a trend suggesting that those with atopic illness, including eczema, allergic rhinosinusitis, and/or asthma, were less adherent to CPAP therapy. Nasal complaints are common and contribute to poor tolerance in those treated with CPAP,38 which may be partially explained by discomfort associated with air forced into an inflamed and/or congested airway as found in those with atopic illness. Indeed, the addition of humidification and treatment with topical nasal steroids are recommended practices to improve compliance.38,39
Strengths of the current study include a large sample size and the inclusion of a diverse and medically complex patient population. These characteristics allowed for a comparison between groups of subjects based on demographics, characteristics of OSA and CPAP therapy, and medical comorbidities.
Limitations of the current study include its retrospective and correlational nature, so discussions of causality remain speculative. Difficulty obtaining reliable and timely adherence data remains a challenge in sleep medicine, as demonstrated by less than half of the eligible study population having complete adherence data. These results are representative of those families who were able to provide downloads, either by communicating with the DME company or coming to their clinic appointment and patients with new initiation or adjustments of CPAP. However, this inadvertently excluded patients and families who refused treatment or did not attend regular clinic appointments, or who were on stable, long-term CPAP therapy without recent clinic follow-up. Also, our catchment has a small but disproportionately represented African-American population. Therefore, our results may be an overestimate or underestimate and may not be generalizable to all pediatric patients with OSA.
Our study reinforces the challenges associated with CPAP use and suggests that more needs to be done in order to optimize adherence, particularly in high-risk populations. Our pediatric sleep medicine program is making efforts aimed at improving adherence for our patients. Though the current analyses were not performed to evaluate the role of our CPAP desensitization program, it is worth mentioning that patients are generally referred for any of three reasons: condition(s) that we suspected may predispose to difficulty tolerating (such as development delay), poor response to mask introduction, or demonstration of poor adherence. Our study suggests higher adherence in those with developmental delays, therefore we may be missing opportunities to improve adherence in others. Additional research is needed to develop individualized treatment strategies to promote regular use of CPAP, as well as the ability to target interventions specific to the subgroups most at risk for poor adherence. We speculate that the ability to recognize and address barriers to adherence prior to initiating treatment; to reduce non-adherence with the help of automated, timely, and robust databases; to intervene with targeted desensitization; and to consider CPAP alternatives when needed will lead to improved outcomes in the treatment of pediatric obstructive sleep apnea.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported in part by NIH/ NCRR Colorado CTSI Grant Number UL1 RR025780 and use of the REDCap database is supported by grant funding: NIH/NCRR Colorado CTSI Grant Number UL1 TR001082. Contents are the authors' sole responsibility and do not necessarily represent the official NIH views. The authors have indicated no financial conflicts of interest. All subjects treated at the Children's Hospital Colorado, Aurora, CO.
ABBREVIATIONS
- AHI
apnea-hyponea index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- DD
developmental delay
- OSA
obstructive sleep apnea
- RDI
respiratory disturbance index
- SD
standard deviation
- TST
total sleep time
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database System Rev. 2006:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 4.Marcus CL, Radcliffe J, Konstantinopoulou S, et al. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:998–1003. doi: 10.1164/rccm.201112-2167OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basoglu OK, Midilli M, Midilli R, Bilgen C. Adherence to continuous positive airway pressure therapy in obstructive sleep apnea syndrome: effect of visual education. Sleep Breath. 2012;16:1193–200. doi: 10.1007/s11325-011-0631-9. [DOI] [PubMed] [Google Scholar]
- 6.Beebe DW, Byars KC. Adolescents with obstructive sleep apnea adhere poorly to positive airway pressure (PAP), but PAP users show improved attention and school performance. PLoS One. 2011;6:e16924. doi: 10.1371/journal.pone.0016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29:1115–34. doi: 10.1093/sleep/29.9.1115. [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shott SR. Evaluation and management of pediatric obstructive sleep apnea beyond tonsillectomy and adenoidectomy. Curr Opin Otolaryngol Head Neck Surg. 2011;19:449–54. doi: 10.1097/MOO.0b013e32834c1728. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg R, Doghramji P. Optimal treatment of obstructive sleep apnea and excessive sleepiness. Adv Ther. 2009;26:295–312. doi: 10.1007/s12325-009-0016-7. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell AR, Bjornson CL, Bohn SG, Kirk VG. Compliance rates in children using noninvasive continuous positive airway pressure. Sleep. 2006;29:651–8. [PubMed] [Google Scholar]
- 13.DiFeo N, Meltzer LJ, Beck SE, et al. Predictors of positive airway pressure therapy adherence in children: a prospective study. J Clin Sleep Med. 2012;8:279–86. doi: 10.5664/jcsm.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon SL, Duncan CL, Janicke DM, Wagner MH. Barriers to treatment of paediatric obstructive sleep apnoea: development of the adherence barriers to continuous positive airway pressure (CPAP) questionnaire. Sleep Med. 2012;13:172–7. doi: 10.1016/j.sleep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao KH, Nixon GM. The effect of treatment of obstructive sleep apnea on quality of life in children with cerebral palsy. Rev Dev Disabil. 2008;29:133–140. doi: 10.1016/j.ridd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Kanimozhi S, Balaji C, Saravanan A, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res. 2015;9:CC07–10. doi: 10.7860/JCDR/2015/13301.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhillon S, Chung SA, Fargher T, Huterer N, Shapiro CM. Sleep apnea, hypertension, and the effects of continuous positive airway pressure. Am J Hypertens. 2005;18:594–600. doi: 10.1016/j.amjhyper.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 19.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27:453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 21.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quittner A, Espelage D, levers-Landis C, Drotar D. Measuring adherence to medical treatments in childhood chronic illness: considering multiple methods and sources of information. J Clin Psychol Med Settings. 2000;7:41–54. [Google Scholar]
- 23.DiMatteo MR. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ Couns. 2004;55:339–44. doi: 10.1016/j.pec.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Ryan S, Garvey JF, Swan V, Behan R, McNicholas WT. Nasal pillows as an alternative interface in patients with obstructive sleep apnoea syndrome initiating continuous positive airway pressure therapy. J Sleep Res. 2011;20:367–73. doi: 10.1111/j.1365-2869.2010.00873.x. [DOI] [PubMed] [Google Scholar]
- 25.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloia MS. Understanding the problem of poor CPAP adherence. Sleep Med Rev. 2011;15:341–2. doi: 10.1016/j.smrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14:323–35. doi: 10.1007/s11325-010-0391-y. [DOI] [PubMed] [Google Scholar]
- 28.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–4. [PubMed] [Google Scholar]
- 29.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez A, Khirani S, Aloui S, et al. Continuous positive airway pressure and noninvasive ventilation adherence in children. Sleep Med. 2013;14:1290–4. doi: 10.1016/j.sleep.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Russell T. Enhancing adherence to positive airway pressure therapy for sleep disordered breathing. Semin Respir Crit Care Med. 2014;35:604–12. doi: 10.1055/s-0034-1390070. [DOI] [PubMed] [Google Scholar]
- 32.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 33.Rapoff MA. Adherence to pediatric medical regimens. 2nd ed. New York, NY: Springer; 2010. [Google Scholar]
- 34.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–9. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 35.Mohsenin V. Gender differences in the expression of sleep-disordered breathing : role of upper airway dimensions. Chest. 2001;120:1442–7. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 36.Weinstock TG, Rosen CL, Marcus CL, et al. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep. 2014;37:261–9. doi: 10.5665/sleep.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balachandran JS, Yu X, Wroblewski K, Mokhlesi B. A brief survey of patients' first impression after CPAP titration predicts future CPAP adherence: a pilot study. J Clin Sleep Med. 2013;9:199–205. doi: 10.5664/jcsm.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 39.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031–5. [PubMed] [Google Scholar]
- 40.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]