Graphical abstract
Keywords: Social anxiety, Eye gaze, Vigilance, Amygdala, Emotional expression, Gaze perception
Highlights
-
•
Patients with social phobia show hypervigilance for the eyes of emotional faces.
-
•
This hypervigilance is evident in reflexive attentional orienting.
-
•
Exploration of diagnostically relevant facial cues is not impaired in social phobia.
-
•
Patients with social phobia demonstrate delayed gaze-following.
Abstract
Previous studies of social phobia have reported an increased vigilance to social threat cues but also an avoidance of socially relevant stimuli such as eye gaze. The primary aim of this study was to examine attentional mechanisms relevant for perceiving social cues by means of abnormalities in scanning of facial features in patients with social phobia. In two novel experimental paradigms, patients with social phobia and healthy controls matched on age, gender and education were compared regarding their gazing behavior towards facial cues. The first experiment was an emotion classification paradigm which allowed for differentiating reflexive attentional shifts from sustained attention towards diagnostically relevant facial features. In the second experiment, attentional orienting by gaze direction was assessed in a gaze-cueing paradigm in which non-predictive gaze cues shifted attention towards or away from subsequently presented targets. We found that patients as compared to controls reflexively oriented their attention more frequently towards the eyes of emotional faces in the emotion classification paradigm. This initial hypervigilance for the eye region was observed at very early attentional stages when faces were presented for 150 ms, and persisted when facial stimuli were shown for 3 s. Moreover, a delayed attentional orienting into the direction of eye gaze was observed in individuals with social phobia suggesting a differential time course of eye gaze processing in patients and controls. Our findings suggest that basic mechanisms of early attentional exploration of social cues are biased in social phobia and might contribute to the development and maintenance of the disorder.
1. Introduction
Social phobia is an anxiety disorder characterized by an intense and persistent fear of social situations and negative evaluation by others often provoking a significant anxiety response and avoidance of social situations (World Health Organisation, 1992). Initial attendance to and subsequent avoidance of social threat stimuli have been suggested to contribute to the development and maintenance of social phobia (Amir, Foa, & Coles, 1998; Heinrichs & Hofmann, 2001). Empirical evidence is largely consistent with this proposal as hypervigilance for social threat cues, such as social threat words and emotional faces, was observed in several studies (Mogg, Philippot, & Bradley, 2004; Seefeldt, Krämer, Tuschen-Caffier, & Heinrichs, 2014; Spector, Pecknold, & Libman, 2003). Moreover, the avoidance of disorder-relevant stimuli following the initial hypervigilance towards such cues has been demonstrated when the time course of attention was taken into account in social phobia and anxiety (Garner, Mogg, & Bradley, 2006; Schofield, Inhoff, & Coles, 2013; Wieser, Pauli, Weyers, Alpers, & Mühlberger, 2009), although evidence for avoidance of these stimuli is less consistent (e.g., Gamble & Rapee, 2010).
The eyes convey important information about the emotional state of conspecifics, their focus of attention and their intentions. Research in social phobia has therefore particularly focused on investigating the relationship between social anxiety and attention towards the eye region. Self-reports and behavioral studies in social phobia and anxiety revealed a marked tendency to avoid eye contact in social situations (Baker & Edelmann, 2002; Daly, 1978; Schneier, Rodebaugh, Blanco, Lewin, & Liebowitz, 2011). Studies employing eye-tracking technology and computerized faces have supported these findings by demonstrating a lower amount of fixations and dwell times on the eye region for patients suffering from social phobia (Horley, Williams, Gonsalvez, & Gordon, 2003, Horley, Williams, Gonsalvez, & Gordon, 2004; Moukheiber et al., 2010; Moukheiber, Rautureau, Perez-Diaz, Jouvent, & Pelissolo, 2012; Weeks, Howell, & Goldin, 2013). However, these studies employed relatively long stimulus presentation times (∼10 s) and analyzed gazing behavior across the whole viewing period. As a result it remains unclear whether initial hypervigilance to the eye region as reported for other social threat cues might precede later avoidance. Moreover, increased rather than decreased attention towards the eyes of facial expressions has been reported in shy children and socially anxious individuals (Brunet, Heisz, Mondloch, Shore, & Schmidt, 2009; Wieser, Pauli, Alpers, & Mühlberger, 2009).
In the current study, we employed two novel eye-tracking paradigms to examine the gazing behavior of patients with social phobia compared to healthy controls in response to emotional facial expressions. In the first experiment, angry, fearful, happy and neutral faces were presented either briefly or for a longer duration and we manipulated whether participants initially fixated on the eye or the mouth region of facial expressions. Thus, contrary to previous experiments investigating attention to the eyes in patients with social phobia, our paradigm allowed for investigating early, reflexive shifts of attention towards the eyes or towards the mouth. It further enabled us to dissociate these reflexive aspects of visual orienting from sustained attention towards specific facial features. We expected that early, reflexive attentional shifts towards the eye region would be more pronounced in patients with social phobia as compared to controls. An avoidance of the eye region as reported in previous studies based on free-viewing conditions however (Horley et al., 2003, Horley et al., 2004, Moukheiber et al., 2010, Moukheiber et al., 2012, Weeks et al., 2013), was expected to occur only subsequent to the initially increased attention towards the eyes in patients with social phobia.
Our task additionally allowed for determining whether the hypothesized preferential processing of the eye region in social phobia is restricted to specific emotions for which the eye region possesses a higher diagnostic relevance than the mouth region. This applies to fearful and angry, but not to happy faces for which the mouth region is diagnostically most relevant (Smith, Cottrell, Gosselin, & Schyns, 2005). Notably, in previous applications of this paradigm initial gaze shifting has been shown to vary as a function of these diagnostic facial features (Boll and Gamer, 2014, Gamer and Büchel, 2009; Scheller, Büchel, & Gamer, 2012). Thus, we expected that the supposed group differences regarding attention towards the eyes would interact with the type of emotional expression if patients with social phobia orient their gaze more frequently towards diagnostically relevant facial features such as the eyes of fearful faces and the mouth of happy faces.
The second experiment was a gaze-cueing paradigm, in which non-predictive gaze cues were supposed to trigger gaze-following and facilitate identification of targets appearing at the gazed-at location. It was hypothesized that enhanced reflexive attention towards the eyes and later avoidance of the eye region would result in differences in gaze-following and gaze-cueing effects between patients and controls.
2. Methods and materials
2.1. Participants
Based on the sample sizes of previous eye-tracking studies in social phobia (e.g., Horley et al., 2003, Horley et al., 2004, Moukheiber et al., 2010, Weeks et al., 2013) as well as our experience with the experimental paradigm, we aimed at recruiting a minimum of 20 participants for each group. Since eye-tracking measures between factor levels are substantially correlated (r > 0.70 in our previous studies, e.g. Boll & Gamer, 2014), the power for detecting medium effects (f = 0.25) in interactions between group and within-subject factors is larger than 0.90 (Faul, Erdfelder, Lang, & Buchner, 2007).
In total, 22 patients with social phobia and 22 healthy controls carefully matched with respect to age, gender and education participated in this study. Initially, 24 patients and 24 controls were recruited. One patient was excluded because of acute psychotic symptoms and another one because he was illiterate and unable to complete the questionnaires. Additionally, two control participants had to be replaced because they reached high values on the social anxiety questionnaires (see Section 3.1). Patients were recruited when seeking treatment from the psychotherapeutic outpatient clinic Falkenried in Hamburg, Germany. All of them had a primary diagnosis of social phobia (ICD-10; see Table 1 and Supplement for further details on the sample), which was established during an initial interview by a clinically trained psychologist who checked relevant ICD-10 criteria for social phobia as well as potential comorbid disorders. This interview was conducted in a similar manner (standardized operating procedures of the respective outpatient clinic) for all participants and diagnoses were verified in a second interview by the clinically trained psychologist who was responsible for organizing further treatment. Participants in the control group were recruited via advertisement and screened for any current or past psychiatric symptoms using the MINI-international neuropsychiatric interview (Sheehan et al., 1998). Four of the social phobia subjects were on medication, including three on selective serotonin reuptake inhibitors (escitalopram and citalopram), one on a serotonin-norepinephrine reuptake inhibitor (venlafaxine) and two of them were additionally treated with anticonvulsant drugs (pregabalin). All patients and controls had normal or corrected-to-normal vision and gave written informed consent as approved by the local ethics committee of the Medical Board in Hamburg, Germany.
Table 1.
Socio-demographic and questionnaire data for patients with social phobia and healthy controls.
| Patients | Controls | Group comparison | |
|---|---|---|---|
| Age (years) | 32.18 (8.59) | 31.91 (8.38) | t(42) = 0.11, ns |
| Sex (male/female) | 12/10 | 12/10 | |
| Education in years | 11.36 (1.53) | 11.82 (1.44) | t(42) = 1.02, ns |
| % Comorbid diagnosis | 50.00 | 0 | |
| % Depression | 31.82 | 0 | |
| % Other anxiety disorders | 9.09 | 0 | |
| % Anxious (avoidant) personality disorder |
9.09 | 0 | |
| % Other | 22.72 | 0 | |
| SPAI | 161.16 (28.78) | 72.04 (23.64) | t(42) = 11.22*** |
| SIAS | 46.45 (12.63) | 12.14 (8.79) | t(39) = 10.14*** |
| BDI | 18.45 (9.50) | 3.72 (3.28) | t(42) = 6.88*** |
| STAI-T | 55.36 (9.06) | 32.18 (8.64) | t(42) = 8.68*** |
| TAS-20 | 56.64 (11.68) | 37.45 (10.23) | t(42) = 5.79*** |
| SDS-17 | 9.68 (3.06) | 9.91 (3.13) | t(42) = 0.24, ns |
Notes. Values in parentheses indicate standard deviations. SPAI: Social Phobia and Anxiety Inventory, SIAS: Social Interaction Anxiety Scale; BDI: Beck’s Depression Inventory; STAI-T: State-Trait Anxiety Inventory, TAS-20: Toronto Alexithymia Scale, SDS-17: Social Desirability Scale. ns: not significant, ***p < 0.001.
2.2. Apparatus and procedure
Volunteers performed two eye-tracking tasks as described in the following. Questionnaires were completed in the break between the experiments. Eye movements were monitored using a video-based eye tracking system (EyeLink 1000, SR Research, Ontario, Canada) with a sampling rate of 1000 Hz. The Software Presentation (Neurobehavioral Systems, Albany, CA, USA) was used to present the stimuli on a 20′′ Samsung SyncMaster 204B display (40.64 × 30.48 cm) with a resolution of 1600 by 1200 pixels and a refresh rate of 60 Hz. Participants viewed the screen from a distance of 51 cm and the head location was fixed using a chin rest and a forehead bar. The physical size of the depicted faces in both experiments amounted to 9.7 × 13.9 cm which yielded a visual angle of 10.9 × 15.6°. To ensure that participants fixated the middle of the screen before the facial stimuli were presented, they were instructed to look at the fixation cross whenever it was shown on the screen in both experiments. When the fixation cross disappeared (e.g., when faces were shown), volunteers were free to look wherever they wanted to. In both paradigms, participants were verbally instructed before the start of the experiment and familiarized with the task in a short training session using a different set of stimuli.
All statistical tests were performed using the statistical programming language R (www.r-project.org). An a priori significance level of α = 0.05 was applied. A multivariate analysis approach as implemented in the car package was used for all repeated-measures analyses of variance (ANOVAs). Partial Cohen’s f is reported as an effect size estimate (Cohen, 1988).
2.3. Measures
2.3.1. Assessment of social anxiety
The level of social anxiety was assessed using German versions of the Social Phobia and Anxiety Inventory (SPAI; Turner, Beidel, Dancu, & Stanley, 1989) and the Social Interaction Anxiety Scale (SIAS; Mattick & Clarke, 1998). The SPAI assesses specific somatic symptoms, cognitions, and behaviors across a range of potentially fear-producing situations to measure social anxiety. It has high test-retest reliability as well as good internal consistency and enables the discrimination of social phobics from individuals with other anxiety disorders as well as healthy controls (Turner et al., 1989). The German version of the inventory (Fydrich, 2002) differs from the original version as the number of items has been reduced from 32 to 22 and the agoraphobia subscale has been removed because contrary to the original version its suppressor function could not be verified in a German sample. To provide SPAI sum scores comparable to the original version, values were linearly transformed in the current study.
The SIAS is a self-report scale that measures fear of interacting with other people. The German version of the SIAS includes 20 items which are evaluated on five-point response scales. Its test-retest reliability is high (r > 0.90) for a four-weeks interval and Cronbach’s α values range between 0.86 and 0.90 for patients with social phobia and healthy controls (Mattick & Clarke, 1998). The SPAI and the SIAS were included in order to control for social anxiety among control participants. Two of the initially recruited control participants were excluded because they scored too high on these social anxiety measures (see Section 2.1).
2.3.2. Assessment of depression
All volunteers completed the Beck’s Depression Inventory (BDI; Hautzinger, Bailer, Worall, & Keller, 1994) to control for the current degree of depression. The BDI is a self-report inventory which measures the severity of depression and comprises 21 items. Internal consistency of the BDI is sufficiently high with Cronbach’s α = 0.88 and the questionnaire has been validated for use in German clinical and non-clinical samples (Hautzinger et al., 1994; Richter, Werner, Heerlein, Kraus, & Sauer, 1998).
2.3.3. Assessment of anxiety
We used the State-Trait Anxiety Inventory (STAI-T; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) to assess trait anxiety as a control variable. The trait subscale of the STAI measures habitual anxiety with short descriptions of emotional states which have to be rated on a 4-item Likert scale. It has excellent internal consistency (average 0.89) and test-retest reliability (average 0.88) and evidenced convergent and discriminant validity (Barnes, Harp, & Woo, 2002; Spielberger et al., 1983).
2.3.4. Assessment of alexithymia
Based on previously reported associations with social anxiety (Dalbudak et al., 2013), alexithymia was assessed using the Toronto Alexithymia Scale (TAS-20; Bagby, Parker, & Taylor, 1994). The TAS-20 comprises three subscales (difficulty describing feelings, difficulty identifying feelings and externally oriented thinking) with high scores indicating high levels of alexithymia. It shows good psychometric properties (Cronbach’s α > 0.80; Bagby et al., 1994) that have been examined in healthy samples.
2.3.5. Assessment of social desirability
To control for social desirability in both groups of volunteers we assessed the Social Desirability Scale (SDS-17; Stöber, 2001)—a 17-item inventory with a true/false answer format with good psychometric properties (Cronbach’s α of 0.72, a test-retest correlation of 0.82 across four weeks and evidenced convergent and discriminant validity).
2.4. Experiment 1: emotion classification task
2.4.1. Stimuli and design
The emotion classification experiment was based on a 2 × 2 × 4 × 2-design with the within-subject factors initial fixation (eyes vs. mouth), presentation time (150 vs. 3000 ms), emotional expression (angry, fearful, happy, neutral) and the between-subjects factor group. Male and female faces unambiguously depicting angry, fearful, happy and neutral expressions were selected on the basis of validation studies from several established data sets. Stimuli were chosen from the following picture sets: KDEF (http://www.emotionlab.se/resources/kdef); the NimStim Face Stimulus Set (http://www.macbrain.org/); Pictures of facial affect (Ekman & Friesen, 1971), and the FACES database (Ebner, Riediger, & Lindenberger, 2010). All images were converted to grayscale and the cumulative brightness was normalized across images. Additionally, images were slightly rotated and cropped with an elliptic mask resulting in images containing only the face with both eyes at exactly the same height during presentation. For each participant, a sample of 40 individual faces (20 male, 20 female) for each emotional expression was drawn from the stimulus pool. These faces were assigned to the experimental conditions in a balanced way (i.e., the male/female ratio was constant across conditions). The resulting 160 trials were split into three experimental sessions with 55, 55, and 50 trials, respectively. Trial order was randomized.
Each trial consisted of the presentation of a fixation cross for 1 s, a facial stimulus shown for 150 or 3000 ms and a blank grey screen displayed for either 3850 or 1000 ms, respectively. The inter-trial interval (ITI), during which a fixation cross was shown again, varied randomly between 1 and 3 s. During stimulus presentation, we controlled for the initial fixation by unpredictably shifting the images upwards or downwards in each trial. As a consequence of this manipulation either the eyes or the mouth were presented at the position of the fixation cross for half of the stimuli within each emotion category, respectively.
Whenever a facial stimulus appeared on the screen, participants were ask to classify the depicted emotion as quickly and accurately as possible by pressing one of four keys on a standard computer keyboard with the index or the middle finger of both hands.
2.4.2. Data analysis
Emotion recognition accuracy was determined by calculating the proportion of correct emotion classifications for each experimental condition. Additionally, we analyzed response latencies for trials with correct emotion classifications and corrected for outliers by removing all trials in which reaction times (RTs) exceeded the individual mean latency plus or minus three standard deviations.
With respect to the eye-tracking data, eye movements were parsed into saccades and fixations using velocity and acceleration thresholds of 30°/s and 8000°/s2, respectively, for saccade detection. Time intervals between saccades were defined as fixation. Using similar procedures as in previous studies (e.g., Boll and Gamer, 2014, Scheller et al., 2012), we analyzed the direction of the first saccade following stimulus onset as well as dwell times on the eye and mouth region when faces were presented for 3000 ms. For all eye-tracking measures, we initially removed trials with saccades > 1° or eye blinks during a baseline period of −300 to 150 ms relative to the onset of facial stimuli from further analyses. To quantify initial gaze orienting towards the eyes and towards the mouth we extracted the first (reflexive) saccade after stimulus onset. These initial fixation changes were detected, when they occurred within a time interval of 150–1000 ms after stimulus onset and their amplitude exceeded 1°. Only vertical gaze shifts were included in the analyses. When the eyes were initially fixated, downward fixation changes towards the mouth were scored. When the mouth was initially fixated, upward fixation changes towards the eyes were scored. The total numbers of these initial gaze changes across trials were divided by the number of valid trials per experimental condition resulting in proportional values. Additionally, we identified the amount of time participants spent looking at either the eye or the mouth region during the long stimulus duration. In more detail, the cumulative fixation time on predefined rectangular regions of interest centred on the respective facial feature was assessed during six successive time bins of 500 ms each and divided by the respective overall fixation time within the bin corrected for the occurrence of blinks and saccades. The analysis of the fixation time course using bins of 500 ms was conducted to evaluate whether fixation preferences changed during the total 3000 ms viewing duration. Moreover, potential hyperscanning of emotional faces was examined by determining the scanpath length during the long stimulus presentation. This was done by summing up the length of all saccades irrespective of their amplitude across the entire stimulus duration when faces were presented for 3000 ms.
We calculated repeated measures 2 × 2 × 4 × 2-ANOVAs with within-subject factors initial fixation, presentation time, emotional expression and the between-subjects factor group for hit rates, RTs and initial fixation changes. Face dwell times were pooled across initial fixation position and analyzed using a 2 × 4 × 6 × 2-ANOVA with within-subject factors fixated region, emotional expression, time bin and the between-subjects factor group. A 2 × 4 × 2-ANOVA with factors initial fixation, emotional expression and group was applied to examine potential differences in hyperscanning. Significant main (regarding factors with more than two levels) or interaction effects were followed by post-hoc pairwise comparisons with p-values adjusted according to Tukey's honest significant difference method.
2.5. Experiment 2: emotional gaze-cueing task
2.5.1. Stimuli and design
The emotional gaze-cueing task was based on a fully-crossed 3 × 5 × 2-design with within-subject factors gaze direction (left, right, frontal), emotional expression (angry, fearful, happy, neutral, or neutral arrow cue) and the between-subjects factor group. Eight male and eight female faces gazing either to the left, to the right or displaying a frontal gaze direction were selected from the Radboud Faces Database (RaFD; Langner et al., 2010). Faces depicted angry, fearful, happy, and neutral expressions. For each facial expression, pictures were available that either showed leftward, rightward, or frontal gaze direction. All faces were converted to grayscale, the cumulative brightness was normalized across images and an elliptical mask was applied to each image to hide hair, ears and shoulders. As a control condition, arrows pointing either to the left, to the right or having an inconclusive, straight line on both sides as an arrowhead were presented during the task in addition to the faces. The 240 experimental trials were randomly assigned to four experimental sessions with 60 trials each. Trial order within each session was randomized.
In each trial, emotional facial expressions or arrows were presented for 2 s after a fixation period of 1 s. At 700 ms after the onset of the faces, a target stimulus (the letter E or F) appeared on the left- or on the right-hand side of the pictures. ITIs varied randomly from 3 to 5 s. Faces were displayed centrally such that the tip of the nose appeared at the position of the fixation cross. Arrows were shown slightly above the fixation cross at the height of the eye region. Gaze direction and target location were fully counterbalanced across all factors. Importantly, gaze direction was not predictive of the target location resulting in equal proportions of valid (i.e., target appearing at the gazed-at location) and invalid trials (i.e., target appearing at the opposite location). Participants were asked to identify the target letter as quickly and as accurately as possible by pressing the corresponding key on a standard computer keyboard. Two stacked keys (H and space) were chosen as response buttons instead of keys aligned left and right from each other (Driver et al., 1999). This was done to reduce the Simon effect which triggers faster and more accurate responses when target location and motor response are spatially congruent.
2.5.2. Data analysis
Arrows represented a non-social control cue and did not reveal any group effects when analyzed separately. Therefore, we restricted our analyses to facial cues. For all measures, we only analyzed trials in which a correct answer was given and removed trials in which RTs were smaller or larger than the individual mean latency plus or minus three standard deviations.
To investigate gaze-cueing effects, response latencies were analyzed as a function of experimental conditions using a 2 × 4 × 2 repeated measures ANOVA with within-subject factors validity (valid vs. invalid gaze cues), emotional expression (angry, fearful, happy, neutral) and the between subjects factor group. Similar to experiment 1, eye-tracking data were corrected for trials in which saccades with an amplitude of >1° or eye blinks occurred during a baseline period of −300 to 150 ms relative to stimulus onset. For all remaining trials, gaze-following effects were examined by determining the proportion of gaze congruent and incongruent saccades relative to all first and second saccades, respectively, within a scoring window of 150–700 ms relative to face onset (i.e., before target onset). Only saccades with a horizontal amplitude of >1° were evaluated. Gaze-following effects were statistically analyzed using a 2 × 4 × 2 × 2-ANOVA with within-subject factors gaze congruency (congruent, incongruent), emotional expression (angry, fearful, happy, neutral), saccade (first, second) and the between subjects factor group. The significant interaction of group, gaze congruency and saccade (see Section 3) was followed by separate 2 × 4 × 2-ANOVAs within each group as well as post-hoc pairwise comparisons with p-values adjusted according to Tukey's honest significant difference method.
The frontal gaze direction was included in the paradigm to increase the variance of facial gaze cues and to control for effects of eye gaze. As neither gaze-cueing nor gaze-following can occur with direct-gazing faces, the frontal gaze direction was not considered in the above mentioned analyses. However, in a supplementary analysis we analyzed saccades towards the eyes and evaluated whether patients tend to avoid the eyes more frequently than controls when they were looking at them (see Supplement). This analysis did not reveal evidence for such response pattern.
3. Results
3.1. Sample characteristics
The social phobia group reported significantly higher levels for social anxiety, depression, anxiety and alexithymia indices as shown in Table 1, whereas scores of the control group were all within normal range. Only for social desirability scores no significant group differences were observed. As shown in supplementary analyses, depression, trait anxiety and alexithymia do not account for differences between social phobia patients and healthy controls in the eye-tracking results of both experiments (see Supplement).
3.2. Experiment 1: emotion classification task
3.2.1. Behavioural data
Overall, classification accuracy was high for patients (M = 93.55%, SD = 3.95%) as well as for controls (M = 92.95%, SD = 6.36%). No significant group difference was found. In both groups, participants achieved higher hit rates during the long than during the short presentation time (main effect presentation time: F(1,42) = 4.65, p < 0.05, f = 0.33), and less correct responses were observed for angry faces as compared to the other facial expressions (main effect emotional expression: F(3,40) = 8.82, p < 0.001, f = 0.47; all pairwise comparisons to angry faces p < 0.01). Response latencies were also comparable between both groups (Patients: M = 1219.15 ms, SD = 217.32 ms, controls: M = 1137.61 ms, SD = 156.80 ms). In general, RTs were faster during the short than during the long stimulus presentation (main effect presentation time: F(1,42) = 13.95, p < 0.001, f = 0.58), and when the eye region was initially fixated (main effect initial fixation: F(1,42) = 4.66, p < 0.05, f = 0.33). Moreover, response latencies differed significantly between emotional expressions (main effect emotional expression: F(3,40) = 18.40, p < 0.001, f = 0.80). Participants responded fastest to happy faces, followed by neutral expressions, and slowest to angry and fearful faces (p < 0.05 for the respective pairwise comparisons). All other effects did not reach statistical significance.
3.2.2. Eye movement data
Participants in both groups made more initial saccades towards the eyes than towards the mouth (main effect initial fixation: F(1,42) = 73.26, p < 0.001, f = 1.32, see Fig. 1).
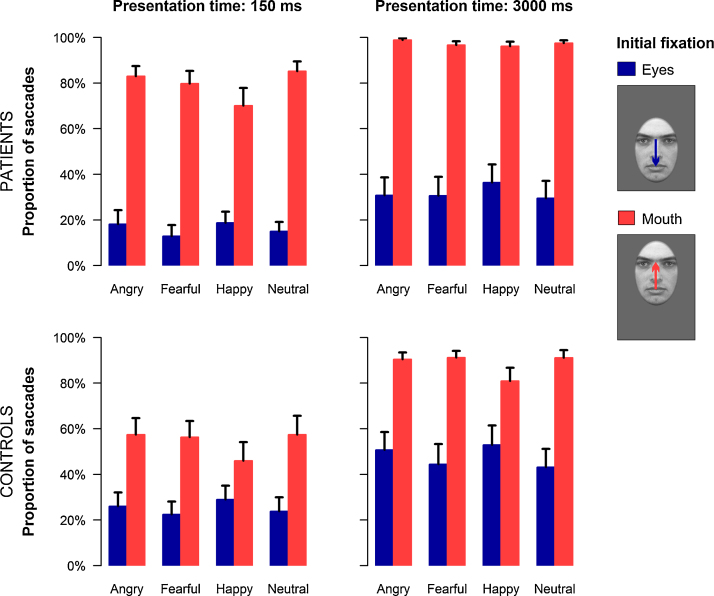
Fig. 1.
Emotion classification task (experiment 1): Proportion of saccades as a function of initial fixation and emotional expression are depicted for patients and controls. Data for both groups are shown separately for the short (left side) and the long (right side) stimulus duration. Proportions reflect the number of first, reflexive saccades relative to the number of valid trials for each experimental condition. Error bars indicate SEM.
However, this gaze bias towards the eye region differed between groups (interaction of group and initial fixation: F(1,42) = 6.52, p < 0.05, f = 0.39) with social phobia patients showing significantly more saccades towards the eyes as compared to healthy controls (p < 0.05). For both groups, the pattern of initial fixation changes followed the distribution of diagnostically relevant facial features (interaction of emotional expression and initial fixation: F(3,40) = 13.39, p < 0.001, f = 0.61). Thus, patients and controls showed more saccades towards the eye region of fearful and neutral as compared to happy faces (all p < 0.01) as well as more saccades towards the mouth of happy faces as contrasted to all other facial expressions (all p < 0.001). Additionally, a greater number of fixation changes irrespective of the initially fixated feature was observed during the long stimulus presentation (main effect presentation time: F(1,42) = 64.33, p < 0.001, f = 1.24). This latter effect was also qualified by a significant interaction of group and presentation time (F(1,42) = 4.27, p < 0.05, f = 0.32) but post-hoc pairwise comparisons within each presentation time condition did not yield significant group differences. Taken together, these results demonstrate a clear hypervigilance for the eye relative to the mouth region in patients with social phobia.
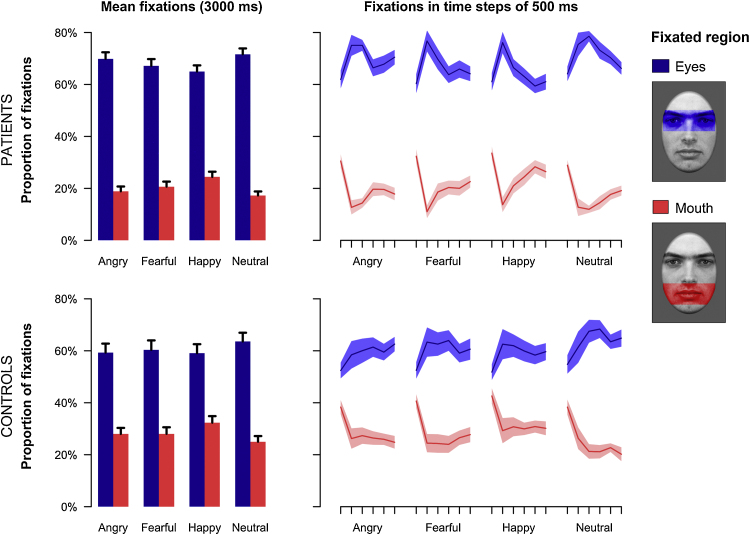
Fig. 2 indicates that participants mainly fixated on the eyes and the mouth during the whole 3 s stimulus presentation. In line with the early attentional bias for the eye region evident in the first saccades after stimulus onset, total dwell times for the long stimulus duration were also higher on the eyes as compared to the mouth region (main effect fixated region: F(1,42) = 144.10, p < 0.001, f = 1.85, see Fig. 3). Again, patients with social phobia were found to fixate the eye region longer (p < 0.05) and the mouth region shorter (p < 0.05) than healthy controls (interaction of group and fixated region: F(1,42) = 5.38, p < 0.05, f = 0.36). Mirroring the results obtained for the initial fixation changes, the fixation bias for the eye region differed between facial expressions (interaction of emotional expression and fixated region: F(3,40) = 16.60, p < 0.001, f = 0.69). The eye region was fixated shorter (all p < 0.05) and the mouth region longer (all p < 0.001) for happy as compared to all other facial expressions indicating that fixation durations were increased for facial features with higher diagnostic relevance. Our data revealed no evidence for a later avoidance of the eye region in patients with social phobia as no significant interaction of group, time bin and fixated feature was found (p > 0.05). Overall, fixation durations decreased after the first time bin (main effect time bin: F(5,38) = 13.57, p < 0.001, f = 0.54), although the strongest bias to primarily fixate the eye region was observed during the second time bin and the strongest bias for the mouth region was found for the first time bin (interaction of time bin and fixated region: F(5,38) = 20.84, p < 0.001, f = 0.67). Moreover, the time course of dwell times on the eye and mouth region varied as a function of emotional expression as can be seen in the right panel of Fig. 3 (interaction of time bin, emotional expression and fixated region: F(15,28) = 3.22, p < 0.01, f = 0.32). No further significant effects were observed for both eye-tracking measures.
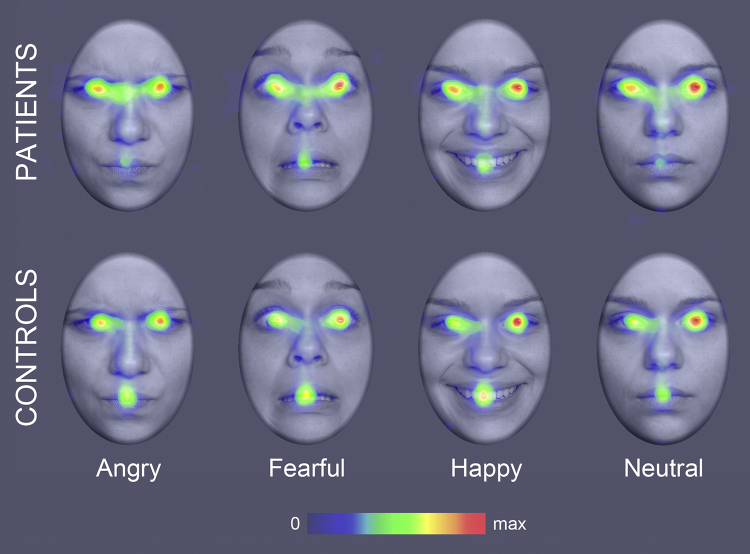
Fig. 2.
Emotion classification task (experiment 1): Heatmaps illustrating the fixation density on different emotional expressions for patients and controls. Data were only analyzed for the long stimulus duration.
Fig. 3.
Emotion classification task (experiment 1): Dwell times on the eye and mouth region for patients and controls. On the left, average dwell times during the whole 3000 ms interval are shown as a function of the fixated region and emotional expression. In the right panel, dwell times are depicted for six time bins of 500 ms each. Error bars (left) or colored bands (right) indicate SEM.
No evidence for hyperscanning of emotional faces in social phobia was found in the current data set. The average length of the fixation scanpath was slightly larger for patients (M = 9.02°, SD = 2.68°) than for controls (M = 7.94°, SD = 2.68°), but the group effect was not significant (p > 0.1). Generally, the fixation scanpath length was reduced for happy faces as compared to all other facial expressions (main effect emotional expression: F(3,40) = 15.63, p < 0.001, f = 0.67; all pairwise comparisons to happy faces p < 0.001), and for trials in which the eye region was initially fixated (main effect initial fixation: F(1,42) = 5.25, p < 0.05, f = 0.35; see also Supplement Fig. S1).
3.3. Experiment 2: gaze-cueing task
3.3.1. Behavioural data
Patients with social phobia identified the target letter correctly in 97.32% (SD = 1.68%) of the trials and controls hit the correct button in 96.15% (SD = 2.85%) of all trials. The two groups did not differ significantly (p > 0.1). Patients with social phobia (M = 713.03 ms, SD = 96.91 ms) were significantly slower in detecting the target letter than control participants (M = 624.73 ms, SD = 90.30 ms; main effect group: F(1,42) = 9.77, p < 0.01, f = 0.48). The gaze-cueing effect did not reach statistical significance, nor did any of the other effects in the repeated measures ANOVA model (see Supplemental Fig. S2). As RTs in the current study were generally rather slow as compared to previous studies and gaze-cueing effects have been reported to depend on brief stimulus onset asynchronies (SOAs) between cue and target presentation (see Frischen, Bayliss, & Tipper, 2007 for a review), we additionally performed separate analyses for fast and slow RTs (below and above median RT of 665.14 ms). When considering fast RTs relative to the median, a significant gaze-cueing effect was observed (main effect validity: F(1,40) = 6.92, p < 0.05, f = 0.42) in addition to the group effect (F(1,40) = 14.86, p < 0.001, f = 0.61), whereas no significant effects were observed for slow RTs relative to the median. When only RTs faster than the median of 665.14 ms were analyzed, more trials were excluded for patients (M = 55.28, SD = 22.48) than for controls (M = 33.47, SD = 22.67), due to the significantly prolonged RTs in the former group.
3.3.2. Eye movement data
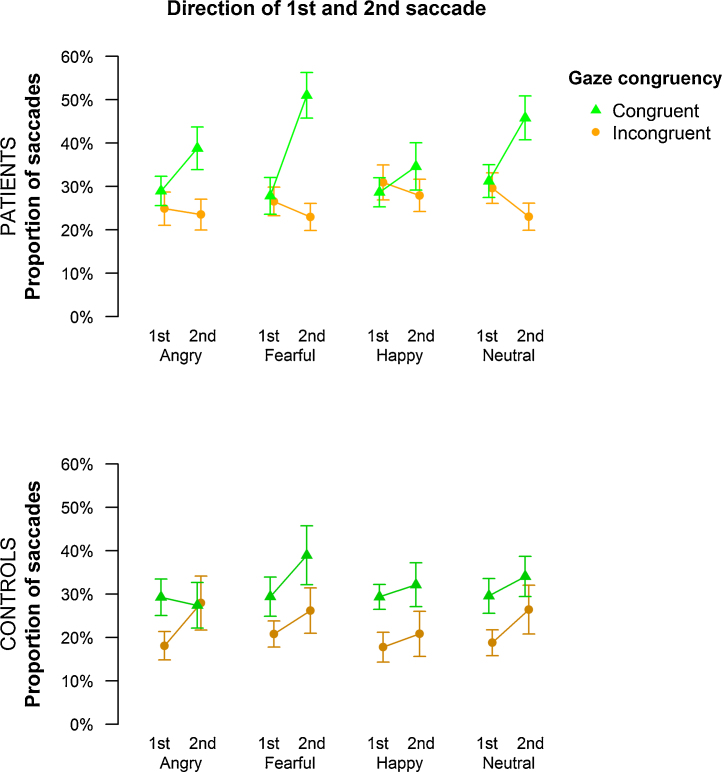
The average number of saccades did not differ between patients with social phobia (M = 1.53, SD = 0.71) and controls (M = 1.19, SD = 0.64, p > 0.1), and the likelihood for observing more than two saccades was low (patients: M = 13.93%, SD = 22.77%; controls: M = 7.40%, SD = 12.34%). Thus, we focused our analyses on first and second congruent and incongruent saccades and observed a gaze-following effect indicating that participants directed their attention more frequently into the gaze direction of the depicted faces than into the opposite direction (main effect congruency: F(1,42) = 22.49, p < 0.001, f = 0.73; see Fig. 4). Interestingly, gaze-following varied as a function of time (interaction of congruency and saccade: F(1,42) = 4.48, p < 0.05, f = 0.33), and this time course differed between the two study groups (interaction of congruency, saccade and group: F(1,42) = 8.59, p < 0.01, f = 0.45). The likelihood of showing lateralized saccades was increased for the second relative to the first saccade (main effect of saccade: F(1,42) = 4.76, p < 0.05, f = 0.34). To deconstruct the observed three-way interaction, separate ANOVAs were calculated within each group. For healthy controls, only the main effect of congruency reached significance (F(1,21) = 10.66, p < 0.01, f = 0.71), thus indicating similar gaze following across both saccades and all facial expressions. Patients with social phobia also exhibited gaze following (main effect congruency: F(1,21) = 11.84, p < 0.01, f = 0.75) but this effect was markedly reduced for the first (no significant difference between congruent and incongruent saccades) as compared to the second saccade (p < 0.001) as reflected in an interaction of congruency and saccade (F(1,21) = 9.56, p < 0.01, f = 0.67). Finally, gaze following differed between emotional expressions in the group of patients (interaction of emotional expression and congruency, F(1,42) = 3.30, p < 0.05, f = 0.44) with relatively more congruent than incongruent saccades occurring for angry (p < 0.05) and fearful emotional expressions (p < 0.001).
Fig. 4.
Gaze-cueing task (experiment 2): Gaze-following effects in patients and controls. The time course of gaze congruent and incongruent saccades is shown as a function of emotional expressions. Here, the term congruency refers only to the gaze direction of the faces and not to the location of the target letter. Gaze congruent and incongruent saccades were assessed when the cue was presented and before the target letter appeared on the screen. As the figure illustrates, gaze-following effects for 1st and 2nd saccades (more congruent than incongruent saccades) can be seen for controls, whereas patients only show gaze-following for the 2nd saccade. Error bars indicate SEM.
4. Discussion
The current results demonstrate a clear hypervigilance for the eye relative to the mouth region in patients with social phobia and show no evidence for a later avoidance of eye gaze during the entire 3 s stimulus presentation interval. Regardless of the type of emotional expression patients as compared to controls reflexively shifted their attention more frequently towards the eye region suggesting that automatic attentional orienting towards the eyes but not towards diagnostically relevant facial features in general is biased in social phobia. Moreover, we provide evidence for a modulation of eye gaze processing in participants with social phobia by revealing a differential time course of gaze-following in patients and controls.
The currently observed enhanced tendency to reflexively shift attention towards the eyes of emotional faces in patients suffering from social phobia is in line with previous reports on hypervigilance for socially threatening stimuli in social anxiety (Mogg et al., 2004, Spector et al., 2003). Thus, initial attentional orienting towards the eye region might reflect an increased sensitivity for socially relevant and potentially threatening cues in individuals with social phobia and contribute to the development of social fears. However, our data argue against a later avoidance of eye gaze, as dwell times on the eye relative to the mouth region were also increased in participants suffering from social phobia when stimuli were presented for a longer duration of 3 s. This finding is rather inconsistent with previous studies showing that social anxiety leads to an avoidance of eye gaze. Most likely, such avoidance primarily occurs when participants are engaged in social interactions instead of simply observing computerized faces (Baker & Edelmann, 2002; Farabee, Melvin, Ramsey, & Cole, 1993), and when emotional faces are presented for longer time intervals than in the current paradigm (Horley et al., 2003, Horley et al., 2004, Moukheiber et al., 2010, Weeks et al., 2013). Prolonged stimulus presentation times in earlier studies may also account for previous findings of hyperscanning of emotional faces (Horley et al., 2003, Horley et al., 2004), that were also absent in the current investigation.
Contrary to previous studies, the current emotion classification paradigm was optimized for differentiating reflexive from sustained attentional processes and thereby revealed an early attentional bias in patients that persisted, when stimuli were presented for 3 s. Furthermore, it enabled us to differentiate whether diagnostically most relevant facial features or the eyes in general are processed preferentially in social phobia. In accordance with previous studies, the attentional bias towards the eyes was attenuated when happy faces were presented, because the mouth region is diagnostically more relevant for this emotion (Boll and Gamer, 2014, Gamer and Büchel, 2009; Gamer, Schmitz, Tittgemeyer, & Schilbach, 2013; Gamer, Zurowski, & Büchel, 2010; Scheller et al., 2012). Yet, this interaction of emotion and initial fixation was comparable between both groups suggesting that only processing of the eye region but not of diagnostically relevant facial features is affected in social phobia. Moreover, emotion recognition accuracy was comparable between patients and controls, which is largely in line with previous studies which often failed to detect group differences in social phobia and anxiety (Arrais et al., 2010; Heuer, Lange, Isaac, Rinck, & Becker, 2010) or reported group differences only for evaluating negative compared to positive stimuli (Foa, Gilboa-Schechtman, Amir, & Freshman, 2000; Joormann & Gotlib, 2006). Thus, beyond facilitating emotion recognition, the eyes of emotional faces are of special relevance for individuals with social phobia presumably by conveying important information about other peoples’ intentions and thoughts. Increased reflexive vigilance towards the eyes in concert with interpretation biases characteristic for the disorder (Clark & McManus, 2002) might therefore reinforce fear of scrutiny and negative evaluation in social phobia.
If attention to the eye region is biased in patients with social phobia, the perception of eye gaze might also be affected. Gaze signals the focus of attention and therefore acts as an important clue to guide and interpret social behavior of conspecifics. Several studies have focused on investigating eye gaze perception in social phobia showing for instance a more liberal criterion for perceiving eye contact in social phobia/anxiety (Gamer, Hecht, Seipp, & Hiller, 2011; Jun, Mareschal, Clifford, & Dadds, 2013), and differences in the processing of direct versus averted gaze as a function of social anxiety (Schmitz, Scheel, Rigon, Gross, & Blechert, 2012; Wieser, Pauli, Alpers et al., 2009). Yet, to the best of our knowledge, attentional orienting by gaze direction has never been studied in social phobia. In healthy participants, gaze-cueing paradigms as employed in the current study consistently revealed attention shifting into the direction of eye gaze followed by substantial gaze-cueing effects, i.e. faster detection of validly compared to invalidly cued targets (for review see Frischen et al., 2007). Here, we observed differences in the time course of attentional orienting triggered by gaze cues between patients and controls suggesting a differential processing of eye gaze. Whereas control participants followed the gaze of emotional faces immediately and directed already their first saccade more frequently into the direction of eye gaze than into the opposite direction, individuals with social phobia only showed a delayed gaze-following effect.
Gaze-following as a fundamental component of joint attention has already been shown to be altered in neurodevelopmental disorders involving severe social deficits such as autism and schizophrenia (Langdon, Corner, McLaren, Coltheart, & Ward, 2006; Ristic et al., 2005; Vlamings, Stauder, Son, & Mottron, 2005). The current finding of delayed gaze-following in patients with social phobia is therefore highly interesting. Apparently, gaze-following occurs more automatically in healthy participants, whereas attention in social phobic individuals is initially captured by socially meaningful cues such as the eyes per se along with difficulties to disengage attention from them as also suggested by traditional models of social phobia (Rapee & Heimberg, 1997). This idea is consistent with the observation that initial saccades were mainly directed towards the eye region in the gaze-cueing paradigm (see Supplement), whereas subsequent saccades were more lateralized in both groups. Moreover, a marked tendency to initially direct attention towards the eye region in social phobia was observed in the emotion classification paradigm, although no significant group differences in the frequency of saccades towards the eye region were observed in the gaze-cueing paradigm. However, as the stimulus position was highly predictable in the gaze-cueing in contrast to the emotion classification paradigm, the task was probably less suitable to detect group differences in reflexive orienting towards specific facial features.
Differences in the time course of attentional orienting towards gaze cues did not translate into group differences with respect to gaze-cueing behavior. Shorter SOAs between cue and target presentation might however capture solely immediate gaze-following effects as observed for healthy controls and might therefore result in differences in gaze-cueing effects between patients and controls. Notably, across both groups faster detection of validly compared to invalidly cued targets was only observed for fast reaction times in the gaze-cueing paradigm presumably due to generally high response latencies across both groups and a rather long SOA of 700 ms.
The current results also fit with the neuroscientific literature on amygdala hyperactivity for emotional faces in social phobia (Lira Yoon, Fitzgerald, Angstadt, McCarron, & Phan, 2007; Phan, Fitzgerald, Nathan, & Tancer, 2006; Straube, Mentzel, & Miltner, 2005; Veit et al., 2002). Recent studies on the functional role of amygdala responses to facial expressions suggest that activity in this brain region is implicated in triggering reflexive gaze shifts towards the eyes (Gamer and Büchel, 2009, Gamer et al., 2013). Hence, exaggerated amygdala activation in social phobia may also underlie the observed hyperactivity towards the eye region in the current emotion classification paradigm. Moreover, our finding of a delayed gaze-following effect in patients as compared to controls suggests dysfunctions in other neural circuits that are implicated in perceptual processing of eye gaze besides the amygdala (Akiyama et al., 2007). One such candidate region is the posterior superior temporal sulcus (pSTS) that was found to mediate gaze following in macaques (Roy, Shepherd, & Platt, 2014). Future studies investigating the neural underpinnings of social phobia should therefore clarify the individual contribution of amygdala and pSTS recruitment during face perception to emotional expression and eye gaze processing.
Some limitations of our study should be noted. First, this study included well-matched patient and control groups but it has still to be elucidated how specific the current findings are for social phobia in comparison to other anxiety disorders. Second, the stimulus duration in experiment 1 did not exceed 3000 ms. It seems very interesting to examine whether hypervigilance for the eye region persists for even longer viewing durations in the current emotion recognition task. Third, to enable an analysis of eye movement data, we used a rather long SOA between cues and targets in experiment 2. Since we only observed a behavioral gaze-cueing effect for short reaction times, a shorter SOA would help to elucidate behavioral differences in gaze-cueing between patients and controls.
In summary, we provide evidence for a marked hypervigilance towards the eye region in social phobia. This increased vigilance represents an early, reflexive attentional mechanism that persists at least during the first 3 s of stimulus presentation and does not directly relate to the detection of diagnostically relevant facial features. We further show that eye gaze processing is biased in social phobia by reporting delayed gaze-following effects. Thus, our findings show that basic attentional mechanisms relevant for adequately perceiving and interpreting social cues are impaired in social phobia. A crucial question for future research is how these attentional biases are represented at the neural level and how they contribute to the development and maintenance of social fears.
Acknowledgements
This work was supported by grants from the German Research Foundation (GA 1621/2-1) and the European Research Council (ERC-2013-StG-336305).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.janxdis.2016.04.004.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Akiyama T., Kato M., Muramatsu T., Umeda S., Saito F., Kashima H. Unilateral amygdala lesions hamper attentional orienting triggered by gaze direction. Cerebral Cortex. 2007;17(11):2593–2600. doi: 10.1093/cercor/bhl166. [DOI] [PubMed] [Google Scholar]
- Amir N., Foa E.B., Coles M.E. Automatic activation and strategic avoidance of threat-relevant information in social phobia. Journal of Abnormal Psychology. 1998;107(2):285–290. doi: 10.1037//0021-843x.107.2.285. [DOI] [PubMed] [Google Scholar]
- Arrais K.C., Machado-de-Sousa J.P., Trzesniak C., Filho A.S., Ferrari M.C.F., Osório F.L., MCrippa J.A.S. Social anxiety disorder women easily recognize fearfull, sad and happy faces: the influence of gender. Journal of Psychiatric Research. 2010;44(8):535–540. doi: 10.1016/j.jpsychires.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Bagby R.M., Parker J.D.A., Taylor G.J. The twenty-item Toronto Alexithymia scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Baker S.R., Edelmann R.J. Is social phobia related to lack of social skills? Duration of skill-related behaviours and ratings of behavioural adequacy. The British Journal of Clinical Psychology/the British Psychological Society. 2002;41(Pt 3):243–257. doi: 10.1348/014466502760379118. [DOI] [PubMed] [Google Scholar]
- Barnes L., Harp D., Woo S.J. Reliability generalization of scores on the Spielberger state-trait anxiety inventory. Educational and Psychological Measurement. 2002;62:603. [Google Scholar]
- Boll S., Gamer M. 5-HTTLPR modulates the recognition accuracy and exploration of emotional facial expressions. Frontiers in Behavioral Neuroscience. 2014;8:255. doi: 10.3389/fnbeh.2014.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet P.M., Heisz J.J., Mondloch C.J., Shore D.I., Schmidt L.A. Shyness and face scanning in children. Journal of Anxiety Disorders. 2009;23(7):909–914. doi: 10.1016/j.janxdis.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Clark D.M., McManus F. Information processing in social phobia. Biological Psychiatry. 2002;51(1):92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- Cohen J. 2nd ed. Routledge; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Dalbudak E., Evren C., Aldemir S., Coskun K.S., Yıldırım F.G., Ugurlu H. Alexithymia and personality in relation to social anxiety among university students. Psychiatry Research. 2013;209(2):167–172. doi: 10.1016/j.psychres.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Daly S. Behavioral correlates of social anxiety. The British Journal of Social and Clinical Psychology. 1978;17(2):117–120. doi: 10.1111/j.2044-8260.1978.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Driver J., Davis G., Ricciardelli P., Kidd P., Maxwell E., Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6(5):509–540. [Google Scholar]
- Ebner N.C., Riediger M., Lindenberger U. FACES—a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behavior Research Methods. 2010;42(1):351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. Constants across cultures in the face and emotion. Journal of Personality and Social Psychology. 1971;17(2):124–129. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Farabee D.J., Melvin Holcom L., Ramsey S.L., Cole S.G. Social anxiety and speaker gaze in a persuasive atmosphere. Journal of Research in Personality. 1993;27(4):365–376. [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Gilboa-Schechtman E., Amir N., Freshman M. Memory bias in generalized social phobia: remembering negative emotional expressions. Journal of Anxiety Disorders. 2000;14(5):501–519. doi: 10.1016/s0887-6185(00)00036-0. [DOI] [PubMed] [Google Scholar]
- Frischen A., Bayliss A.P., Tipper S.P. Gaze cueing of attention. Psychological Bulletin. 2007;133(4):694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fydrich T. Diagnostische verfahren in der psychotherapie. Hogrefe; Göttingen, Germany: 2002. Soziale phobie und angst inventar (SPAI) [Social phobia and anxiety inventory (SPAI)] pp. 335–338. [Google Scholar]
- Gamble A.L., Rapee R.M. The time-course of attention to emotional faces in social phobia. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41(1):39–44. doi: 10.1016/j.jbtep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Gamer M., Büchel C. Amygdala activation predicts gaze toward fearful eyes. The Journal of Neuroscience. 2009;29(28):9123–9126. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Hecht H., Seipp N., Hiller W. Who is looking at me? The cone of gaze widens in social phobia. Cognition & Emotion. 2011;25(4):756–764. doi: 10.1080/02699931.2010.503117. [DOI] [PubMed] [Google Scholar]
- Gamer M., Schmitz A.K., Tittgemeyer M., Schilbach L. The human amygdala drives reflexive orienting towards facial features. Current Biology. 2013;23(20):R917–R918. doi: 10.1016/j.cub.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences. 2010;107(20):9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M., Mogg K., Bradley B.P. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115(4):760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M., Worall H., Keller F. Huber; Bern, Switzerland: 1994. Beck depression inventory (BDI) [Google Scholar]
- Heinrichs N., Hofmann S.G. Information processing in social phobia: a critical review. Clinical Psychology Review. 2001;21(5):751–770. doi: 10.1016/s0272-7358(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Heuer K., Lange W.-G., Isaac L., Rinck M., Becker E.S. Morphed emotional faces: emotion detection and misinterpretation in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41(4):418–425. doi: 10.1016/j.jbtep.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Horley K., Williams L.M., Gonsalvez C., Gordon E. Social phobics do not see eye to eye: a visual scanpath study of emotional expression processing. Journal of Anxiety Disorders. 2003;17(1):33–44. doi: 10.1016/s0887-6185(02)00180-9. [DOI] [PubMed] [Google Scholar]
- Horley K., Williams L.M., Gonsalvez C., Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Research. 2004;127(1–2):43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Joormann J., Gotlib I.H. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. Journal of Abnormal Psychology. 2006;115(4):705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- Jun Y.Y., Mareschal I., Clifford C.W.G., Dadds M.R. Cone of direct gaze as a marker of social anxiety in males. Psychiatry Research. 2013;210(1):193–198. doi: 10.1016/j.psychres.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Langdon R., Corner T., McLaren J., Coltheart M., Ward P.B. Attentional orienting triggered by gaze in schizophrenia. Neuropsychologia. 2006;44(3):417–429. doi: 10.1016/j.neuropsychologia.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Langner O., Dotsch R., Bijlstra G., Wigboldus D.H.J., Hawk S.T., van Knippenberg A. Presentation and validation of the radboud faces database. Cognition & Emotion. 2010;24(8):1377–1388. [Google Scholar]
- Lira Yoon K., Fitzgerald D.A., Angstadt M., McCarron R.A., Phan K.L. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Research: Neuroimaging. 2007;154(1):93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Mattick R.P., Clarke J.C. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy. 1998;36(4):455–470. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- Mogg K., Philippot P., Bradley B.P. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113(1):160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Moukheiber A., Rautureau G., Perez-Diaz F., Jouvent R., Pelissolo A. Gaze behaviour in social blushers. Psychiatry Research. 2012;200(2–3):614–619. doi: 10.1016/j.psychres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Moukheiber A., Rautureau G., Perez-Diaz F., Soussignan R., Dubal S., Jouvent R., Pelissolo A. Gaze avoidance in social phobia: objective measure and correlates. Behaviour Research and Therapy. 2010;48(2):147–151. doi: 10.1016/j.brat.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Rapee R.M., Heimberg R.G. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35(8):741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Richter P., Werner J., Heerlein A., Kraus A., Sauer H. On the validity of the Beck depression inventory. A review. Psychopathology. 1998;31(3):160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- Ristic J., Mottron L., Friesen C.K., Iarocci G., Burack J.A., Kingstone A. Eyes are special but not for everyone: the case of autism. Cognitive Brain Research. 2005;24(3):715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Roy A., Shepherd S.V., Platt M.L. Reversible inactivation of pSTS suppresses social gaze following in the macaque (Macaca mulatta) Social Cognitive and Affective Neuroscience. 2014;9(2):209–217. doi: 10.1093/scan/nss123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller E., Büchel C., Gamer M. Diagnostic features of emotional expressions are processed preferentially. Public Library of Science. 2012;7(7):e41792. doi: 10.1371/journal.pone.0041792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Scheel C.N., Rigon A., Gross J.J., Blechert J. You don’t like me, do you? Enhanced ERP responses to averted eye gaze in social anxiety. Biological Psychology. 2012;91(2):263–269. doi: 10.1016/j.biopsycho.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Rodebaugh T.L., Blanco C., Lewin H., Liebowitz M.R. Fear and avoidance of eye contact in social anxiety disorder. Comprehensive Psychiatry. 2011;52(1):81–87. doi: 10.1016/j.comppsych.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield C.A., Inhoff A.W., Coles M.E. Time-course of attention biases in social phobia. Journal of Anxiety Disorders. 2013;27(7):661–669. doi: 10.1016/j.janxdis.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Seefeldt W.L., Krämer M., Tuschen-Caffier B., Heinrichs N. Hypervigilance and avoidance in visual attention in children with social phobia. Journal of Behavior Therapy and Experimental Psychiatry. 2014;45(1):105–112. doi: 10.1016/j.jbtep.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl. 20):22–33. [quiz 34–57]. [PubMed] [Google Scholar]
- Smith M.L., Cottrell G.W., Gosselin F., Schyns P.G. Transmitting and decoding facial expressions. Psychological Science. 2005;16(3):184–189. doi: 10.1111/j.0956-7976.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- Spector I.P., Pecknold J.C., Libman E. Selective attentional bias related to the noticeability aspect of anxiety symptoms in generalized social phobia. Journal of Anxiety Disorders. 2003;17(5):517–531. doi: 10.1016/s0887-6185(02)00232-3. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the state-trait anxiety inventory. [Google Scholar]
- Stöber J. The Social Desirability Scale-17 (SDS-17): convergent validity, discriminant validity, and relationship with age. European Journal of Psychological Assessment. 2001;17(3):222. [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Turner, Beidel, Dancu, Stanley An empirically derived inventory to measure social fears and anxiety: the Social Phobia and Anxiety Inventory. Psychological Assessment. 1989;1:35–40. [Google Scholar]
- Veit R., Flor H., Erb M., Hermann C., Lotze M., Grodd W., Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328(3):233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Vlamings P.H.J.M., Stauder J.E.A., van Son I.A.M., Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high functioning autism. Journal of Autism and Developmental Disorders. 2005;35(3):267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Weeks J.W., Howell A.N., Goldin P.R. Gaze avoidance in social anxiety disorder. Depression and Anxiety. 2013;30(8):749–756. doi: 10.1002/da.22146. [DOI] [PubMed] [Google Scholar]
- Wieser M.J., Pauli P., Alpers G.W., Mühlberger A. Is eye to eye contact really threatening and avoided in social anxiety?—An eye-tracking and psychophysiology study. Journal of Anxiety Disorders. 2009;23(1):93–103. doi: 10.1016/j.janxdis.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Wieser M.J., Pauli P., Weyers P., Alpers G.W., Mühlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: an eye-tracking study. Journal of Neural Transmission. 2009;116(6):717–723. doi: 10.1007/s00702-008-0101-0. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 1992. ICD-10classifications of mental and behavioural disorder: clinical descriptions and diagnostic guidelines. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.