Abstract
Paenibacillus antibioticophila strain GD11T sp. nov. is the type strain of a new species within the genus Paenibacillus. This strain, whose genome is described here, was isolated from human faeces of a 63-year-old woman with multidrug-resistant tuberculosis who was receiving numerous antibiotics at the time of stool collection. P. antibioticophila is a Gram-positive aerobic bacterium. We describe here the features of this bacterium, together with the complete genome sequence and annotation. The 5 562 631 bp long genome contains 5084 protein-coding and 71 RNA genes.
Keywords: Culturomics, genome, Paenibacillus antibioticophila, taxonogenomics
Introduction
Paenibacillus antibioticophila strain GD11T (= DSM 28228 = CSUR P1358) is the type strain of Paenibacillus antibioticophila sp. nov. It is a Gram-positive, aerobic, indole-negative rod-shaped bacterium isolated as part of a culturomics study [1], [2]. This bacterium was isolated from a 63-year-old woman with multidrug-resistant tuberculosis who was receiving a broad-spectrum antibiotic regimen at the time of stool collection, as recently reported [2].
The current classification of prokaryotes is based on a combination of phenotypic and genotypic characteristics [3], [4] which includes 16S rRNA gene phylogeny, G+C content and DNA–DNA hybridization. Although these tools are considered to be the reference standard, they have several pitfalls [5], [6]. As a result of the declining cost of sequencing, there has been rapid growth in the number of bacterial genomes being sequenced [7], and we thus recently proposed to add genomic information to phenotypic criteria for the description of new bacterial species [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36].
The genus Paenibacillus initially included Gram-variable, facultative anaerobic endospore-forming bacteria that were reclassified as a separate genus in 1993 [37] after being grouped with the genus Bacillus. To date, the genus consists of 170 described species and four subspecies that have been isolated from a variety of environments such as soil, water, rhizosphere, vegetable matter, forage and insect larvae [38], [39], [40]. Isolation from human specimens has also been described [41], [42]. One Paenibacillus species was cultured for the first time from human faeces as a part of a culturomics study [14].
Here we present a summary of the classification and set of features for Paenibacillus antibioticophila sp. nov. strain GD11T, together with the description of the complete genomic sequencing and annotation. These characteristics support the creation of the Paenibacillus antibioticophila species.
Organism information
Classification and features
A stool sample was collected from a 63-year-old woman with a pulmonary form of multidrug-resistant tuberculosis [2]. The study was approved by the ethics committee of the Institut Fédératif de Recherche IFR48, Faculty of Medicine, Marseille, France, under agreement 09-002. The faecal specimen was preserved at −80°C after collection. Strain GD11T (Table 1) was isolated in March 2012 by cultivation on 5% sheep's blood agar in aerobic conditions at 37°C after a 21-day preincubation in a blood culture bottle with sterile cow rumen fluid and sheep's blood.
Table 1.
Classification and general features of Paenibacillus antibioticophila strain GD11T according to MIGS recommendations [48].
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Current classification | Domain: Bacteria | TAS [43] | |
| Phylum: Firmicutes | TAS [44] | ||
| Class: Bacillus | TAS [45] | ||
| Order: Bacillales | TAS [46] | ||
| Family: Paenibacillaceae | TAS [45] | ||
| Genus: Paenibacillus | TAS [37] | ||
| Species: Paenibacillus antibioticophila | IDA | ||
| Type strain: GD11T | IDA | ||
| Gram stain | Positive | IDA | |
| Cell shape | Bacilli | IDA | |
| Motility | Motile | IDA | |
| Sporulation | Nonsporulating | IDA | |
| Temperature range | Mesophile | IDA | |
| Optimum temperature | 37°C | IDA | |
| MIGS-6.3 | Salinity | Unknown | |
| MIGS-22 | Oxygen requirement | Aerobic | IDA |
| Carbon source | Unknown | IDA | |
| Energy source | Unknown | IDA | |
| MIGS-6 | Habitat | Human gut | IDA |
| MIGS-15 | Biotic relationship | Free-living | IDA |
| MIGS-14 | Pathogenicity | Unknown | |
| Biosafety level | 2 | ||
| Isolation | Human faeces | ||
| MIGS-4 | Geographic location | Marseille, France | IDA |
| MIGS-5 | Sample collection time | March 2012 | IDA |
| MIGS-4.1 | Latitude | 43.296482 | IDA |
| MIGS-4.1 | Longitude | 5.36978 | IDA |
| MIGS-4.3 | Depth | Surface | IDA |
| MIGS-4.4 | Altitude | 0 m above sea level | IDA |
MIGS, minimum information about a genome sequence.
Evidence codes are as follows: IDA, inferred from direct assay; TAS, traceable author statement (i.e., a direct report exists in the literature); NAS, nontraceable author statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species or anecdotal evidence). These evidence codes are from the Gene Ontology project (http://www.geneontology.org/GO.evidence.shtml) [47]. If the evidence code is IDA, then the property should have been directly observed, for the purpose of this specific publication, for a live isolate by one of the authors, or by an expert or reputable institution mentioned in the acknowledgements.
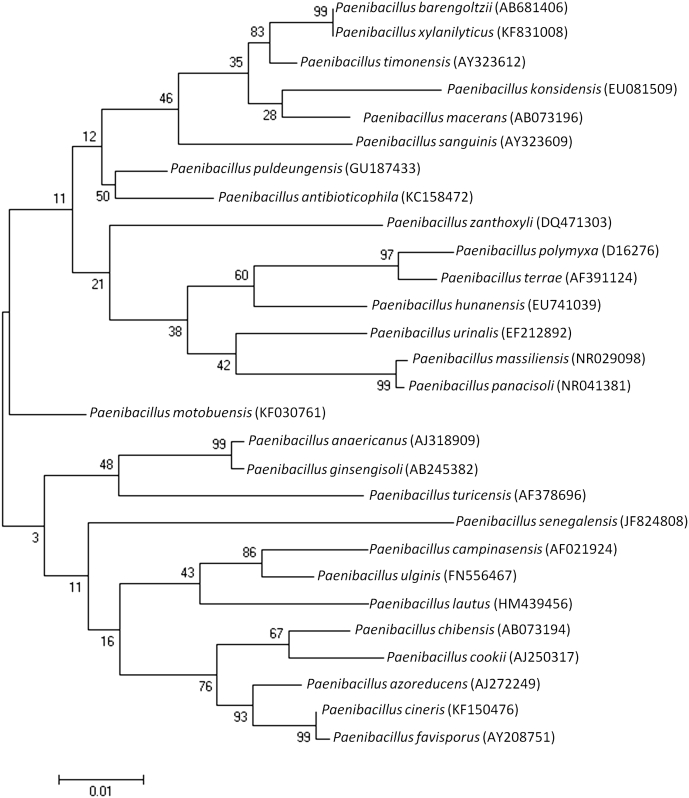
Strain GD11T exhibited a 97.6% 16S rRNA sequence identity with P. puldeungensis (GenBank accession no. NR117451), the phylogenetically closest bacterial species with standing in nomenclature (Fig. 1). Its 16S rRNA sequence was deposited in GenBank under accession number KC158472. This value was lower than the 98.7% 16S rRNA gene sequence threshold recommended by Stackebrandt and Ebers [4] to delineate a new species without carrying out DNA-DNA hybridization.
Fig. 1.
Phylogenetic tree showing position of Paenibacillus antibioticophila strain GD11T relative to other type strains within Paenibacillaceae. The strains and their corresponding GenBank accession numbers for 16S rRNA genes are (type = T): P. sanguinis strain 2301083T, AY323612; P. macerans strain ATCC 8244T, AB073196; P. timonensis strain 2301032T, AY323612; P. barengoltzii strain SAFN-016T, AY167814; P. antibioticophila strain GD11T, KC158472; P. puldeungensis strain CAU 9324T, GU187433; P. motobuensis strain MC10T, AY741810; P. senegalensis strain JC66T, JF824808; P. zanthoxyli strain JH29T, DQ471303; P. sabinae strain T27T, DQ338444; P. lautus strain ATCC 43898T, AB073188; P. terrae strain AM141T, AF391124; P. polymyxa strain ATCC 842T, D16276; P. xylanilyticus strain XIL14T, AY427832; P. massiliensis strain 2301065T, AY323608; P. panacisoli strain Gsoil 1411T, AB245384; Bacillus subtilis ATCC 6051T, AJ276351. Sequences were aligned using CLUSTALW, and phylogenetic inferences were obtained using the maximum likelihood method within MEGA6. Numbers at nodes are percentages of bootstrap values obtained by repeating analysis 1000 times to generate majority consensus tree. Bacillus subtilis ATCC 6051T (AJ276351) was used as outgroup. Scale bar = 1% nucleotide sequence divergence.
Growth at different temperatures (25, 30, 37, 45 and 56°C) was tested; no growth was observed at 45°C or 56°C. Growth occurred between 25°C and 37°C, after 24 to 48 hours of incubation. Colonies were 0.5 μm in diameter on blood-enriched Columbia agar. Growth of the strain was tested in 5% sheep's blood–enriched Columbia agar (bioMérieux, Marcy l’Etoile, France) under anaerobic and microaerophilic conditions using the GENbag anaer and GENbag microaer systems, respectively (bioMérieux), and under aerobic conditions, with or without 5% CO2. Growth was achieved only both aerobically and anaerobically. Gram staining showed Gram-positive bacilli (Fig. 2). A motility test was positive. Cells grown on agar were soft and translucent after 24 hours and had a mean width of 0.49 μm and mean length of 2.67 μm (Fig. 3).
Fig. 2.
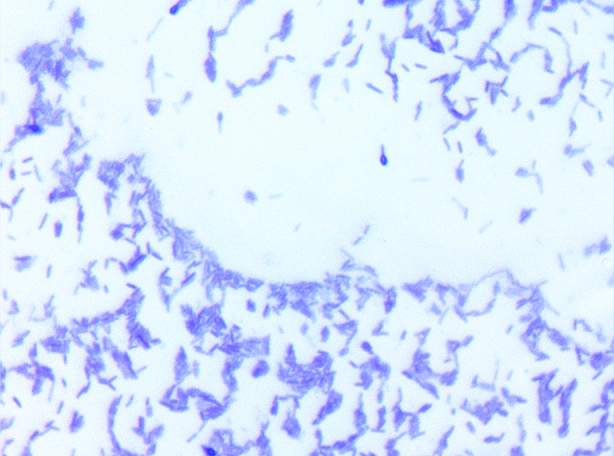
Gram staining of Paenibacillus antibioticophila strain GD11T (= DSM 28228 = CSUR P1358).
Fig. 3.
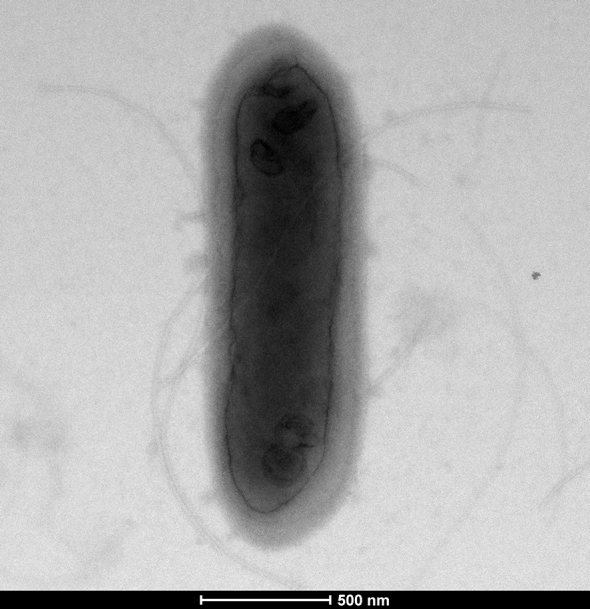
Transmission electron microscopy of P. antibioticophila strain GD11T with Morgani 268D device (Philips, Amsterdam, The Netherlands) at operating voltage of 60 kV. Scale bar = 200 nm.
Strain GD11T exhibits neither catalase nor oxidase activity. Using an API ZYM strip (bioMérieux), positive reactions were observed for esterase (C4), esterase lipase (C8), naphthol-AS-BI-phosphohydrolase, β-galactosidase, α-galactosidase and α-glucosidase. Using rapid ID32A, positive reactions were observed for α-glucosidase, α-arabinosidase, β-glucuronidase, N-acetyl-β-glucosaminidase, nitrate reduction, glutamic acid decarboxylase, fermentation of mannose and raffinose.
Using an API 50 CH strip (bioMérieux), positive reactions were recorded for esculin hydrolysis and fermentation of l-arabinose, d-ribose, d-xylose, methyl-βd-xylopranoside, d-galactose, d-glucose, d-fructose, d-mannose, l-rhamnose, d-mannitol, N-acetylglucosamine, amygdalin, arbutin, salicin, d-cellobiose, d-maltose, d-lactose, d-melibiose, d-saccharose, d-trehalose, inulin, d-melezitose, d-raffinose, starch, glycogen and d-lyxose.
Using an API ZYM strip (bioMérieux), negative reactions were observed for acid phosphatase, alkaline phosphatase, leucine arylamidase, valine arylamidase, cystine arylamidase, lipase (C14), trypsin, α-chymotrypsin, β-glucosidase, α-mannosidase, and α-fucosidase. Using rapid API 32A, negative reactions were observed for arginine dihydrolase, urease, production of indole, leucine arylamidase, histidine arylamidase, phenylalanine arylamidase, tyrosin arylamidase, alanine arylamidase α-mannosidase, β-glucosidase, α-fucosidase. an API 50 CH strip (bioMérieux), negative reactions were recorded for fermentation of erythritol, d-arabinose, l-xylose, d-adonitol, l-sorbose, dulcitol, inositol, d-sorbitol, xylitol, d-turanose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium gluconate, potassium 2-ketogluconate, potassium 5-ketogluconate and potassium-5-ketogluconate.
Cells are susceptible to penicillin G, amoxicillin, amoxicillin–clavulanic acid, ceftriaxone, imipenem, vancomycin, rifampicin, erythromycin, gentamicin, ciprofloxacin and trimethoprim–sulfamethoxazole, but resistant to metronidazole.
By comparison with P. puldeungensis strain CAU 9324T, its phylogenetically closest neighbor, P. antibioticophila differed in alkaline phosphates, acid phosphatase, oxidase and β-glucosidase (Table 2).
Table 2.
Differential characteristics of Paenibacillus antibioticophila strain GD11T (data from this study) with P. sanguinis strain 2301083T, P. zanthoxyli strain JH29T, P. puldeungensis strain CAU 9324T, P. terrae strain AM141T
| Property | P. antibioticophila | P. sanguinis | P. zanthoxyli | P. puldeungensis | P. terrae |
|---|---|---|---|---|---|
| Cell diameter (μm) | 0.49 × 2.67 | 0.5 × 2–3 | 0.4 × 4–4.8 | 0.3 × 1.3–2.3 | 1.5 × 4–7 |
| Oxygen requirement | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic |
| Gram stain | + | + | + | + | + |
| Salt requirement | No | No | No | No | |
| Motility | + | + | + | − | − |
| Endospore formation | NA | + | + | + | + |
| Production of: | |||||
| Alkaline phosphatase | − | + | NA | + | − |
| Acid phosphatase | − | + | NA | + | − |
| Catalase | − | − | + | − | + |
| Oxidase | − | − | − | + | + |
| Nitrate reductase | + | + | NA | + | + |
| Urease | − | − | NA | NA | NA |
| α-Galactosidase | + | NA | NA | + | NA |
| β-Galactosidase | + | NA | NA | + | NA |
| β-Glucuronidase | + | NA | NA | + | NA |
| α-Glucosidase | + | NA | NA | + | NA |
| β-Glucosidase | − | NA | NA | + | NA |
| Esterase | + | NA | NA | + | NA |
| Esterase lipase | + | NA | NA | − | NA |
| Naphthol-AS-BI-phosphohydrolase | + | NA | NA | NA | NA |
| N-acetyl-β-glucosaminidase | − | NA | NA | − | NA |
| Pyrazinamidase | NA | NA | NA | NA | NA |
| α-Mannosidase | − | NA | NA | − | NA |
| α-Fucosidase | − | NA | NA | − | NA |
| Leucine arylamidase | + | NA | NA | + | NA |
| Valine arylamidase | − | NA | NA | + | NA |
| Cystine arylamidase | − | NA | NA | + | NA |
| α-Chymotrypsin | − | NA | NA | + | NA |
| Trypsin | − | NA | NA | + | NA |
| Utilization of: | |||||
| 5-Keto-gluconate | − | NA | NA | NA | NA |
| d-Xylose | − | − | − | NA | + |
| d-Fructose | − | + | − | NA | + |
| d-Glucose | − | − | − | NA | − |
| d-Mannose | − | − | NA | NA | + |
| Habitat | Human gut | Human | Soil | Soil | Algae |
+, positive result; −, negative result; NA, data not available.
Extended features descriptions
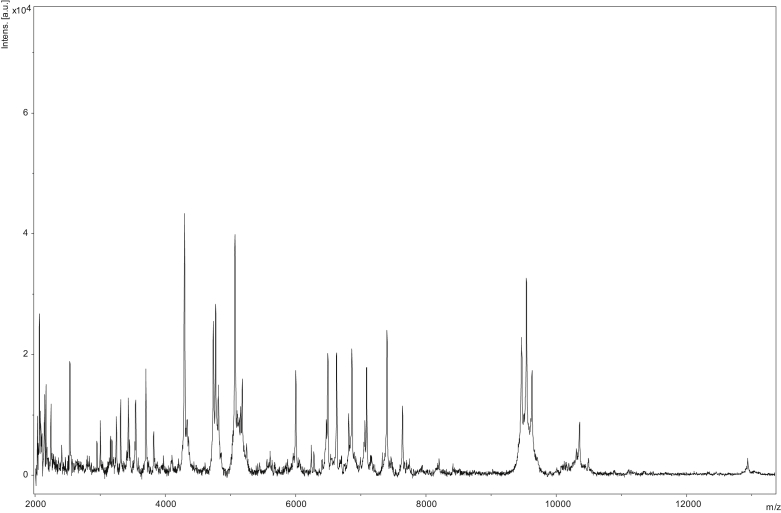
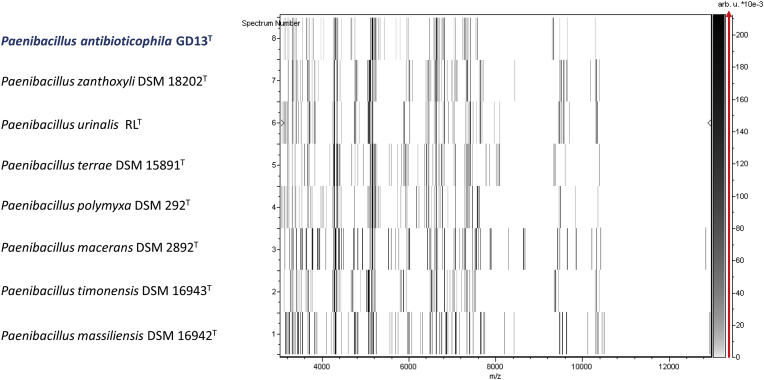
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) protein analysis was carried out as previously described [48]. Briefly, using a pipette tip, one isolated bacterial colony from a culture on agar plate was transferred and spread as a thin film on a MSP 96 MALDI-TOF target plate (Bruker Daltonics, Leipzig, Germany). Twelve distinct deposits from 20 isolated colonies were performed for strain GD11T. Each smear was overlaid with 2 μL of matrix solution (saturated solution of alpha-cyano-4-hydroxycinnamic acid) in 50% acetonitrile, 2.5% trifluoroacetic acid, and dried for 5 minutes. Microflex spectrometer (Bruker) was used for measurements, and spectra were then recorded in the positive linear mode for the mass range of 2000 to 20 000 Da (parameter settings: ion source 1 (ISI), 20 kV; IS2, 18.5 kV; lens, 7 kV). A spectrum was obtained after 240 shots with variable laser power. The 20 GD11T spectra were imported into MALDI BioTyper 3.0 software (Bruker) and analysed by standard pattern matching (with default parameter settings) against the main spectra of 7379 bacteria, including 129 spectra from 70 Paenibacillus species. The method of identification included the m/z from 3000 to 15 000 Da. A maximum of 100 peaks were compared with spectra in the database for every spectrum. The resulting score enabled the identification of tested species (or not): a score of ≥2 with a validly published species enabled identification at the species level; a score of ≥1.7 but <2 enabled identification at the genus level; and a score of <1.7 did not enable any identification. No significant MALDI-TOF score was obtained for strain GD11T against the Bruker database, suggesting that our isolate was not a member of a known species. We added the spectrum from strain GD11T to our database (Fig. 4). Finally, the spectral differences with other members of the genus Paenibacillus were highlighted by gel view (Fig. 5).
Fig. 4.
Reference mass spectrum from Paenibacillus antibioticophila strain GD11T (= DSM 28228 = CSUR P1358). Spectra from 16 individual colonies were compared and reference spectrum generated.
Fig. 5.
Gel view comparing Paenibacillus antibioticophila strain GD11T. Gel view displays raw spectra of loaded spectrum files arranged in pseudo gel-like look. The x-axis records m/z value. Left y-axis displays running spectrum number originating from subsequent spectra loading. Peak intensity is expressed by greyscale scheme code. Color bar and right y-axis indicating relation between color peak are displayed, with peak intensity in arbitrary units. Displayed species are indicated at left, with GD11T highlighted in blue.
Genome sequencing information
Genome project history
As part of a culturomics study isolating all bacterial species from the human digestive flora from patients with multidrug-resistant tuberculosis and treated with broad-spectrum antibiotics, this organism was isolated and selected for sequencing on the basis of its phenotypic differences, phylogenetic position and 16S rRNA sequence similarity to other members of the genus Paenibacillus. It is the first sequenced genome of P. antibioticophila sp. nov. The GenBank Bioproject number is PRJEB1962 and consists of 131 large contigs in nine scaffolds. Table 3 shows the project information and its association with minimum information about a genome sequence (MIGS) 2.0 compliance [49].
Table 3.
Project information
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | High-quality draft |
| MIGS-28 | Libraries used | 454 paired-end 5 kb library |
| MIGS-29 | Sequencing platform | 454 Roche Titanium |
| MIGS-31.2 | Fold coverage | 19.96× |
| MIGS-30 | Assemblers | Newbler |
| MIGS-32 | Gene calling method | Prodigal |
| GenBank date of release (04-06-2013) | ||
| NCBI project ID (PRJEB1771) | ||
| EMBL accession (CBLK000000000) | ||
| MIGS-13 | Project relevance | Study of human gut microbiome |
EMBL, European Molecular Biology Laboratory; MIGS, minimum information about a genome sequence; NCBI, National Center for Biotechnology Information.
Growth conditions and DNA isolation
Paenibacillus antibioticophila strain GD11T (= DSM 28228 = CSUR P1358) was cultured aerobically on Columbia agar (bioMérieux). A total of 200 μL of bacterial suspension was diluted in 1 mL Tris-EDTA (TE) buffer for lysis treatment. After a lysozyme incubation of 30 minutes at 37°C, the lysis was performed with laurylsarcosylby 1% final and RNAseA treatment at 50 μG/μL final concentration during 1 hour at 37°C, followed by an overnight proteinase K incubation at 37°C.The DNA was purified three times by phenol–chloroform extractions, and ethanolic precipitation was performed at −20°C overnight. After centrifugation, the DNA was resuspended in 150 μL TE buffer. The concentration was measured by the Quant-it Picogreen kit (Invitrogen; Life Technologies, Carlsbad, CA, USA) on the Genios_Tecan fluorometer at 48 ng/μL.
Genome sequencing and assembly
Genomic DNA of Paenibacillus antibioticophila strain GD11T was sequenced on 454_Roche_Titanium (Roche, Basel, Switzerland).
A paired end library was pyrosequenced on the 454_Roche_Titanium. This project was loaded twice on a ¼ region for the 5 kb insert libraries on PTP PicoTiterPlates. The library was constructed with 2.5 μg of DNA according to the 454_Roche_Titanium paired end protocol and manufacturer. It was mechanically fragmented on the Covaris device (KBioScience–LGC Genomics, Teddington, UK) through miniTUBE-Red 5 kb. The DNA fragmentation was visualized through the Agilent 2100 BioAnalyzer on a DNA labchip 7500, with an optimal size of 4.7 kb. The library was constructed according to the 454_Roche_Titanium paired end protocol and manufacturer. Circularization and nebulization were performed and generated a pattern with optimal at 490 bp. After PCR amplification through 17 cycles followed by double size selection, the library was then quantified on the Quant-it Ribogreen kit (Invitrogen) on the Genios_Tecan fluorometer at 759 pg/μL. The library concentration equivalence was calculated as 2.84 × 109 molecules/μL. The library was stocked at −20°C until use.
The 5 kb paired end library was clonal amplified with 0.25 and 0.5 cpb in 4 emPCR reactions per condition, with the GS Titanium SV emPCR Kit (Lib-L) v2. The yield of the emPCR was 5.21% and 5.69%, respectively, according to the quality expected by the range of 5 to 20% from the Roche procedure.
Twice, 790 000 beads were loaded on the GS Titanium PicoTiterPlates PTP Kit 70 × 75 and sequenced with the GS Titanium Sequencing Kit XLR70.
The runs were performed overnight and then analysed through the gsRunBrowser and Newbler assembler _Roche. The global 411 382 passed filter sequences generated 150.40 Mb with a length average of 354 bp. These sequences were assembled on the gsAssembler from Roche with 90% identity and 40 bp as overlap. It led to nine scaffolds and 131 large contigs (>1500 bp), generating a genome size of 5.6 Mb, which corresponds to a coverage of 19.96 genome equivalent.
Genome annotation
Open reading frames (ORFs) were predicted using Prodigal [50] with default parameters, but the predicted ORFs were excluded if they were spanning a sequencing gap region. The predicted bacterial protein sequences were searched against the GenBank database [51] and the Clusters of Orthologous Groups (COGs) database using BLASTP. The tRNAScanSE tool [52] was used to find tRNA genes, whereas ribosomal RNAs were found by using RNAmmer [53] and BLASTn against the GenBank database. Lipoprotein signal peptides and the number of transmembrane helices were predicted using SignalP [54] and TMHMM [55], respectively. ORFans were identified if their BLASTP E value was lower than 1e-03 for alignment length greater than 80 aa. If alignment lengths were smaller than 80 aa, we used an E value of 1e-05. Such parameter thresholds have already been used in previous works to define ORFans. Here, we compared the genome sequence of P. antibioticophila strain GD11T with those of Paenibacillus barengoltzii strain G22 (GenBank accession no. ASSZ00000000.1), Paenibacillus massiliensis strain 2301065T (GenBank accession no. ARIL00000000.1), Paenibacillus panacisoli strain DSM 21345 (GenBank accession no. AUFO00000000.1), Paenibacillus polymyxa strain ATCC 842T (GenBank accession no. AFOX00000000.1), Paenibacillus sanguinis strain 2301083T (GenBank accession no. ARGO00000000.1), Paenibacillus senegalensis strain JC66T (GenBank accession no. CAES00000000.1), Paenibacillus terrae strain HPl-003 (GenBank accession no. CP003107.1) and Paenibacillus zanthoxyli strain JH29T (GenBank accession no. ASSD00000000.1), which were identified using the Proteinortho software (version 1.4) [56] using a 30% protein identity and 1e-05 E value. The average percentage of nucleotide sequence identity (Table 4) between corresponding orthologous sets was determined using the Needleman-Wunsch algorithm global alignment technique. Artemis [57] was used for data management, and DNA Plotter [58] was used for visualization of genomic features. The Mauve alignment tool was used for multiple genomic sequence alignment and visualization [59]. PHAST (PHAge search Tool) was employed to identify phage sequences [60].
Table 4.
Nucleotide content and gene count levels of genome
| Attribute | Value | % of totala |
|---|---|---|
| Size (bp) | 5 562 631 | 100 |
| G+C content (bp) | 2 731 251 | 49.1 |
| Coding region (bp) | 4 803 279 | 86.34 |
| Total genes | 5155 | 100 |
| RNA genes | 71 | 1.37 |
| Protein-coding genes | 5084 | 98.62 |
| Genes assigned to COGs | 3814 | 73.98 |
| Genes with peptide signals | 415 | 8.05 |
| Genes with transmembrane helices | 1508 | 29.25 |
COGs, Clusters of Orthologous Groups database.
Total is based on either size of genome (in base pairs) or total number of protein-coding genes in annotated genome.
Genome properties
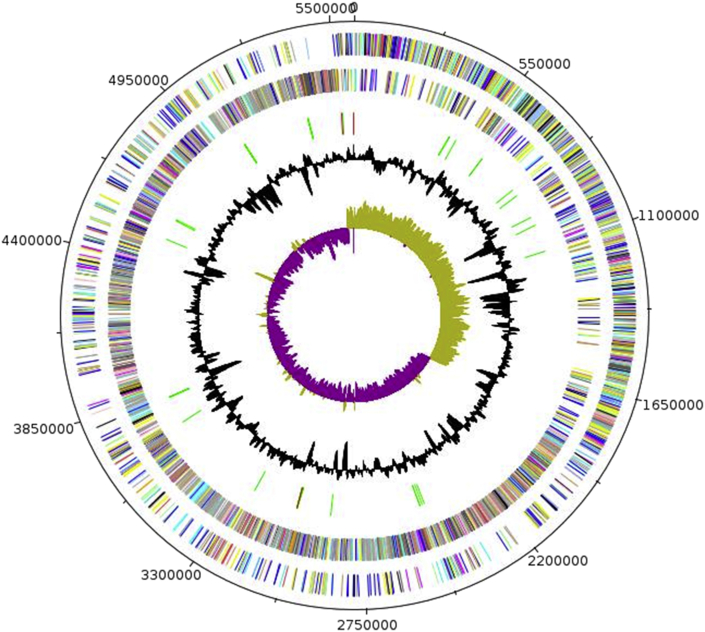
The genome of P. antibioticophila strain GD11T is 5 562 631 bp long with a 49.1% G+C content (Fig. 6). Of the 5155 predicted genes, 5084 were protein-coding genes and three were RNAs. Three rRNA genes (one 16S rRNA, one 23S rRNA and one 5S rRNA) and 68 predicted tRNA genes were identified in the genome. A total of 3814 genes (73.98%) were assigned a putative function. One hundred forty-three genes (2.77%) were identified as ORFans. The remaining genes were annotated as hypothetical proteins. The properties and the statistics of the genome are summarized in Table 5, Table 6. The distribution of genes into COGs functional categories is presented in Table 5.
Fig. 6.
Graphical circular map of chromosome. From outside in, outer two circles show ORFs oriented clockwise (colored by COGs categories) and anticlockwise (colored by COGs categories) direction, respectively. Third circle marks rRNA gene operon (red) and tRNA genes (green). Fourth circle shows GC% content plot. Innermost circle shows GC skew, with purple indicating negative values and olive positive values.
Table 5.
Number of genes associated with 25 general COGs functional categories
| Code | Value | % of totala | Description |
|---|---|---|---|
| J | 184 | 3.61 | Translation |
| A | 0 | 0 | RNA processing and modification |
| K | 486 | 9.55 | Transcription |
| L | 154 | 3.02 | Replication, recombination and repair |
| B | 1 | 0.01 | Chromatin structure and dynamics |
| D | 35 | 0.68 | Cell cycle control, mitosis and meiosis |
| Y | 0 | 0 | Nuclear structure |
| V | 132 | 2.59 | Defense mechanisms |
| T | 314 | 6.17 | Signal transduction mechanisms |
| M | 179 | 3.52 | Cell wall/membrane biogenesis |
| N | 70 | 1.37 | Cell motility |
| Z | 2 | 0.03 | Cytoskeleton |
| W | 0 | 0 | Extracellular structures |
| U | 50 | 0.98 | Intracellular trafficking and secretion |
| O | 110 | 2.16 | Posttranslational modification, protein turnover, chaperones |
| C | 148 | 2.91 | Energy production and conversion |
| G | 637 | 12.52 | Carbohydrate transport and metabolism |
| E | 296 | 5.82 | Amino acid transport and metabolism |
| F | 89 | 1.75 | Nucleotide transport and metabolism |
| H | 122 | 2.39 | Coenzyme transport and metabolism |
| I | 88 | 1.73 | Lipid transport and metabolism |
| P | 266 | 5.23 | Inorganic ion transport and metabolism |
| Q | 77 | 1.51 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 576 | 11.32 | General function prediction only |
| S | 323 | 6.35 | Function unknown |
| — | 1,27 | 24.98 | Not in COGs |
COGs, Clusters of Orthologous Groups database.
Total is based on total number of protein-coding genes in annotated genome.
Table 6.
Numbers of orthologous proteins shared between genomes
| PN | PB | PM | PPA | PP | PS | PSE | PT | PZ | |
|---|---|---|---|---|---|---|---|---|---|
| PN | 5084 | 2553 | 2421 | 2381 | 2242 | 2562 | 1883 | 2318 | 2078 |
| PB | 72.85 | 4307 | 2357 | 2304 | 2249 | 2456 | 1833 | 2292 | 2063 |
| PM | 69.61 | 69.12 | 5055 | 3881 | 3084 | 2339 | 1900 | 3142 | 2283 |
| PPA | 69.60 | 69.14 | 96.47 | 5059 | 3038 | 2302 | 1894 | 3104 | 2267 |
| PP | 69.11 | 69.12 | 71.29 | 71.23 | 5068 | 2167 | 1798 | 3279 | 2210 |
| PS | 73.96 | 75.72 | 69.47 | 69.42 | 69.29 | 4093 | 1802 | 2228 | 1948 |
| PSE | 67.11 | 67.15 | 66.78 | 66.75 | 66.74 | 67.17 | 4278 | 1797 | 1651 |
| PT | 69.62 | 69.55 | 71.63 | 71.53 | 86.20 | 69.68 | 66.99 | 5525 | 2240 |
| PZ | 69.97 | 70.46 | 69.28 | 69.26 | 69.32 | 69.99 | 67.16 | 69.85 | 4676 |
Average percentage similarity of nucleotides corresponding to orthologous protein shared between genomes (below diagonal) and numbers of proteins per genome (bold).
PA, Paenibacillus antibioticophila; PB, P. barengoltzii; PM, P. massiliensis; PP, P. polymyxa; PPA, P. panacisoli; PS, P. sanguinis; PSE, P. senegalensis; PT, P. terrae; PZ, P. zanthoxyli.
Insights from genome sequence
Extended insights
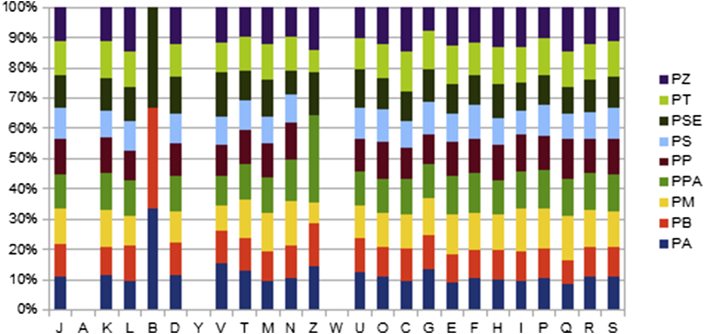
The genome of P. antibioticophila strain GD11T was compared to each of Paenibacillus barengoltzii strain G22 (GenBank accession no. ASSZ00000000.1), Paenibacillus massiliensis strain 2301065T (GenBank accession no. ARIL00000000.1), Paenibacillus panacisoli strain DSM 21345 (GenBank accession no. AUFO00000000.1), Paenibacillus polymyxa strain ATCC 842T (GenBank accession no. AFOX00000000.1), Paenibacillus sanguinis strain 2301083T (GenBank accession no. ARGO00000000.1), Paenibacillus senegalensis strain JC66T (GenBank accession no. CAES00000000.1), Paenibacillus terrae strain HPl-003 (GenBank accession no. CP003107.1) and Paenibacillus zanthoxyli strain JH29T (GenBank accession no. ASSD00000000.1). The draft genome of P. antibioticophila has a larger size than that of P. barengoltzii, P. sanguinis and P. zanthoxyli (5.56, 4.75, 4.8 and 5.05 Mb, respectively). The G+C content of P. antibioticophila is higher than those of P. massiliensis, P. panacisoli, P. polymyxa, P. senegalensis and P. terrae (49.1, 48.5, 48.3, 44.9, 48.2 and 46.8%, respectively) but less than that of P. barengoltzii, P. sanguinis and P. zanthoxyli (51.9, 49.3 and 50.9%, respectively). The gene content of P. antibioticophila is larger than those of P. barengoltzii, P. sanguinis, P. senegalensis and P. zanthoxyli (5155, 4394, 4209, 4422 and 4878, respectively). However, the distribution of genes into COGs categories was similar in all nine compared genomes (Fig. 7). In addition, P. antibioticophila shared 5084, 4307, 5055, 5059, 5068, 4093, 4278, 5525 and 4676 orthologous genes with P. barengoltzii, P. massiliensis, P. panacisoli, P. polymyxa, P. sanguinis, P. senegalensis, P. terrae and P. zanthoxyli, respectively (Table 6). The average nucleotide sequence identity ranged from 96.47 to 66.74% between the species. Finally, no sequences coding for nonribosomal peptide synthetases or polyketide synthases were found within the P. antibioticophila genome. The analysis of virome revealed the presence of one intact phage of 20.8 kb, and other three incomplete phages, of, respectively, 17, 15.4 and 14.3 kb with 43.96, 50.60 and 49.84% G+C content (Table 7).
Fig. 7.
Distribution of functional classes of predicted genes of Paenibacillus chromosomes according to clusters of orthologous groups of proteins. PA, P. antibioticophila; PB, P. barengoltzii; PM, P. massiliensis; PP, P. polymyxa; PPA, P. panacisoli; PS, P. sanguinis; PSE, P. senegalensis; PT, P. terrae; PZ, P. zanthoxyli.
Table 7.
Characteristics associated to phages in Paenibacillus antibioticophila strain GD11T
| Region length (bp) | Completeness | GC% |
|---|---|---|
| 20.8 | Intact | 48.31 |
| 17 | Incomplete | 43.96 |
| 15.4 | Incomplete | 50.60 |
| 14.3 | Incomplete | 49.84 |
Conclusions
On the basis of phenotypic, phylogenetic and genomic analyses, we formally propose the creation of Paenibacillus antibioticophila sp. nov. The strain has been isolated from the stool sample of a 63-year-old woman with multidrug-resistant tuberculosis in Marseille, France. Several other previously undescribed bacterial species were also cultivated from different faecal samples through diversification of culture conditions [1], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], thus suggesting that the human faecal flora of humans remains partially unknown.
Description of Paenibacillus antibioticophila strain GD11T sp. nov.
Paenibacillus antibioticophila strain GD11T (= DSM 28228 = CSUR P1358) is the type strain of the genus Paenibacillus. It was isolated from the stool samples of a 63-year-old woman with a pulmonary form of multidrug-resistant tuberculosis hospitalized in an infectious diseases ward in Marseille, France. The main scope of the culturomics study is to cultivate all the species within the human faeces. P. antibioticophila is a Gram-positive bacilli that does exhibits neither catalase nor oxidase activity. Colonies were 0.5 mm in diameter, and cells have a mean width of 0.49 μm and a mean length of 2.67 μm. Esterase (C4), esterase lipase (C8), naphthol-AS-BI-phosphohydrolase, β-galactosidase, α-galactosidase and α-glucosidase, α-arabinosidase, β-glucuronidase, N-acetyl-β-glucosaminidase, nitrate reduction, glutamic acid decarboxylase, fermentation l-arabinose, d-ribose, d-xylose, methyl-βd-xylopranoside, d-galactose, d-glucose, d-fructose, d-mannose, l-rhamnose, d-mannitol, N-acetylglucosamine, amygdalin, arbutin, salicin, d-cellobiose, d-maltose, d-lactose, d-melibiose, d-saccharose, d-trehalose, inulin, d-melezitose, d-raffinose, starch, glycogen and d-lyxose were positive. Acid phosphatase, alkaline phosphatase, leucine arylamidase, valine arylamidase, cystine arylamidase, lipase (C14), trypsin, α-chymotrypsin, β-glucosidase, α-mannosidase, α-fucosidase, arginine dihydrolase, urease, production of indole, leucine arylamidase, histidine arylamidase, phenylalanine arylamidase, tyrosin arylamidase, alanine arylamidase α-mannosidase, fermentation erythritol, d-arabinose, l-xylose, d-adonitol, l-sorbose, dulcitol, inositol, d-sorbitol, xylitol, d-turanose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium gluconate, potassium 2-ketogluconate, potassium 5-ketogluconate and potassium-5-ketogluconate were negative. Cells are susceptible to penicillin G, amoxicillin, amoxicillin–clavulanic acid, ceftriaxone, imipenem, vancomycin, rifampicin, erythromycin, gentamicin, ciprofloxacin and trimethoprim–sulfamethoxazole and resistant to metronidazole.
The G+C content of the genome is 49.1%. The 16S rRNA gene sequence and whole-genome shotgun sequence of P. antibioticophila strain GD11T is deposited in GenBank under accession number KC158472. The type strain is GD11T (= DSM 28228 = CSUR P1358).
Acknowledgements
The authors thank the Xegen Company (http://www.xegen.fr/) for automating the genomic annotation process. This study was funded by the Méditerranée Infection Foundation.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.nmni.2015.10.006.
Conflict of Interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Lagier J.C., Armougom F., Million M., Hugon P., Pagnier I., Robert C. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 2.Dubourg G., Lagier J.C., Armougom F., Robert C., Hamad I., Brouqui P. The gut microbiota of a patient with resistant tuberculosis is more comprehensively studied by culturomics than by metagenomics. Eur J Clin Microbiol Infect Dis. 2013;32:637–645. doi: 10.1007/s10096-012-1787-3. [DOI] [PubMed] [Google Scholar]
- 3.Tindall B.J., Rosselló-Móra R., Busse H.J., Ludwig W., Kämpfer P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol. 2010;60:249–266. doi: 10.1099/ijs.0.016949-0. [DOI] [PubMed] [Google Scholar]
- 4.Stackebrandt E., Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152–155. [Google Scholar]
- 5.Wayne L.G., Brenner D.J., Colwell P.R., Grimont P.A.D., Kandler O., Krichevsky M.I. Report of the ad hoc committee on reconciliation of approaches to bacterial systematic. Int J Syst Bacteriol. 1987;37:463–464. [Google Scholar]
- 6.Rossello-Móra R. Molecular identification, systematics, and population structure of prokaryotes. Springer Berlin; Heidelberg: 2006. DNA-DNA reassociation methods applied to microbial taxonomy and their critical evaluation; pp. 23–250. [Google Scholar]
- 7.Genomes OnLine Database (GOLD). https://gold.jgi.doe.gov/index.
- 8.Lagier J.C., El Karkouri K., Nguyen T.T., Armougom F., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Bacillus timonensis sp. nov. Stand Genomic Sci. 2012;6:346–355. doi: 10.4056/sigs.2776064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagier J.C., El Karkouri K., Nguyen T.T., Armougom F., Raoult D., Fournier P.E. Non-contiguous finished genome sequence and description of Anaerococcus senegalensis sp. nov. Stand Genomic Sci. 2012;6:116–125. doi: 10.4056/sigs.2415480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra A.K., Gimenez G., Lagier J.C., Robert C., Raoult D., Fournier P.E. Genome sequence and description of Alistipes senegalensis sp. nov. Stand Genomic Sci. 2012;6:1–16. doi: 10.4056/sigs.2625821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagier J.C., Armougom F., Mishra A.K., Nguyen T.T., Raoult D., Fournier P.E. Non-contiguous finished genome sequence and description of Alistipes timonensis sp. nov. Stand Genomic Sci. 2012;6:315–324. doi: 10.4056/sigs.2685971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra A.K., Lagier J.C., Robert C., Raoult D., Fournier P.E. Non-contiguous finished genome sequence and description of Clostridium senegalense sp. nov. Stand Genomic Sci. 2012;6:386–395. doi: 10.4056/sigs.2766062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra A.K., Lagier J.C., Robert C., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Peptoniphilus timonensis sp. nov. Stand Genomic Sci. 2012;7:1–11. doi: 10.4056/sigs.2956294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra A.K., Lagier J.C., Rivet R., Raoult D., Fournier P.E. Non-contiguous finished genome sequence and description of Paenibacillus senegalensis sp. nov. Stand Genomic Sci. 2012;7:70–81. doi: 10.4056/sigs.3056450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagier J.C., Gimenez G., Robert C., Raoult D., Fournier P.E. Non-contiguous finished genome sequence and description of Herbaspirillum massiliense sp. nov. Stand Genomic Sci. 2012;7:200–209. doi: 10.4056/sigs.3086474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roux V., El Karkouri K., Lagier J.C., Robert C., Raoult D. Non-contiguous finished genome sequence and description of Kurthia massiliensis sp. nov. Stand Genomic Sci. 2012;7:221–232. doi: 10.4056/sigs.3206554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokcha S., Ramasamy D., Lagier J.C., Robert C., Raoult D., Fournier P.E. Non-contiguous finished genome sequence and description of Brevibacterium senegalense sp. nov. Stand Genomic Sci. 2012;7:233–245. doi: 10.4056/sigs.3256677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramasamy D., Kokcha S., Lagier J.C., Nguyen T.T., Raoult D., Fournier P.E. Genome sequence and description of Aeromicrobium massiliense sp. nov. Stand Genomic Sci. 2012;7:246–257. doi: 10.4056/sigs.3306717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagier J.C., Ramasamy D., Rivet R., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Cellulomonas massiliensis sp. nov. Stand Genomic Sci. 2012;7:258–270. doi: 10.4056/sigs.3316719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagier J.C., Elkarkouri K., Rivet R., Couderc C., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Senegalemassilia anaerobia gen. nov., sp. nov. Stand Genomic Sci. 2013;7:343–356. doi: 10.4056/sigs.3246665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra A.K., Hugon P., Lagier J.C., Nguyen T.T., Robert C., Couderc C. Non contiguous-finished genome sequence and description of Peptoniphilus obesi sp. nov. Stand Genomic Sci. 2013;7:357–369. doi: 10.4056/sigs.3276687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra A.K., Lagier J.C., Nguyen T.T., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Peptoniphilus senegalensis sp. nov. Stand Genomic Sci. 2013;7:370–381. doi: 10.4056/sigs.3366764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagier J.C., El Karkouri K., Mishra A.K., Robert C., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Enterobacter massiliensis sp. nov. Stand Genomic Sci. 2013;7:399–412. doi: 10.4056/sigs.3396830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugon P., Ramasamy D., Lagier J.C., Rivet R., Couderc C., Raoult D. Non contiguous-finished genome sequence and description of Alistipes obesi sp. nov. Stand Genomic Sci. 2013;7:427–439. doi: 10.4056/sigs.3336746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra A.K., Hugon P., Robert C., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Peptoniphilus grossensis sp. nov. Stand Genomic Sci. 2012;7:320–330. doi: 10.4056/sigs.3076460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra A.K., Hugon P., Lagier J.C., Nguyen T.T., Couderc C., Raoult D. Non contiguous-finished genome sequence and description of Enorma massiliensis gen. nov., sp. nov., a new member of the family Coriobacteriaceae. Stand Genomic Sci. 2013;8:290–305. doi: 10.4056/sigs.3426906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramasamy D., Lagier J.C., Gorlas A., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Bacillus massiliosenegalensis sp. nov. Stand Genomic Sci. 2013;8:264–278. doi: 10.4056/sigs.3496989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramasamy D., Lagier J.C., Nguyen T.T., Raoult D., Fournier P.E. Non contiguous-finished genome sequence and description of Dielma fastidiosa gen. nov., sp. nov., a new member of the family Erysipelotrichaceae. Stand Genomic Sci. 2013;8:336–351. doi: 10.4056/sigs.3567059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra A.K., Lagier J.C., Robert C., Raoult D., Fournier P.E. Genome sequence and description of Timonella senegalensis gen. nov., sp. nov., a new member of the suborder Micrococcinae. Stand Genomic Sci. 2013;8:318–335. doi: 10.4056/sigs.3476977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux V., Lagier J.C., Gorlas A., Robert C., Raoult D. Non-contiguous finished genome sequence and description of Kurthia senegalensis sp. nov. Stand Genomic Sci. 2014;9(3) doi: 10.4056/sigs.5078947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padhmanabhan R., Dubourg G., Lagier J.C., Nguyen T.T., Couderc C., Rossi-Tamisier M. Non-contiguous finished genome sequence and description of Collinsella massiliensis sp. nov. Stand Genomic Sci. 2014;9(3) doi: 10.4056/sigs.5399696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasamy D., Dubourg G., Robert C., Caputo A., Papazian L., Raoult D. Non contiguous-finished genome sequence and description of Enorma timonensis sp. nov. Stand Genomic Sci. 2014;9(3) doi: 10.4056/sigs.4878632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edouard S., Bibi F., Ramasamy D., Lagier J.C., Azhar E.I., Robert C. Non-contiguous finished genome sequence and description of Corynebacterium jeddahense sp. nov. Stand Genomic Sci. 2014;9(3) doi: 10.4056/sigs.5561028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagnier I., Croce O., Robert C., Raoult D., La Scola B. Non-contiguous finished genome sequence and description of Anaerococcus provencensis sp. nov. Stand Genomic Sci. 2014;9(3) doi: 10.4056/sigs.5501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagnier I., Croce O., Robert C., Raoult D., La Scola B. Non-contiguous finished genome sequence and description of Fenollaria massiliensis gen. nov., sp. nov., a new genus of anaerobic bacterium. Stand Genomic Sci. 2014;9(3) doi: 10.4056/sigs.3957647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeiderer A., Robert C., Caputo A., Raoult D., Fournier P.E. Non-contiguous finished genome sequence and description of Alistipes massilioanorexius sp. nov. Stand Genomic Sci. 2014;9(3) doi: 10.4056/sigs.4698398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ash C., Priest F.G., Collins M.D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek. 1993;64:253–260. doi: 10.1007/BF00873085. [DOI] [PubMed] [Google Scholar]
- 38.Lal S., Tabacchioni S. Ecology and biotechnological potential of Paenibacillus polymyxa: a minireview. Indian J Microbiol. 2009;49:2–10. doi: 10.1007/s12088-009-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McSpadden Gardener B.B. Ecology of Bacillus and Paenibacillus spp. in agricultural systems. Phytopathology. 2004;94:1252–1258. doi: 10.1094/PHYTO.2004.94.11.1252. [DOI] [PubMed] [Google Scholar]
- 40.Montes M.J., Mercade E., Bozal N., Guinea J. Paenibacillus antarcticus sp. nov., a novel psychrotolerant organism from the Antarctic environment. Int J Syst Evol Microbiol. 2004;54:1521–1526. doi: 10.1099/ijs.0.63078-0. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang J., Pei Z., Lutwick L., Dalal S., Yang L., Cassai N. Case report: Paenibacillus thiaminolyticus: a new cause of human infection, inducing bacteremia in a patient on hemodialysis. Ann Clin Lab Sci. 2008;38:393–400. [PMC free article] [PubMed] [Google Scholar]
- 42.Glaeser S.P., Falsen E., Busse H.J., Kämpfer P. Paenibacillus vulneris sp. nov., isolated from a necrotic wound. Int J Syst Bacteriol. 2013;63(pt 2):777–782. doi: 10.1099/ijs.0.041210-0. [DOI] [PubMed] [Google Scholar]
- 43.Woese C.R., Kandler O., Wheelis M.L. Towards a natural system of organisms: proposal for the domains Archae, Bacteria, and Eukarya. Proc Natl Acad Sci U S A. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skerman V.B.D., Sneath P.H.A. Approved list of bacterial names. Int J Syst Bact. 1980;30:225–420. [Google Scholar]
- 45.Garrity G.M., Holt J. Taxonomic outline of the Archae and Bacteria. In: Garrity G.M., Boone D.R., Castenholz R.W., editors. Bergey’s manual of systematic bacteriology. Springer Verlag; New York: 2001. pp. 155–166. [Google Scholar]
- 46.Prevot A.R. Dictionnaire des bactéries pathogènes. In: Hauduroy P., Ehringer G., Guillot G., Magrou J., Prevot A.R., Rosset A., editors. Masson; Paris: 1953. pp. 1–692. [Google Scholar]
- 47.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.E., Rolain J.M. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 49.Field D., Garrity G., Gray T., Morrison N., Selengut J., Sterk P. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol. 2008;26:541–547. doi: 10.1038/nbt1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prodigal. http://prodigal.ornl.gov/.
- 51.Benson D.A., Karsch-Mizrachi I., Clark K., Lipman D.J., Ostell J., Sayers E.W. GenBank. Nucleic Acids Res. 2012;40:D48–D53. doi: 10.1093/nar/gkr1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowe T.M., Eddy S.R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lagesen K., Hallin P., Rodland E.A., Staerfeldt H.H., Rognes T., Ussery D.W. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bendtsen J.D., Nielsen H., von Heijne G., Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 55.TMHMM. http://www.cbs.dtu.dk/services/TMHMM/.
- 56.Lechner M., Findeib S., Steiner L., Marz M., Stadler P.F., Prohaska S.J. Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics. 2011;12:124. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutherford K., Parkhill J., Crook J., Horsnell T., Rice P., Rajandream M.A. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 58.Carver T., Thomson N., Bleasby A., Berriman M., Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics. 2009;25:119–120. doi: 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darling A.C., Mau B., Blattner F.R., Perna N.T. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y., Liang Y., Lynch K.H., Dennis J.J., Wishart D.S. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39(Web server issue):W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.