Abstract
Objective To find an efficient set of diagnostic indicators that are optimally informative in the diagnosis of a bacterial origin of acute infectious conjunctivitis.
Design Cohort study involving consecutive patients. Results of index tests and reference standard were collected independently from each other.
Setting 25 Dutch health centres.
Participants 184 adults presenting with a red eye and either (muco)purulent discharge or glued eyelid(s), not wearing contact lenses.
Main outcome measures Probability of a positive bacterial culture, given different combinations of index test results; area under receiver operating characteristics curve.
Results Logistic regression analysis showed optimal diagnostic discrimination for the combination of early morning glued eye(s), itch, and a history of conjunctivitis. The first of these indicators increased the likelihood of a bacterial cause, whereas the other two decreased it. The area under the receiver operating characteristics curve for this combination of symptoms was 0.74 (95% confidence interval 0.63 to 0.80). The overall prevalence of bacterial involvement of 32% could be lowered to 4% or raised to 77%, depending on the pattern of index test results.
Conclusion A bacterial origin of complaints indicative of acute infectious conjunctivitis can be made much more likely or unlikely by the answers to three simple questions posed during clinical history taking (possibly by telephone). These results may have consequences for more targeted prescription of ocular antibiotics.
Introduction
In the developed world, acute infectious conjunctivitis is a common disorder with an annual incidence of 1.5-2% in primary care.1-5 Randomised trials in patients with suspected acute bacterial conjunctivitis show a pooled prevalence of bacterial pathogens of 50% (95% confidence interval 45% to 54%).6-9 No more than half of the cases of acute infectious conjunctivitis in primary care probably have a bacterial origin. Confronted with acute infectious conjunctivitis, most general practitioners feel unable to discriminate between a bacterial and a viral cause. In practice, more than 80% of such patients receive antibiotics.1,5 Hence, in cases of acute infectious conjunctivitis, many unnecessary ocular antibiotics are prescribed. In 2001 in the Netherlands, more than 900 000 prescriptions for topical ocular antibiotics were issued, at a cost of €8.85 million (£5.9 million, $10.9 million). In England 3.4 million community prescriptions for these antibiotics are issued each year, at a cost to the NHS of £4.7 million (€7.1 million, $8.7 million).10,11
To select those patients who might benefit most from antibiotic treatment, the general practitioner needs an informative diagnostic tool to determine a bacterial cause. With such a tool, antibiotic prescriptions may be reduced and better targeted. Most general practitioners make the distinction between a bacterial cause and another cause on the basis of signs and symptoms. Additional diagnostic investigations, such as a culture of the conjunctiva, are seldom done, mostly because of the resulting diagnostic delay. Can general practitioners actually differentiate between bacterial and viral conjunctivitis on the basis of signs and symptoms alone? Major textbooks list several signs and symptoms that are supposed to be diagnostic for the cause of acute infectious conjunctivitis.12-14 A recently published systematic literature search summed up the signs and symptoms and found no evidence for these assertions.15 This paper presents what seems to be the first empirical study on the diagnostic informativeness of signs and symptoms in acute infectious conjunctivitis.
Methods
Participants
We asked nine designated general practitioners, working in 25 care centres with a total of 41 general practitioners, in the Amsterdam and Alkmaar region to include patients with a red eye and either (muco)purulent discharge or sticking of the eyelids. The exclusion criteria were age younger than 18 years, pre-existing symptoms for longer than seven days, acute loss of vision, wearing of contact lenses, use of systemic or local antibiotics within the previous two weeks, ciliary redness, eye trauma, and a history of eye surgery. All eligible patients were referred to one of the nine designated general practitioners for enrolment in the study. Patients were recruited during office hours only. Participants gave written informed consent. We collected the data for this diagnostic study as part of the baseline measurements for a randomised trial on the treatment of acute infectious conjunctivitis. We used the complete cohort for this analysis.
Data collection
At inclusion of each participant, general practitioners completed a standardised questionnaire and physical examination (index tests). The questionnaire contained questions about medical history (self reported), duration of symptoms (days), self medication and self treatment, itching, burning sensation, foreign body sensation, and the number of glued eyes in the morning (0, 1, or 2). The physical examination included investigation of the degree of redness (peripheral, whole conjunctiva, or whole conjunctiva and pericorneal), the presence of periorbital oedema, the kind of discharge (watery, mucous, or purulent), and bilateral involvement (yes or no). The general practitioner then took one conjunctival sample of each eye for a bacterial culture (reference standard). The general practitioners did not receive the culture results, and the microbiologist who analysed the cultures had no knowledge of the results of the index tests.
For each patient one eye was designated as the “study eye.” In the case of two diseased eyes, the diseased eye with worse signs or symptoms was the study eye. In the case of two equally affected eyes, the first affected eye was the study eye.
Microbiological procedures
General practitioners took one sample of the conjunctiva of each eye by rolling a cotton swab (Laboratory Service Provider, Velzen-Noord, Netherlands) over the conjunctiva of the lower fornix. They put the swabs into transport medium and sent them to the investigating laboratory in Alkmaar. Directly after arrival, we inoculated the swabs on to blood agar enriched with 5% sheep blood, MacConkey agar, and chocolate agar. All media were made at the laboratory with standard ingredients (Becton Dickinson, Cockeysville, MD, USA). After standard inoculation, we incubated the blood agar and MacConkey agar plates for 48 hours at 35°C; we incubated the chocolate agar plates for the same period and at the same temperature, but in a 7% CO2 atmosphere. We analysed cultures daily according to the guidelines of the American Society for Microbiology.16 We identified all pathogens by using routine standard biochemical procedures. Colonies suspected to be pathogens were selected and investigated by Gram stain. Depending on the results of the Gram stain, we did additional tests. In the case of Gram positive cocci, we did a catalase test followed by, for example, a coagulase test (staphylococci) or an optochine (pneumococci) test. In the case of Gram negative rods or cocci, we did sugar tests.
Statistical analysis
We assessed the associations between findings from the index tests and the presence of a positive bacterial culture in the study eye by using a stepwise forward logistic regression analysis.17 The dependent variable was the presence or absence of a bacterium. We entered variables with a univariate P value of ≤ 0.10 into the model. We considered variables with a multivariate P value of < 0.15 to be independent indicators of the presence of bacteria and retained them in the final model. We modelled determinants with more than two categories as dummy variables.17 We assessed all second order interactions between the variables entered into the final model. We deemed interaction to be present if the P value associated with an interaction term was < 0.05.
We quantified the ability of the final model to discriminate between patients with and without a positive bacterial culture by using the area under the receiver operating characteristics curve with 95% confidence intervals.18 We quantified the reliability or calibration of the model by using the Hosmer-Lemeshow goodness of fit test. Finally, we bootstrapped the receiver operating characteristics curve a thousand times to counteract potential undue influence of atypical patients on the predictions of the final model.18
We used the final model to estimate the probability of a positive bacterial culture for each individual patient given his or her combination of test results. For each test result we generated a clinical score by using the rounded regression coefficients associated with these test results. All possible combinations of test results led to different clinical scores, which could be used for treatment decisions depending on the choice of a treatment cutoff point. In this way, we could calculate the numbers of correctly treated patients (sensitivity of the chosen cut-off point) and correctly untreated patients (specificity) and the reduction in prescriptions with different treatment cut-off points. We illustrated this by use of an example.
We used SPSS (version 11.5.2) to do the statistical analyses. We used Stata (version 7) to calculate the 95% confidence intervals around the predicted probabilities and to do the bootstrapping.
Results
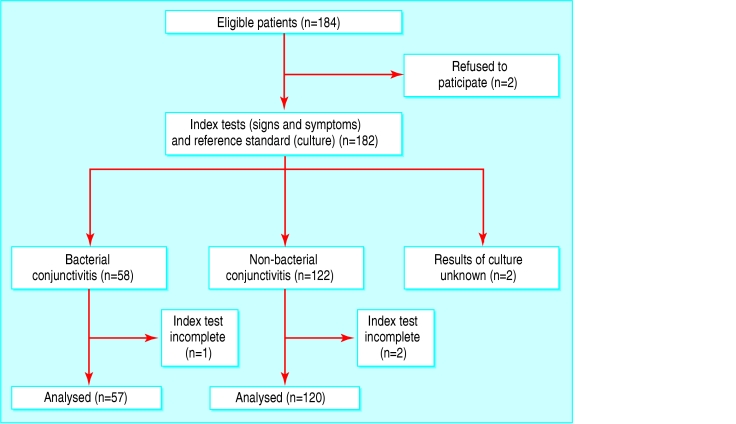
Between September 1999 and December 2002 we enrolled 184 patients; data from 177 (96%) of these could be analysed (fig). The reasons for non-inclusion were refusal (n = 2) or incompleteness of data (n = 5). Three patients had incomplete index tests, and the culture results for two patients were unknown because the culture samples never arrived at the laboratory.
Figure 1.
Flow of participants through the study
The prevalence of a positive bacterial culture in the study eye was 32% (57/177). The groups (culture positive and culture negative) were comparable with respect to baseline demographics, but some notable differences existed in the results of index tests (table 1). A history of conjunctivitis occurred more often in participants with a negative culture (21% v 9%). In the group with a positive culture, more patients had two glued eyes in the morning (39% v 11%) and bilateral involvement (37% v 16%). The most prevalent species was Streptococcus pneumoniae, which accounted for 27/57 of the positive cultures (table 2).
Table 1.
Baseline demographic characteristics and index test results and their univariate odds ratios. Values are numbers (percentages) unless stated otherwise. The prevalence of a positive culture was 32% (57/177)
| Characteristic | Culture positive (n=57) | Culture negative (n=120) | Odds ratio (95% CI) |
|---|---|---|---|
| Mean (SD) age (years) | 47 (17) | 42 (14) | - |
| Median (range) duration of symptoms (days) | 2 (1-7) | 3 (1-7) | - |
| Female | 36 (63) | 68 (57) | - |
| History of hay fever | 9 (16) | 18 (15) | 1.06 (0.45 to 2.54) |
| History of conjunctivitis | 5 (9) | 25 (21) | 0.37 (0.13 to 1.01) |
| History of allergic conjunctivitis | 3 (5) | 6 (5) | 1.06 (0.25 to 4.38) |
| Self treatment* | 45 (79) | 85 (71) | 1.54 (0.73 to 3.26) |
| Redness: | |||
| Peripheral | 16 (28) | 50 (42) | 1 |
| Whole conjunctiva | 29 (51) | 50 (42) | 1.81 (0.88 to 3.74) |
| Conjunctival and pericorneal | 12 (21) | 20 (17) | 1.88 (0.75 to 4.66) |
| Periorbital oedema | 20 (35) | 41 (34) | 1.04 (0.54 to 2.02) |
| Secretion: | |||
| None or water | 20 (35) | 47 (39) | 1 |
| Mucus | 26 (46) | 43 (36) | 1.42 (0.70 to 2.90) |
| Purulent | 11 (19) | 30 (25) | 0.86 (0.36 to 2.05) |
| Bilateral involvement | 21 (37) | 19 (16) | 3.10 (1.50 to 6.42) |
| Itching | 33 (58) | 76 (63) | 0.80 (0.42 to 1.52) |
| Foreign body sensation | 23 (40) | 48 (40) | 1.02 (0.53 to 1.93) |
| Burning sensation | 37 (65) | 69 (58) | 1.37 (0.71 to 2.63) |
| Glued eyes: | |||
| None | 5 (8) | 33 (27) | 1 |
| One in the morning | 30 (53) | 74 (62) | 2.68 (0.95 to 7.51) |
| Two in the morning | 22 (39) | 13 (11) | 11.17 (3.49 to 35.77) |
Cleaning with water.
Table 2.
Culture results
| Pathogen | No (%) (n=57) |
|---|---|
| Streptococcus pneumoniae | 27 (47) |
| Staphylococcus aureus | 13 (23) |
| Haemophilus influenzae | 9 (16) |
| Coagulase negative staphylococci | 5 (9) |
| Streptococcus haemolyticus, group C | 1 (2) |
| Other bacteria | 2 (4) |
Three determinants were retained in the multivariable regression analysis: history of conjunctivitis (yes or no), itch (yes or no), and glued eyes in the morning (0, 1, or 2). Table 3 lists the odds ratios of these independent indicators of a positive bacterial culture and their clinical scores. We found no statistical interactions.
Table 3.
Results of logistic regression analysis. Independent indicators of positive bacterial culture and their clinical score
| Indicator | Odds ratio (95% CI) | Regression coefficient | Clinical score* |
|---|---|---|---|
| Two glued eyes | 14.99 (4.36 to 51.53) | 2.707 | 5 |
| One glued eye | 2.96 (1.03 to 8.51) | 1.086 | 2 |
| Itching | 0.54 (0.26 to 1.12) | −0.61 | −1 |
| History of conjunctivitis | 0.31 (0.10 to 0.96) | −1.161 | −2 |
| Area under ROC curve (95% CI) | 0.74 (0.65 to 0.82) | - | - |
ROC=receiver operating characteristics.
Clinical scores of every symptom present are added up. For example, a patient with two glued eyes, itch, and no history of conjunctivitis has a clinical score of: 5 + −1 = 4.
The area under the receiver operating characteristics curve of the final model was 0.74 (95% confidence interval 0.65 to 0.82). The Hosmer-Lemeshow goodness of fit test had a P value of 0.117, indicating that the model does not misrepresent the data.17 Validation of this model with the bootstrap technique showed hardly any indication of undue influence by particular patients (corrected 95% confidence interval of area under curve 0.63 to 0.80).
The logistic regression analysis generated 12 different combinations of test results. These combinations corresponded to nine different clinical scores, varying from +5 to -3. For each clinical score, we calculated the probability of a positive culture. For a patient with a clinical score of +5, this probability was increased from 32% (prevalence in this study) to 77% (table 4). By contrast, a clinical score of -3 lowered this probability to 4%. Table 4 allows the calculation of the numbers of correctly treated patients (sensitivity) and correctly untreated patients (specificity) and the reduction of prescriptions with different treatment cut-off points. For example, if the treatment cut-off point is set at +2, indicating that only patients with a clinical score of +2 or higher receive ocular antibiotics, 38/57 (67%) of patients are correctly treated and 87/120 (73%) patients are correctly untreated. If applied to our study population, the cut-off point of +2 would lead to a reduction in prescriptions of antibiotics from more than 80% (current practice) to 40% (71/177).
Table 4.
Clinical scores and their associated probabilities of a positive culture, sensitivity, and specificity
| Clinical score | Percentage (No) observed positive cultures* | Percentage (95% CI) predicted positive cultures† | Percentage correctly treated (sensitivity)‡ | Percentage correctly untreated (specificity)§ |
|---|---|---|---|---|
| +5 | 100 (5/5) | 77 (57 to 90) | 9 | 100 |
| +4 | 71 (17/24) | 65 (47 to 79) | 39 | 94 |
| +3 | 0 (0/3) | 51 (23 to 79) | 39 | 92 |
| +2¶ | 41 (16/39) | 40 (26 to 55) | 67 | 73 |
| +1 | 20 (10/51) | 27 (17 to 39) | 84 | 38 |
| 0 | 13 (3/23) | 18 (7 to 38) | 89 | 22 |
| −1 | 20 (5/25) | 11 (4 to 26) | 98 | 5 |
| −2 | 0 (0/1) | 7 (2 to 28) | 98 | 4 |
| −3 | 17 (1/6) | 4 (1 to 15) | 100 | 0 |
Overall prevalence of positive culture=32% (57/177). In parenthesis are the number of positive cultures (numerator) and total number of cultures (denominator) in that row.
Predicted probability is the probability of a positive culture calculated by regression analysis.
Fraction of patients with a positive culture who would be correctly treated if the clinical score in that row was used as treatment cut-off point.
Fraction of all patients with a negative culture who would be correctly untreated if the clinical score in that row was used as treatment cut-off point.
Clinical score of +2 used in the text to illustrate its use for treatment decisions.
Discussion
This study seems to be the first empirical study on the informativeness of combinations of signs and symptoms to estimate the probability of a positive bacterial culture in adult patients who present to their general practitioner with a red eye and either (muco)purulent discharge or glued eyes. In contrast to what is stated in most textbooks, for example, purulent secretion seems to be diagnostically almost non-informative.12-14 However, the combination of three diagnostic indicators—glued eyes, itch, and a history of conjunctivitis—provided optimal discrimination between patients with and without a positive culture. It is of practical interest that these indicators may all be collected by clinical history taking or by telephone interview.
A history of infectious conjunctivitis and itch both made the probability of current bacterial involvement less likely. This may be explained by assuming that a viral conjunctivitis is more prevalent or has a stronger tendency to recur than a bacterial conjunctivitis and that itch indicates an allergic cause.
The use of the logistic regression model allows for the flexible creation of easy to use clinical rules. However, as long as more formal decision analyses do not exist, the choice of a rational treatment threshold remains somewhat arbitrary. We used the example of a treatment cut-off point of +2 to illustrate an approximate reduction of antibiotic prescriptions from more than 80% to 40%. These data indicate that in the absence of “alarm symptoms” the decision whether to prescribe antibiotics could be made without any additional diagnostic tests. This could lead to a substantial reduction in the costs associated with prescription of topical antibiotics. Use of a treatment cut-off point means that some patients with a positive culture will not receive treatment. The question is whether this is acceptable. A meta-analysis indicated that suspected acute bacterial conjunctivitis is mostly a self limiting disorder, with no serious complications in the placebo arms of the included studies. However, this meta-analysis also showed that treatment with antibiotic was associated with significantly better rates of early (days 2 to 5) clinical remission (relative risk 1.3, 95% confidence interval 1.1 to 1.6).4
Doctors who feel inclined to use these results in their daily practice should be aware of several factors. Firstly, the clinical domain to which our results apply does, of course, not formally include patient types that were excluded—for example, patients with acute loss of vision and contact lens wearers. Secondly, as we instructed the general practitioners especially for the study, the results may not be fully replicated if used by general practitioners not similarly instructed. Thirdly, an independent replication of our study would be useful, as other diagnostic indicators may perform better in other populations or the same indicators may be associated with different degrees of informativeness.18 On the other hand, we took some precautions against overoptimism in regression analysis by limiting the number of variables to four, which is below the rule of thumb stating that this number should be no bigger than the number of cases of the target disease divided by 10.18 As we had 57 cases, the use of four variables complies with that rule. In addition, the bootstrap procedure should be a safeguard against finding a regression model that is influenced too much by particular patients that are not found outside our dataset.
This study was limited to adult patients. The incidence of acute infectious conjunctivitis in children is higher than in adults, and the spectrum of causative micro-organisms may differ from that in adults. Therefore, these results cannot automatically be applied in children.
The low prevalence of a positive bacterial culture found in this study indicates that general practitioners unnecessarily prescribe topical ocular antibiotics in most cases of acute infectious conjunctivitis.1,5 This prescription policy may increase the risk of antibiotic resistance,19-21 and the number of patients who experience side effects, and is responsible for relatively high costs associated with prescription of these drugs.10,11 We hope that our approach will stimulate others to replicate our findings, including studies in children. Eventually this may lead to the construction of a reliable and easy to use tool to restrict the prescription of antibiotics in patients presenting with a red eye and conjunctival discharge to those with a suitably high probability of an underlying bacterial cause.
What is already known on this topic
No evidence has been published to show that clinical signs, symptoms, or both help to distinguish bacterial from viral conjunctivitis
General practitioners would benefit from an easy to use diagnostic tool to make this distinction
What this study adds
General practitioners can increase or reduce the chances that conjunctivitis is bacterial by asking about the number of glued eyes, itch, and history of infectious conjunctivitis
General practitioners could use this diagnostic information in their decisions about antibiotic treatment
A considerable reduction in the number of prescriptions for topical ocular antibiotics could be achieved, while avoiding harm or much discomfort
Contributors: HCPMvanW had the original idea. HCPMvanW and PJEB wrote the protocol and gained funding. RPR collected the data. RPR and GterR analysed the data. JHS analysed the bacterial cultures. RPR wrote the first draft of the paper, which was edited by all other authors. HCPMvanW is the guarantor for the study.
Funding: Dutch College of General Practitioners, Utrecht.
Competing interests: None declared.
Ethical approval: The medical ethics committee of the Academic Medical Center, Amsterdam, approved the original trial protocol.
References
- 1.Okkes IM, Oskam SK, Lamberts H. Van klacht naar diagnose: episodegegevens uit de huisartspraktijk. Bussum: Coutinho, 1998.
- 2.Van der Werf GT, Smit RJA, Stewart RE, Meyboom de Jong B. Spiegel op de huisarts: over registratie van ziekte, medicatie en verwijzing in de geautomatiseerde huisartspraktijk. Groningen: Rijksuniversiteit Groningen, 1998.
- 3.Van de Lisdonk EH, Bakx J. Continue morbiditeits registratie: ziekten in de huisartspraktijk. Bunge: Elsevier, 1999.
- 4.Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: a systematic review. Br J Gen Pract 2001;51: 473-7. [PMC free article] [PubMed] [Google Scholar]
- 5.Everitt H, Little P. How do GPs diagnose and manage acute infective conjunctivitis? A GP survey. Fam Pract 2002;19: 658-60. [DOI] [PubMed] [Google Scholar]
- 6.Horven I. Acute conjunctivitis: a comparison of fusidic acid viscous eye drops and chloramphenicol. Acta Ophthalmol 1993;71: 165-8. [DOI] [PubMed] [Google Scholar]
- 7.Miller IM, Wittreich J, Vogel R, Cook TJ. The safety and efficacy of topical norfloxacin compared with placebo in the treatment of acute, bacterial conjunctivitis. Eur J Ophthalmol 1992;2: 58-66. [DOI] [PubMed] [Google Scholar]
- 8.Gallenga PE, Lobefalo L, Colangelo L, Della Loggia G, Orzalesi N, Velati P, et al. Topical lomefloxacin 0.3% twice daily versus tobramycin 0.3% in acute bacterial conjunctivitis: a multicenter double-blind phase III study. Ophthalmologica 1999;213: 250-7. [DOI] [PubMed] [Google Scholar]
- 9.Agius-Fenandez A, Patterson A, Fsadni M, Jauch A, Raj PS. Topical lomefloxacin versus topical chloramphenicol in the treatment of acute bacterial conjunctivitis. Clin Drug Invest 1998;15: 263-9. [DOI] [PubMed] [Google Scholar]
- 10.Genees en hulpmiddelen Informatie Project. Annual report prescription data. College voor zorgverzekeringen, Amstelveen, 2001.
- 11.Department of Health. Prescription cost analysis data. Leeds: Department of Health, 1998.
- 12.Krachmer JH. Cornea. St Louis: Mosby, 1997.
- 13.Kanski JJ. Clinical ophthalmology: a systematic approach. Oxford: Butterworth-Heinemann, 1999.
- 14.Tasman W, Jaeger EA. Duane's clinical ophthalmology on CD-Rom. Lippincot Williams and Wilkins, 2001.
- 15.Rietveld RP, Van Weert HC, Riet ter G, Bindels PJ. Diagnostic impact of signs and symptoms in acute infectious conjunctivitis: systematic literature search. BMJ 2003;327: 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall GS, Pezzlo M. Ocular cultures. In: Isenberg HD, ed. Clinical microbiology procedures handbook. Washington: American Society for Microbiology, 1995.
- 17.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons, 1989.
- 18.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15: 361-87. [DOI] [PubMed] [Google Scholar]
- 19.Brown EM, Thomas P. Fusidic acid resistance in Staphylococcus aureus isolates. Lancet 2002;359: 803. [DOI] [PubMed] [Google Scholar]
- 20.Mills O Jr, Thornsberry C, Cardin CW, Smiles KA, Leyden JJ. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm Venereol 2002;82: 260-5. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 1999;106: 1313-8. [PubMed] [Google Scholar]