Abstract
Gasification of organic wastes coupled to syngas fermentation allows the recovery of carbon in the form of commodity chemicals, such as carboxylates and biofuels. Acetogenic bacteria ferment syngas to mainly two-carbon compounds, although a few strains can also synthesize four-, and six-carbon molecules. In general, longer carbon chain products have a higher biotechnological (and commercial) value due to their higher energy content and their lower water solubility. However, de-novo synthesis of medium-chain products from syngas is quite uncommon in acetogenic bacteria. An alternative to de-novo synthesis is bioproduction of short-chain products (C2 and C4), and their subsequent elongation to C4, C6, or C8 through reversed β-oxidation metabolism. This two-step synergistic approach has been successfully applied for the production of up to C8 compounds, although the accumulation of alcohols in these mixed cultures remained below detection limits. The present work investigates the production of higher alcohols from syngas by open mixed cultures (OMC). A syngas-fermenting community was enriched from sludge of an anaerobic digester for a period of 109 days in a lab-scale reactor. At the end of this period, stable production of ethanol and butanol was obtained. C6 compounds were only transiently produced at the beginning of the enrichment phase, during which Clostridium kluyveri, a bacterium able to carry out carbon chain elongation, was detected in the community. Further experiments showed pH as a critical parameter to maintain chain elongation activity in the co-culture. Production of C6 compounds was recovered by preventing fermentation pH to decrease below pH 4.5–5. Finally, experiments showed maximal production of C6 compounds (0.8 g/L) and alcohols (1.7 g/L of ethanol, 1.1 g/L of butanol, and 0.6 g/L of hexanol) at pH 4.8. In conclusion, low fermentation pH is critical for the production of alcohols, although detrimental to C. kluyveri. Fine control of fermentation pH to final values around 4.8 could allow sustained production of higher alcohols.
Keywords: alcohols, chain elongation, pH, mixed culture, syngas fermentation
Introduction
The current industrial society depends heavily on fossil resources as a source of energy and raw materials. About 80% of the primary energy used worldwide is obtained from fossil fuels (Sims et al., 2013), while oil is an essential part of almost any material we use within our daily lives. Combustion of fossil fuels is a major source of CO2 to the atmosphere (Hartmann et al., 2013), with significant negative impacts on the environment due to air pollution and the enhancement of global warming. In this respect, the depletion of oil reserves and the strategies to mitigate climate change have triggered the development of alternative sources of renewable and carbon-neutral biofuels and chemical building blocks (Marshall et al., 2013).
One of the most promising approaches is the thermal gasification of non-alimentary feedstock sources (i.e., agricultural, forestry, and municipal wastes, biosolids from wastewater treatment plants) to synthesis gas (syngas), a producer gas mainly composed of H2, CO, and CO2 (Hernández et al., 2013), and its subsequent fermentation to organic acids and/or alcohols. Among its key advantages: (i) its unifying nature allows transformation of heterogeneous resources into a fermentable gas stream using a single technology (Daniell et al., 2012); (ii) biocatalysts are tolerant to impurities such as NOx, H2S, and NH3 (Munasinghe and Khanal, 2010; Xu et al., 2011); and (iii) syngas composition may vary considerably due to the heterogeneity of the feedstocks, but biocatalysis is highly independent of CO:CO2:H2 ratios (Heiskanen et al., 2007).
Biological fermentation of syngas is carried out by acetogens, strict anaerobic bacteria able convert CO2 and/or CO to acetyl-CoA through the Wood-Ljungdahl pathway (Ragsdale and Pierce, 2008). Acetyl-CoA can be further transformed to organic acids (i.e., acetate, butyrate), and alcohols (i.e., ethanol, butanol). The latter are of special interest due to their potential as biofuels (Dürre, 2007; Ranjan and Moholkar, 2012). Until now, more than 100 acetogens have been isolated, although not all of them can use CO (or were tested for CO utilization; Diender et al., 2015). Acetic acid is their main end-metabolite, although some acetogenic organisms can produce also ethanol, butyrate, or butanol (Schiel-Bengelsdorf and Dürre, 2012). However, among the plethora of acetogenic bacteria, only two strains, Clostridium carboxidivorans P7 and “Clostridium ragsdalei” P11, have been reported to produce C6 compounds (caproic acid and/or hexanol) under abundant supply of reducing power (Ramachandriya et al., 2013), during incubation at sub-optimal temperatures (Ramió-Pujol et al., 2015), or using mineral medium and controlling the supply of CO (Phillips et al., 2015).
Syngas fermentation has been mainly studied in pure cultures (Daniell et al., 2012). However, mixed cultures enriched from environmental samples have several potential advantages, such as a higher adaptive capacity (Kleerebezem and van Loosdrecht, 2007), and the selection of the most efficient and effective microbial catalysts (Marshall et al., 2013). Such features confer mixed cultures with a high resistance and resilience to changing environmental conditions, and an increased functional redundancy, aspects that are pivotal to maintain or recover bioreactor productivity in front of many operational eventualities (Marshall et al., 2013). Additionally, positive synergistic relationships can be established within complex bacterial communities. It is then likely that in mixed cultures composed of different bacterial species, the carboxydotrophic production of C2 compounds can be coupled to chain elongation processes, by which short-chain molecules are condensed to produce longer carbon chain products (4, 6, and 8 carbon atoms; Agler et al., 2011; Steinbusch et al., 2011). On the downside, it is challenging to ensure that such communities can deliver a stable production over long periods of time. To date, several works have explored the potential of syngas fermentations in mixed cultures, most of them taking sludge from anaerobic reactors treating organic waste as starting point (Guiot et al., 2011; Alves et al., 2013; Zhang et al., 2013). Among them, Zhang and co-workers demonstrated the feasibility of fermenting a mixture of CO2 and H2 into short (7.4 g/L acetate; 1.8 g/L butyrate) and medium-chain organic acids (0.98 g/L caproate; 0.42 g/L caprylate) using a mesophilic methanogenic reactor enrichment. Fermentation was carried out in a membrane bio-reactor with pH controlled to 6, thus only organic acids were produced. Besides, Singla et al. (2014) enriched several mixed cultures and optimized their growth conditions for ethanol production, attaining maximum concentrations of 2.3 g/L of ethanol. However, the production of significant concentrations of higher alcohols (i.e., butanol and hexanol) from syngas fermentation by mixed cultures is yet to be reported. This is the objective of the present work, which targets the production of higher alcohols from syngas by mixed cultures, with special attention to how operational conditions affect production dynamics and bacterial community.
Materials and methods
Source of inoculum and pre-acclimation to syngas
Inoculum was obtained from the anaerobic digester of the wastewater treatment plant of Girona (Catalonia, Spain). Anaerobic sludge was diluted in a proportion of 1:10 with low-strength phosphate buffered medium and incubated in a serum bottle under stirring at 150 rpm and 37°C. This was flushed with syngas (32% H2, 32% CO, 8% CO2, and 28% N2; Praxair, Spain) to an overpressure of 100 kPa every 2 days, and 80% of the liquid phase was replaced every 7 days with fresh medium to maintain activity of the cultures. After 21 days, once autotrophic acetate production was detected (data not shown), the enriched suspension was inoculated into a controlled bioreactor.
Media composition
Enrichment of the mixed culture was conducted using a phosphate buffered medium that contained, per liter: 1 g NaCl, 0.25 g NH4Cl, 0.05 g Mg(OH)2, 0.1 g KCl, and 0.03 g CaCl2, 10 mL of vitamin stock solution, 10 mL of trace elements stock solution, 1 g/L KH2PO4 as a buffer, and 20 mM of 2-bromoethanesulfonate (BES) as a methanogenesis inhibitor (Steinbusch et al., 2009). Vitamin and trace elements stock solutions were prepared according to the ATCC 1754 medium. Medium pH was adjusted to 6.2 with NaOH 40%.
Further reactor experiments with moderate-strength buffered media (25 mM) were conducted using the same medium composition except for the buffers, which were, respectively: (i) phosphate (3.4 g/L KH2PO4); (ii) 2-(N-morpholino)ethanesulfonic acid (MES; 4.88 g/L); and (iii) bicarbonate (2.1 g/L NaHCO3). Additionally, 0.1 g/L of KH2PO4 was added to the media of MES and bicarbonate experiments to serve as a source of phosphorus.
Media for the solvent production experiment had the same composition as the MES medium above, but was assayed at different MES buffer strengths (0, 10, 25, 50, and 100 mM).
Reactor set-up
Reactor experiments were carried out in water-jacketed stirred tank reactors with minimum and maximum working volumes of 0.2 and 1 L, and a total vessel volume of 2 L (Afora, Spain). Mixing was provided by a magnetic stirrer at 250 rpm. Temperature was controlled at 37°C by a water bath. Reactors were operated in batch mode. In every batch, cultures were grown during ~20 days, after which 80% of the fermentation broth was replaced with freshly prepared medium. Previous research showed higher productivity of acetogenic strains at initial fermentation pH of 6–7, while low pH-values are required for solvent production (Ganigué et al., 2015). Thus, no external pH control was applied to allow the initial production of organic acids at initial pH around 6, and their subsequent conversion to alcohols at low pH-values. Liquid samples were taken periodically every 2–3 days for the analysis of pH and product composition.
Enrichment of a syngas fermenting mixed culture
Enrichment of the syngas-fermenting mixed culture was carried out in a 2-L bio-reactor. Syngas (32% H2, 32% CO, 8% CO2, and 28% N2; Praxair, Spain) was injected through a diffusion stone at a flow rate of 2,000 mL/min for a period of 1 min every 4 h. Such high flow rate ensured the complete replacement of the gas headspace with fresh syngas every 4 h (average initial composition: 29.7 ± 2.6% H2, 32.4 ± 2.3% CO, 9.8 ± 0.8% CO2, and 28.0 ± 1.4% N2; n = 17). Pressure in the reactor at the beginning of every 4 h cycle was 1.2 ± 0.1 atm.
The reactor was initially started up with 1% v/v of autotrophic inoculum culture (Section Source of Inoculum and Pre-acclimation to Syngas) and was operated in batch mode for 109 days, divided in five batch runs (B1–B5). Samples for bacterial community analysis were taken for each batch. A volume of 15 mL was aseptically collected and centrifuged at 10,000 rpm during 15 min with a centrifuge 5415D (Eppendorf, Germany). Cell pellets were immediately frozen and kept at −20°C until analysis.
Recovery of chain elongation activity
Three 2-L reactors were used to evaluate how different media buffers (all with initial pHs around 6.1–6.3, but providing different pH profiles throughout the fermentation) affected chain elongation to C6 compounds and the microbial community structure. Reactors were started-up using inoculum from the enrichment reactor (10% v/v) obtained at the end of B5, and were operated in parallel for two consecutive batches. Syngas (32% H2, 32% CO, 8% CO2, and 28% N2;Praxair, Spain) was injected in each reactor through a diffusion stone at a flow rate of 58.3 mL/min for a period of 2 min every 3 h. Such low flows were aimed at decreasing metabolic rates, preventing the fast accumulation of organic acids and the subsequent drop in pH. Samples for bacterial community analysis were taken from each reactor at the end of batch 1 and 2, following the same procedure above-described.
Consumption of CO and CO2/H2
The capacity of the microbial community to consume CO and/or CO2/H2 was assessed in a batch study. Three 500-mL serum bottles containing 90 mL of fresh MES medium were inoculated (10% v/v) with culture from the MES reactor after the completion of the second batch. The three bottles were initially flushed with syngas (32% H2, 32% CO, 8% CO2, and 28% N2; Praxair, Spain) to an overpressure of 180 kPa. Following that, samples for the analysis of gas composition, pH, and culture density (optical density, OD600) were drawn periodically.
Production of higher alcohols using mixed cultures
Batch experiments were performed to further investigate the production of higher alcohols by mixed cultures at different final fermentation pHs. Anaerobic 20 mL glass tubes were filled with 5 mL of mineral media containing different concentrations of MES to dampen the decrease in pH due to the production of organic acids. Five concentrations of MES (0, 10, 25, 50, and 100 mM) were used. A total of 12 tubes were prepared for each MES concentration. All tubes were inoculated using 0.5 mL of mixed culture from the MES reactor after the completion of the second batch. Tubes were thoroughly flushed with syngas (32% H2, 32% CO, 8% CO2, and 28% N2; Praxair, Spain) for 1 min to an overpressure of 200–250 kPa. Following that, three tubes of each treatment were sampled, whereas the remaining nine were incubated at 37°C in a rotary shaker Stuart incubator SI500 (Bibby Scientific Ltd., OSA, UK). Tubes were incubated horizontally to enhance gas–liquid mass transfer. During the batch tests, the headspace of each tube was flushed with syngas twice a day for 1 min to ensure replenishment of gas substrates. The optical density of the tubes was monitored daily to follow the evolution of the experiment. Once all treatments reached the stationary phase, tubes were sampled to determine the final concentration of products, pH, and OD600.
Analytical methods
Concentrations of acetic acid, butyric acid, caproic acid, ethanol, butanol, and hexanol were analyzed using a 7890A gas chromatograph (Agilent, USA) equipped with a DB-FFAP column and a FID detector. Injector and detector were held at 250 and 275°C, respectively. The oven was maintained at 40°C during 1 min, after which temperature increased 5°C/min to 70°C, then 10°C/min to 180°C, and finally 35°C/min to 250°C. This temperature was held during 5 min. Helium was the carrier gas and was injected at 85.11 mL/min. Composition of gas headspace (% vol) was analyzed using a gas chromatograph (Agilent 7890A GC system, Agilent Technologies, Spain) equipped with a fused zeolite capillary column (HP-Molesieve, 30 m × 0.53 mm × 50 μm) and thermal conductivity detector (TCD). The pH of samples was measured using a Basic 20+ pH-meter (Crison, Spain). Optical density (OD600) was monitored by measuring the absorbance at 600 nm using a UV-2501(PC) spectrophotometer. Concentration of biomass was determined by a gravimetric test according to standard methods and correlated to OD600 based on a calibration curve (APHA, 1995).
DNA extraction and PCR amplification of bacterial 16S rRNA
DNA was extracted from cell pellets using the FastDNA® SPIN kit for soils (MP, Biomedicals) following the manufacturer's instructions. Partial 16S rRNA gene fragments were obtained by PCR amplification using the bacterial universal primers 357F and 907R. Reaction mixtures and PCR amplification conditions have been described previously (Lane, 1991). A 44 base pair GC clamp sequence was added to the 5′ end of 357F primer for separation of PCR products by DGGE (Muyzer and Smalla, 1998). All chemicals and Taq polymerase used in PCR amplifications were provided by Qiagen (Qiagen Ltd., Sussex, UK). PCR amplifications were performed in a 9700 GeneAmp thermal cycler (Applied Biosystems, Foster City, CA).
DGGE analysis of 16S rRNA genes
When necessary, different PCR products of the same sample were concentrated and quantified. Twenty-five microliters of concentrated 16S rRNA PCR products (from 400 to 500 ng DNA) were loaded on 6% (v/v) acrylamide-bis-acrylamide gels with a 40–65% urea-formamide denaturing gradient (Bäckman et al., 2003). DGGE was performed in a Ingeny phorU system (INGENY, Netherlands) as described previously (Prat et al., 2009). DGGE gel was run for 17 h at 160 V and stained for 30 min with Sybr® Gold (Molecular Probes Europe, Invitrogen Corporation, UK), for visualization under UV excitation. Images were captured with a GelDoc 2000 system. The more intense bands of every position in the gel were excised using a sterile scalpel. The DNA fragments were recovered by elution in Tris/HCl buffer (10 mM at pH 7.4) at 65°C for 2 h and re-amplified with 357F/907R primer pair.
Digital images of acrylamide gel were analyzed using the GelCompar II v4.0 software (Applied Maths BVBA, Sint-Marteens, Belgium). Original DGGE images are shown as supplementary images (Figures S1, S2), and lanes related to this study, identified. Lanes and bands were manually defined. Band positions in the different lanes were normalized using internal standards within samples.
Sequencing
Sequencing in forward or reverse direction of 16S rRNA gene fragments was performed by the Macrogen service (Macrogen, Korea). Sequences were examined for the presence of chimeras using the Uchime algorithm (Edgar et al., 2011), manually refined using the Bioedit v7.0 package and aligned using the ClustalW software. Aligned sequences were analyzed with the BLASTn® program at NCBI (http://blast.ncbi.nlm.nih.gov/) and bacterial species identified as closer similarities to known sequences using the nucleotide collection database. Partial 16S rRNA gene sequences were submitted to GenBank public database with accession numbers from KM489062 to KM489069.
Results and discussion
Enrichment of a syngas-fermenting mixed culture
Figure 1A depicts the total amount of carbon converted into products and pH in the reactor throughout the enrichment phase—Batch 1 (B1) and Batch 2 (B2)—and stable performance—Batch 3 (B3) to Batch 5 (B5)—of the syngas-fermenting mixed culture. Additionally, Figures 1B,C provide further insights on the product speciation in organic acids and alcohols, and the length of product carbon chain.
Figure 1.
(A) Product profile and pH dynamics throughout the enrichment phase. Inverted white triangles indicate the days when samples were obtained for bacterial community analysis; (B) percentage of the products in the form of organic acids and alcohols; (C) percentage of the products in the form of C2, C4, and C6 compounds.
The total amount of carbon converted into extracellular products increased from B1 to B5 (38 mmol C/L in B1 vs. 118 mmol C/L in B5), indicating an increased performance of the reactor (Figure 1A). Initially, the culture produced mainly acetate (B1), up to concentrations of 20 mmols of carbon as products per liter (mmol C/L). However, as the culture acclimated to the new conditions the reactor reached a stable performance phase, during which product spectrum was dominated by ethanol and butanol. At the end of B3, B4, and B5, the percentage of alcohols represented 83.4 ± 9.8% of the total products, with maximum concentrations of 63 mmol C/L of ethanol and 34 mmol C/L of butanol at the end of B5. Methane production throughout the enrichment phase was negligible (0.09% ± 0.17; n = 20), as expected due to BES addition to the medium.
Low extracellular pH has been acknowledged to be a crucial factor triggering solventogenesis, probably due to the increase in the proportion of unionized acid forms at low pH-values (Grethlein et al., 1990; Lewis et al., 2007). In this study, pH was consistently below 4.5 during most part of B3–B5 due to the low buffering capacity of the media. The average concentration of unionized organic acids (encompassing acetic, butyric and caproic acids) during the solventogenic phases of B3–B5 was 2.91 ± 0.84 mmol/L (n = 13). Under such conditions, direct production of solvents and/or the re-assimilation of the already produced acids and their subsequent transformation into alcohols are favored over acid production to avoid further inhibition (Jones and Woods, 1986). This is clearly illustrated by periods 70–81 and 93–107 days, during which the concentration of organic acids remained fairly constant while the total concentration of carbon as products increased from 23.4 to 72.6 and 25.2 to 88.3 mmol C/L, respectively, due to the production of alcohols.
During the stable performance phase (B3–B5), about 40% of the carbon fixed as end-products was in the form of four-carbon compounds (C4). This is remarkable considering that C2 compounds are usually the main metabolites derived from syngas fermentation (Bruant et al., 2010; Perez et al., 2013). In addition to that, production of hexanol and caproate was also observed during the transient phase (B1 and B2), although the concentrations of hexanol and caproate, 9 and 6 mmol C/L (0.15 and 0.12 g/L, respectively), were much lower than those previously reported in literature [0.9 g/L hexanol by Phillips et al. (2015), and 0.98 g/L caproate by Zhang et al. (2013)]. However, production of C6 compounds declined from B3, reaching concentrations close to zero at the end of B5. Among all acetogenic bacteria, only C. carboxidivorans P7 (Ramachandriya et al., 2013; Phillips et al., 2015; Ramió-Pujol et al., 2015) and “C. ragsdalei” P11 (Ramachandriya et al., 2013) have been reported to be capable of directly producing C6 compounds from CO/CO2/H2. Caproic acid and hexanol production can be also achieved by chain-elongation of 2- and 4-carbon compounds through the reversed β-oxidation pathway, which can be carried out by Clostridium kluyveri (Spirito et al., 2014).
To monitor the evolution of the bacterial community structure, samples were taken from the reactor on days 9, 36, 45, and 88, and analyzed by using PCR-DGGE of the 16S rRNA gene. DGGE profiles (Figure 2) showed that the enriched community was rather simple, with few high intensity DGGE bands per sample. Based on UPGMA algorithm using relative band intensities, fairly homogeneous bacterial communities (> 80% similarity) were found for B2, B3 and B4 samples.
Figure 2.

Negative image composition of SybrGold®-stained DGGE gels. Sampling dates of Batch 1, 2, 3, and 4 are indicated in parenthesis. Lanes are organized on a time-scale basis. Bands indicated with a white arrow head were excised and sequenced. Most probable identification of sequenced bands are indicated at right of the image. Numbers in parenthesis indicate the number of bands excised for every position.
Most probable bacterial species identification corresponding to the most prominent DGGE bands was done by sequencing and comparison to public databases (Table 1). Microbial communities were mainly dominated by bacteria belonging to the phylum Firmicutes, which contains most of the identified acetogens and particularly all species related to syngas fermentation (Latif et al., 2014). Only two Proteobacteria could be identified, Acetobacter peroxydans (DGGE band 6) and Paludibacterium yongneupense (DGGE band 8), although they occurred probably as low abundant populations according to band intensities. Precise identifications for DGGE bands 1, 3, and 6, could not be obtained due to more than one exact match to sequences deposited in the NCBI database. Identical 16S rRNA gene sequences have been published for “C. ragsdalei” P11, Clostridium autoethanogenum and Clostridium ljungdahlii (Genbank accession numbers DQ020022, Y18178, and FR733688, respectively). Similar results are obtained with the corresponding 16S rRNA gene sequences of C. carboxidivorans P7 and Clostridium scatologenes (sequence obtained from DGGE band 1). The presence of the organism detected in DGGE band 4, with a close similarity to C. kluyveri, was concomitant to higher chain alcohol and acid production specifically during B2 and to a lesser extent during B3, which supports the hypothesis of the production of C6 compounds through chain elongation of 2- and 4-carbon molecules. DGGE band 4 remained at undetectable levels during B3, B4, and B5, periods in which little or no production of C6 compound was observed. C. kluyveri has an optimum growth pH of 7–7.6 (Weimer and Stevenson, 2012; William, 2014), with its activity being seriously compromised at pH below 5 (William, 2014). Therefore, it can be conjectured that the pH below 4.5 during the majority of B3–B5 could be the key factor leading to C. kluyveri disappearance from the community.
Table 1.
Most probable sequence identification of DGGE bands.
| Band number | Closest bacterial strain | Length (bp) | Similarity (%) |
|---|---|---|---|
| DGGE-Band 1 (KM489065) | Clostridium carboxidivorans P7 | 505 | 100 |
| Clostridium scatologenes K29 | |||
| Clostridium drakei FP | |||
| DGGE-Band 2 (KM489066) | Clostridium aciditolerans JW-YJLB3 | 507 | 98 |
| DGGE-Band 3 (KM489064) | Clostridium ljungdahlii DSM13528 | 543 | 100 |
| “Clostridium ragsdalei” P11 | |||
| Clostridium autoethanogenum DSM10061 | |||
| DGGE-Band 4 (KM489067) | Clostridium kluyveri 15 | 506 | 100 |
| DGGE-Band 5 (KM489068) | Acetobacterium fimetarium DSM8238 | 498 | 98 |
| DGGE-Band 6 (KM489062) | Acetobacter peroxydans JCM25077 | 503 | 99 |
| Acetobacter papayae 1–25 | |||
| DGGE-Band 7 (KM489069) | Uncultured Firmicutes clone | 375 | 94 |
Similarity values and closest match to sequences of cultivated bacteria in GenBank nucleotide collection database are indicated (reference RNA sequences, accession November 2015). Accession numbers appear in parentheses.
Recovery of chain elongation activity
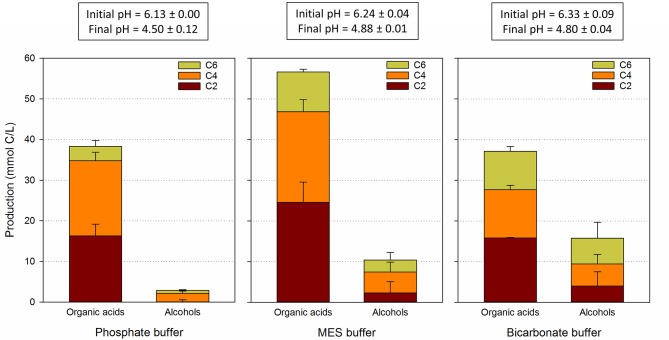
Three identical reactors, each containing mineral media with different buffering agents were started up using enriched carboxydotrophic inoculum from the previous experiment at the end of B5. The three reactors were operated in parallel for two consecutive batch cycles. The net total production of alcohols and acids (based on the initial and final concentrations) in each media and initial and final pHs (average of two batch runs) are depicted in Figure 3. Further details on the evolution of the concentrations of the fermentation end-products and pH throughout each batch are presented in Supplementary Information (Figures S3–S5).
Figure 3.
Net production of C2, C4, and C6 organic acids and alcohols for each media buffer. Results are an average of two consecutive batch runs.
Overall, the reactor with MES buffer fixed a total of 67.1 ± 9.7 mmol C/L, a concentration significantly higher than those of the experiments with phosphate and bicarbonate buffer (40.5 ± 0.81 and 52.8 ± 9.8 mmol C/L, respectively). Such difference in fixation/production was likely due to the higher buffering capacity of MES over the other two buffers in the pH range 5–6 (Figure S6). The product spectra obtained for each buffer was slightly different. Acetate and butyrate were the main fermentation products in all cases. However, significant production of C6 compounds (caproic acid and hexanol) was detected in both MES and bicarbonate experiments, with total C6 concentrations of 12.8 ± 2.5 and 15.7 ± 5.1 mmol C/L, respectively. Caproic acid was also produced in the phosphate buffer experiments (mainly in the first batch), although in lower amounts, 4.2 ± 1.6 mmol C/L. It is important to note that the use of bicarbonate buffer at pH below 6 may have resulted in an additional supply of CO2. However, given the frequent supply of fresh syngas and the high solubility of CO2, this should have not affected the outcome of the experiment in any significant way.
At the end of the first and second batch periods, all bacterial communities were dominated by an acetogenic bacterium with identical 16S rRNA sequence to that observed in DGGE band 3 (Figure 4, Table 1) probably being the key organism fixating inorganic carbon into organic products. Gas consumption experiments conducted in 0.5 L-serum bottles showed that the mixed culture was able to grow on both CO and CO2/H2 (Figure S7). It is thus likely that the abovementioned bacterium was responsible for the consumption of both substrates. Among the three bacterial species showing an identical partial 16S rRNA sequence as the one obtained here, production of C4 and C6 compounds have only been reported for “C. ragsdalei” P11. In contrast to the experiment performed here, C4–C6 production by P11 occurred in abundant supply of reducing power (Phillips et al., 2015). More likely, production of both C4 and C6 in our tests can be attributed to a chain elongation mechanism. Although no reliable data on relative abundance of a certain bacterial species can be obtained from intensity measurements of DGGE bands, the presence of DGGE band 4 at high densities coincided with those periods in which higher amounts of C4 and C6 compounds were produced. DGGE band 4 appeared at higher intensities in MES and bicarbonate buffered media, whereas this band remained almost undetectable in the phosphate buffer reactor.
Figure 4.
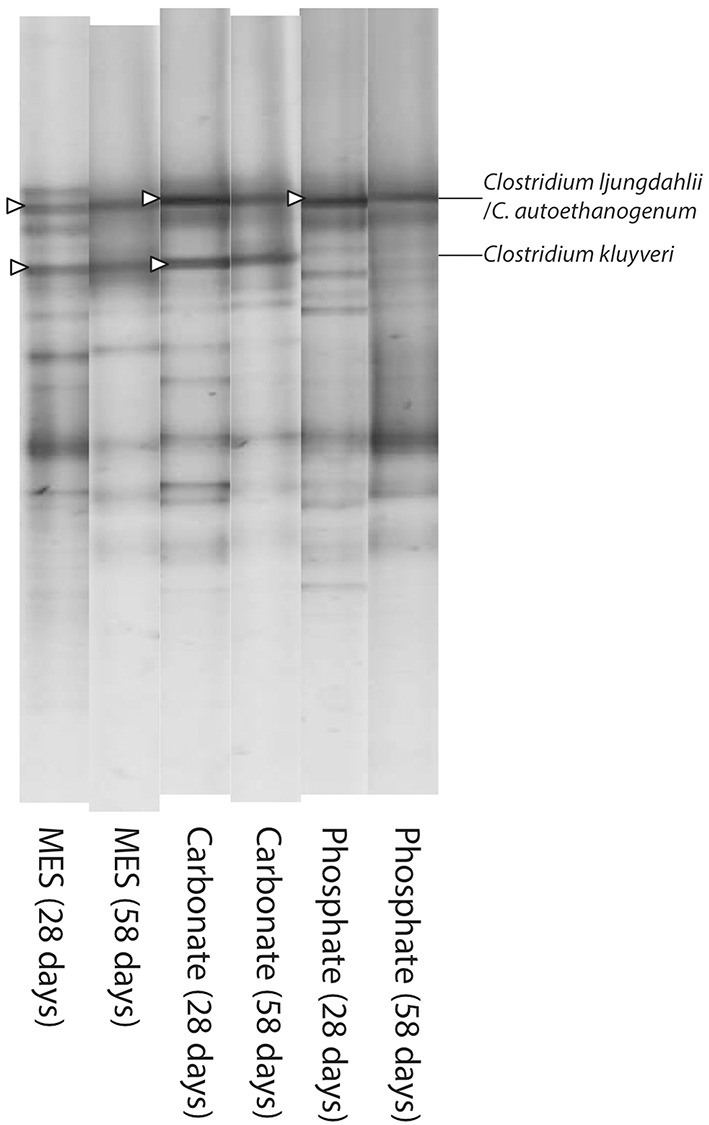
Negative image composition of SybrGold®-stained DGGE gels. Batch experiments are indicated by their buffer (MES, Carbonate or Phosphate) and the sampling date in parenthesis. Bands indicated with an arrow head were excised and sequenced to confirm identifications reported in Table 1.
Changes in DGGE band 4 intensities are hypothesized to be linked to fermentation pH. In the MES and bicarbonate buffer experiments, pH was consistently above 5 throughout most of the fermentation period, and only decreased to 4.88 ± 0.01 and 4.80 ± 0.03, respectively, during the final day of the experiment. The final pH of the phosphate buffer experiments was about 0.2 pH units lower, 4.50 ± 0.12, which may help explaining why C. kluyveri did not regain a significant role in phosphate buffered media. Finally, one could argue that the concentration of undissociated organic acids could have also played a role in defining prominent population in the reactors and determining product speciation, but no significant differences in the concentration of undissociated organic acids profile were observed across the three reactors (Figure S8).
Minimum pH for stable production of higher alcohols using mixed cultures
Batch experiments were performed to further investigate the role of final fermentation pH on product speciation. Several concentrations of MES buffer were used to obtain different buffering capacities and final fermentation pHs. Overall carbon fixation of each test is shown in Figure 5, whereas final concentrations of the different compounds, together with final fermentation pH and biomass are depicted in Figure 6.
Figure 5.
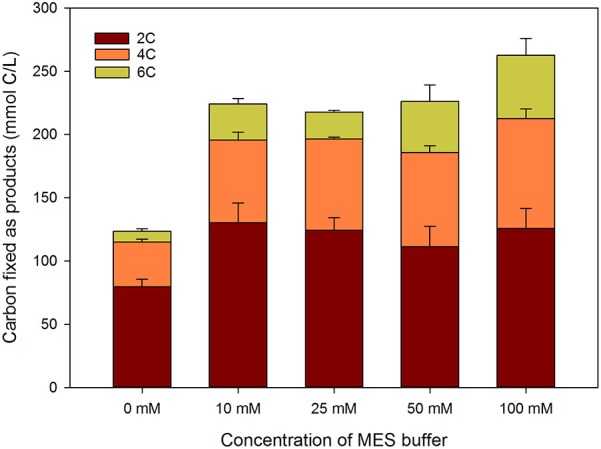
Overall carbon fixation of the batch fermentation experiments with different MES concentrations (n > 6).
Figure 6.
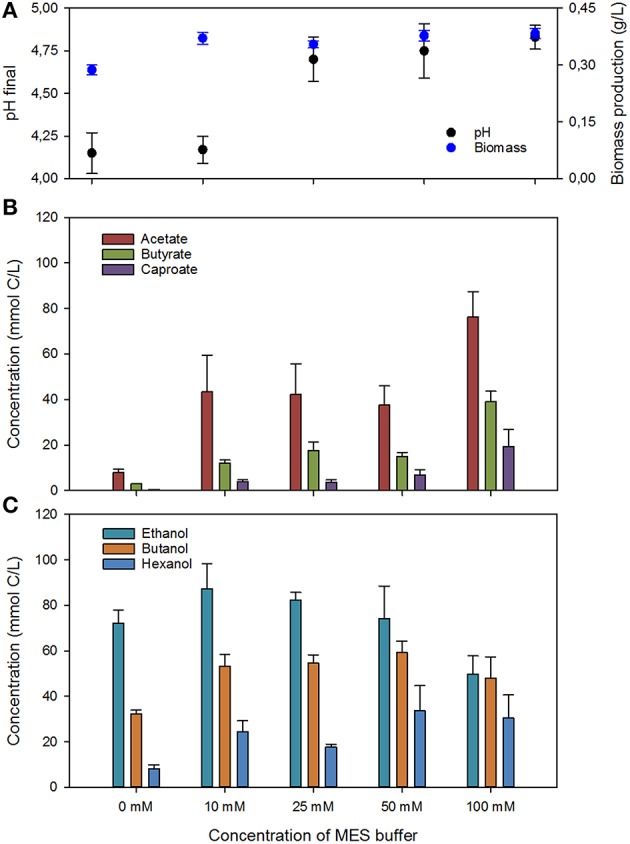
Results of the batch fermentation in media with different MES concentrations (n > 6). (A) Final pH and concentration of biomass as dry weight; (B) net production of organic acids; and (C) net production of alcohols.
Results showed that the better the buffering capacity (and hence the higher the pH during the fermentation), the higher the amount of carbon fixed into products (Figure 5). The batch tests without MES had the lowest productivity, with only 123 ± 15 mmol C/L converted into products. Tests with concentration of MES of 10, 25, and 50 mM had similar carbon fixations (ca. 225 mmol C/L), while the 100 mM MES exhibited the highest carbon fixation (275 mmol C/L).
In terms of product speciation (Figure 6), the highest proportion of alcohols was observed for the unbuffered tests (90.6%), and decreased to around 70% for the experiments at 10, 25, and 50 mM of MES, and further to 45% in case of the 100 mM of MES. Such behavior was likely due to the higher concentrations of buffer providing better pH dampening and delaying the drop in pH and accumulation of high concentration of undissociated organic acids. However, it is important to bear in mind that part of the ethanol could have also been used for chain-elongation, resulting in a decrease in the concentration of ethanol, but an increase in the concentration of C4 and C6 compounds. Production of C2, C4, and C6 compounds was fairly constant in the experiments at 10, 25, 50, and 100 mM of MES, with slightly higher concentrations of C6 compounds being produced in better buffered media. The highest production of C6 compounds (50 mmol C/L) and higher alcohols (59 mmol C/L of butanol and 34 mmol C/L of hexanol) was attained at a final fermentation pH of 4.75. Lower final pH did not increase the concentration of higher alcohols in the fermentation broth, whereas higher final pH of the fermentation test decreased the fraction of C4 and C6 alcohols (i.e., test at 100 mM of MES).
Implications and future prospects
In this study, a stable carboxydotrophic culture able to grow on both CO and CO2/H2 was enriched from a sludge sample obtained from an anaerobic digester of a wastewater treatment plant. Given the global distribution of anaerobic digestion, this source of inoculum could be a suitable platform for syngas fermentation to organic acids or bioalcohols. However, anaerobic digestion is applied to the treatment of a plethora of industrial and agricultural wastes, leading to highly heterogeneous microbiomes (Zhang et al., 2014). Future work should study the enrichment of carboxydotrophic bacteria and mixed microbial communities from anaerobic digester sludge from different origins and characteristics.
The present results prove the feasibility of fermenting syngas to higher alcohols using an enriched mixed culture as a biocatalyst. Butanol can be produced from direct syngas fermentation by several well-known strains. However, the production of C6 compounds, is far more challenging, and to date only two strains have been proven to have the capacity to produce both caproic acid and hexanol under specific conditions (Ramachandriya et al., 2013; Phillips et al., 2015; Ramió-Pujol et al., 2015). The proposed approach takes advantage of the synergistic relationship between acetogenic bacteria and chain elongating bacteria to produce C4 and C6 compounds. Concentrations of butanol and hexanol produced by the mixed culture (1.1 g/L of butanol and 0.6 g/L of hexanol) are comparable to those obtained using pure cultures of C. carboxidivorans—1.09 g/L of butanol and 0.94 g/L of hexanol, Phillips et al. (2015); 1.07 g/L of butanol and 0.84 g/L of hexanol, Ramió-Pujol et al. (2015)—and show the potential of the proposed approach. Recently, Diender et al. proved that C. autoethanogenum and C. kluyveri can grow in a co-culture and produce a mixture of medium chain fatty acids and higher alcohols (Diender et al., 2016). The co-culture produced concentrations of butanol and hexanol of 0.42 and 0.41 g/L, respectively.
Care must be taken in selecting adequate operational conditions for mixed cultures (and co-cultures). Among them, pH is probably the most critical, as low pH are needed to trigger solventogenesis, but pH below 4.5–5 pose a serious threat to the viability of chain elongation in the mixed culture. Experimental data of this work suggest that operation at a minimum fermentation pH of 4.7–4.8 should maximize alcohol production and yet allow the growth of the chain-elongating organisms, such as C. kluyveri. Diender and co-workers screened the viability of the co-culture in the pH range 4–7, concluding that it was only functional at pH 5.5–6.5. Besides, no significant difference in production was observed within the tested range of viable pH (Diender et al., 2016). Such discrepancy is likely linked to the fact that the mixed culture was composed of different species, with slightly different growth pH range as those of the co-culture. Future work should further investigate the synergistic interactions between the different species of the mixed culture, including their long-term stability and how changes in operational conditions (especially pH) shift the carbon and electron flows.
One of the bottlenecks of syngas fermentation is gas solubility, particularly of H2 and CO. Production of higher alcohols relies heavily on the availability of reducing power, and thus production rates and product titers obtained in this study could be substantially improved when using enhanced mass-liquid transfer reactors, such as membrane bio-reactors (Zhang et al., 2013), or systems with cell recirculation (Grethlein et al., 1991; Richter et al., 2013). However, care must be taken that CO is rapidly consumed, as this could be inhibitory to the activity of C. kluyveri (Diender et al., 2016). Performance could be also enhanced by using optimized media (Phillips et al., 2015) and operational conditions (Singla et al., 2014). Future research should aim to establish the potential of the mixed culture approach for industrial application, both in terms of production rates, titers and yields, but also on the long-term stability of the culture. Another important aspect that should not be overlooked is the product specificity. In this study, syngas fermentation using mixed cultures yielded a mixture of products which, together with the low titers obtained, would make the product recovery challenging at best. Optimal reactor design and operation could increase product titers, but efforts should also target a more selective production to make easier the downstream processing of the broth. Finally, it is important to bear in mind that higher alcohols are significantly more toxic than ethanol (Lee et al., 2008), and accumulation of higher concentrations of these will unavoidably end up in decreased performance due to activity inhibition unless in-line product extraction is integrated in the processes design.
Conclusions
This study proved for the first time the fermentation of syngas to higher alcohols by a mixed microbial culture enriched from natural communities, with maximum concentrations of 1.1 g/L of butanol and 0.6 g/L of hexanol.
The enrichment process yielded a bacterial community mainly composed of two carboxydotrophic bacteria, i.e., C. ljungdahli and C. carboxidivorans, and a chain elongating bacteria C. kluyveri, on the basis of 16S rRNA fragment gene sequence comparisons.
Acidic fermentation pH is required to trigger solventogenesis, but fermentation pH around 4.5 are detrimental to C. kluyveri. A minimum fermentation pH of 4.7–4.8 should allow production of solvents at a pH compatible for growth of C. kluyveri.
Author contributions
RG performed the experiments and wrote the manuscript. PS performed part of the experiments. LB conducted the community analysis and reviewed and revised the manuscript. JC reviewed and revised the manuscript. All authors gave approval for publication of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the Spanish Ministry (Best-Energy, CTQ2011-23632 and Bescarbox, CTM2013-43454-R) for its financial support in this study. LEQUIA and EcoAqua have been recognized as consolidated research groups by the Catalan Government (2014-SGR-1168 and 2014-SGR-0484). RG gratefully acknowledges support from Ghent University BOF postdoctoral fellowship (BOF15/PDO/068).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00702
References
- Agler M. T., Wrenn B. A., Zinder S. H., Angenent L. T. (2011). Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol. 29, 70–78. 10.1016/j.tibtech.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Alves J. I., Stams A. J. M., Plugge C. M., Alves M. M., Sousa D. Z. (2013). Enrichment of anaerobic syngas-converting bacteria from thermophilic bioreactor sludge. FEMS Microbiol. Ecol. 86, 590–597. 10.1111/1574-6941.12185 [DOI] [PubMed] [Google Scholar]
- APHA (1995). Standard Methods for the Examination of Water and Wastewater. Washington, DC. [Google Scholar]
- Bäckman J. S. K., Hermansson A., Tebbe C. C., Lindgren P.-E. (2003). Liming induces growth of a diverse flora of ammonia-oxidising bacteria in acid spruce forest soil as determined by SSCP and DGGE. Soil Biol. Biochem. 35, 1337–1347. 10.1016/S0038-0717(03)00213-X [DOI] [Google Scholar]
- Bruant G., Lévesque M.-J., Peter C., Guiot S. R., Masson L. (2010). Genomic analysis of carbon monoxide utilization and butanol production by Clostridium carboxidivorans strain P7. PLoS ONE 5:e13033. 10.1371/journal.pone.0013033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell J., Köpke M., Simpson S. D. (2012). Commercial biomass syngas fermentation. Energies 5, 5372–5417. 10.3390/en5125372 [DOI] [Google Scholar]
- Diender M., Stams A. J. M., Sousa D. Z. (2015). Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front. Microbiol. 6:1275 10.3389/fmicb.2015.01275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diender M., Stams A. J. M., Sousa D. Z. (2016). Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels 9:82 10.1186/s13068-016-0495-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürre P. (2007). Biobutanol: an attractive biofuel. Biotechnol. J. 2, 1525–1534. 10.1002/biot.200700168 [DOI] [PubMed] [Google Scholar]
- Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganigué R., Ramió-Pujol S., Sánchez P., Bañeras L., Colprim J. (2015). Conversion of sewage sludge to commodity chemicals via syngas fermentation. Water Sci. Technol. 72, 415–420. 10.2166/wst.2015.222 [DOI] [PubMed] [Google Scholar]
- Grethlein A. J., Worden R. M., Jain M. K., Datta R. (1991). Evidence for production of n-butanol from carbon monoxide by Butyribacterium methylotrophicum. J. Ferment. Bioeng. 72, 58–60. [Google Scholar]
- Guiot S. R., Cimpoia R., Carayon G. (2011). Potential of wastewater-treating anaerobic granules for biomethanation of synthesis gas. Environ. Sci. Technol. 45, 2006–2012. 10.1021/es102728m [DOI] [PubMed] [Google Scholar]
- Hartmann D. L., Klein Tank A. M. G., Rusticucci M., Alexander L. V., Brönnimann S., Charabi Y., et al. (2013). Observations: atmosphere and surface, in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Stocker T. F., Qin D., Plattner G.-K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P. M. (Cambridge, UK; New York, NY: Cambridge University Press; ), 159–254. [Google Scholar]
- Heiskanen H., Virkajärvi I., Viikari L. (2007). The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzyme Microb. Technol. 41, 362–367. 10.1016/j.enzmictec.2007.03.004 [DOI] [Google Scholar]
- Hernández A. B., Ferrasse J.-H., Roche N. (2013). Limiting the pollutant content in the sewage sludge producer gas through staged gasification. Chem. Eng. Technol. 36, 1985–1996. 10.1002/ceat.201300103 [DOI] [Google Scholar]
- Grethlein A. J., Worden R. M., Jain M. K., Datta R. (1990). Continuous production of mixed alcohols and acids from carbon monoxide. Appl. Biochem. Biotechnol. 24–25, 875–884. [Google Scholar]
- Jones D. T., Woods D. R. (1986). Acetone-butanol fermentation revisited. Microbiol. Rev. 50, 484–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem R., van Loosdrecht M. C. M. (2007). Mixed culture biotechnology for bioenergy production. Curr. Opin. Biotechnol. 18, 207–212. 10.1016/j.copbio.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Lane D. J. (1991). in Nucleic Acid Techniches in Bacterial Systematics, eds Stackebrandt E., Goodfellow M. (New York, NY: John Wiley; Chichester; ), 115–175. [Google Scholar]
- Latif H., Zeidan A., a Nielsen, A. T., Zengler K. (2014). Trash to treasure: production of biofuels and commodity chemicals via syngas fermenting microorganisms. Curr. Opin. Biotechnol. 27, 79–87. 10.1016/j.copbio.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Park J. H., Jang S. H., Nielsen L. K., Kim J., Jung K. S. (2008). Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 101, 209–228. 10.1002/bit.22003 [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Tanner R. S., Huhnke R. L. (2007). Indirect or Direct Fermentation of Biomass to Fuel Alcohol. Patent No. 0275447 A1. [Google Scholar]
- Marshall C. W., LaBelle E. V., May H. D. (2013). Production of fuels and chemicals from waste by microbiomes. Curr. Opin. Biotechnol. 24, 391–397. 10.1016/j.copbio.2013.03.016 [DOI] [PubMed] [Google Scholar]
- Munasinghe P. C., Khanal S. K. (2010). Biomass-derived syngas fermentation into biofuels: opportunities and challenges. Bioresour. Technol. 101, 5013–5022. 10.1016/j.biortech.2009.12.098 [DOI] [PubMed] [Google Scholar]
- Muyzer G., Smalla K. (1998). Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek 73, 127–141. [DOI] [PubMed] [Google Scholar]
- Perez J. M., Richter H., Loftus S. E., Angenent L. T. (2013). Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol. Bioeng. 110, 1066–1077. 10.1002/bit.24786 [DOI] [PubMed] [Google Scholar]
- Phillips J. R., Atiyeh H. K., Tanner R. S., Torres J. R., Saxena J., Wilkins M. R., et al. (2015). Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: medium development and culture techniques. Bioresour. Technol. 190, 114–121. 10.1016/j.biortech.2015.04.043 [DOI] [PubMed] [Google Scholar]
- Prat C., Ruiz-Rueda O., Trias R., Anticó E., Capone D., Sefton M., et al. (2009). Molecular fingerprinting by PCR-denaturing gradient gel electrophoresis reveals differences in the levels of microbial diversity for musty-earthy tainted corks. Appl. Environ. Microbiol. 75, 1922–1931. 10.1128/AEM.02758-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale S. W., Pierce E. (2008). Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation. Biochim. Biophys. Acta 1784, 1873–1898. 10.1016/j.bbapap.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandriya K. D., Kundiyana D. K., Wilkins M. R., Terrill J. B., Atiyeh H. K., Huhnke R. L. (2013). Carbon dioxide conversion to fuels and chemicals using a hybrid green process. Appl. Energy 112, 289–299. 10.1016/j.apenergy.2013.06.017 [DOI] [Google Scholar]
- Ramió-Pujol S., Ganigué R., Bañeras L., Colprim J. (2015). Incubation at 25 °C prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresour. Technol. 192, 296–303. 10.1016/j.biortech.2015.05.077 [DOI] [PubMed] [Google Scholar]
- Ranjan A., Moholkar V. S. (2012). Biobutanol: science, engineering, and economics. Int. J. Energy Res. 36, 277–323. 10.1002/er.1948 [DOI] [Google Scholar]
- Richter H., Martin M., Angenent L. (2013). A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies 6, 3987–4000. 10.3390/en6083987 [DOI] [Google Scholar]
- Schiel-Bengelsdorf B., Dürre P. (2012). Pathway engineering and synthetic biology using acetogens. FEBS Lett. 586, 2191–2198. 10.1016/j.febslet.2012.04.043 [DOI] [PubMed] [Google Scholar]
- Sims R., Schaeffer R., Creutzig F., Cruz-Núñez X., D'Agosto M., Dimitriu D., et al. (2013). Transport, in Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Edenhofer O., Pichs-Madruga R., Sokona Y., Farahani E., Kadner S., Seyboth K., Adler A., Baum I., Brunner S., Eickemeier P., Kriemann B., Savolainen J., Schlömer S., von Stechow C., Zwickel T., Minx J. C. (Cambridge, UK; New York, NY: Cambridge University Press; ), 599–670. [Google Scholar]
- Singla A., Verma D., Lal B., Sarma P. M. (2014). Enrichment and optimization of anaerobic bacterial mixed culture for conversion of syngas to ethanol. Bioresour. Technol. 172, 41–49. 10.1016/j.biortech.2014.08.083 [DOI] [PubMed] [Google Scholar]
- Spirito C. M., Richter H., Rabaey K., Stams A. J., Angenent L. T. (2014). Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 27, 115–122. 10.1016/j.copbio.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Steinbusch K. J. J., Arvaniti E., Hamelers H. V. M., Buisman C. J. N. (2009). Selective inhibition of methanogenesis to enhance ethanol and n-butyrate production through acetate reduction in mixed culture fermentation. Bioresour. Technol. 100, 3261–3267. 10.1016/j.biortech.2009.01.049 [DOI] [PubMed] [Google Scholar]
- Steinbusch K. J. J., Hamelers H. V. M., Plugge C. M., Buisman C. J. N. (2011). Biological formation of caproate and caprylate from acetate: fuel and chemical production from low grade biomass. Energy Environ. Sci. 4, 216 10.1039/C0EE00282H [DOI] [Google Scholar]
- Weimer P. J., Stevenson D. M. (2012). Isolation, characterization, and quantification of Clostridium kluyveri from the bovine rumen. Appl. Microbiol. Biotechnol. 94, 461–466. 10.1007/s00253-011-3751-z [DOI] [PubMed] [Google Scholar]
- William E. L. (2014). Butyrate producing Clostridium species, Clostridium pharus. US20140242654 A1. [Google Scholar]
- Xu D., Tree D. R., Lewis R. S. (2011). The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 35, 2690–2696. 10.1016/j.biombioe.2011.03.005 [DOI] [Google Scholar]
- Zhang F., Ding J., Zhang Y., Chen M., Ding Z. W., van Loosdrecht M. C. M., et al. (2013). Fatty acids production from hydrogen and carbon dioxide by mixed culture in the membrane biofilm reactor. Water Res. 47, 6122–6129. 10.1016/j.watres.2013.07.033 [DOI] [PubMed] [Google Scholar]
- Zhang W., Werner J. J., Agler M. T., Angenent L. T. (2014). Substrate type drives variation in reactor microbiomes of anaerobic digesters. Bioresour. Technol. 151, 397–401. 10.1016/j.biortech.2013.10.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.