Abstract
Intestinal inflammation is a harmful condition in fish that can be triggered by the ingestion of soybean meal. Due to the positive costs-benefits ratio of including soybean meal in farmed fish diets, identifying additives with intestinal anti-inflammatory effects could contribute to solving the issues caused by this plant protein. This study evaluated the effect of incorporating lactoferrin (LF) into a soybean meal-based diet on intestinal inflammation in zebrafish. Larvae were fed with diets containing 50% soybean meal (50SBM) or 50SBM supplemented with LF to 0.5, 1, 1.5 g/kg (50SBM+LF0.5; 50SBM+LF1.0; 50SBM+LF1.5). The 50SBM+LF1.5 diet was the most efficient and larvae had a reduced number of neutrophils in the intestine compared with 50SBM larvae and an indistinguishable number compared with control larvae. Likewise, the transcription of genes involved in neutrophil migration and intestinal mucosal barrier functions (mmp9, muc2.2, and β-def-1) were increased in 50SBM larvae but were normally expressed in 50SBM+LF1.5 larvae. To determine the influence of intestinal inflammation on the general immune response, larvae were challenged with Edwardsiella tarda. Larvae with intestinal inflammation had increased mortality rate compared to control larvae. Importantly, 50SBM+LF1.5 larvae had a mortality rate lower than control larvae. These results demonstrate that LF displays a dual effect in zebrafish, acting as an intestinal anti-inflammatory agent and improving performance against bacterial infection.
1. Introduction
Intestinal inflammation in fish is a detrimental condition that affects growth and the ability to respond to pathogens [1]. Preventing this pathology is of particular relevance for fish farming as small fish size and/or high fish mortality drastically affect the competitiveness and profitability of the aquaculture industry.
Most commercially important fish are carnivorous and require a high-protein diet, usually provided through fishmeal [2]. However, increased aquaculture production has limited fishmeal availability, leading to the use of plant proteins in fish diets [2]. Soybean meal, which is widely available and economical, has a balanced amino acid profile, and contains a high amount of digestible proteins, is currently the most common plant protein source used in fish feed [3]. Studies in different fish species, such as Atlantic salmon (Salmo salar) [4], rainbow trout (Oncorhynchus mykiss) [5], carp (Cyprinus carpio L.) [6], Nile tilapia (Oreochromis niloticus) [7], gilthead seabream (Sparus aurata) [8], and zebrafish (Danio rerio) [9, 10], have shown that the inclusion of soybean meal in the diet triggers intestinal inflammation [11–14]. Nevertheless, the advantages of soybean meal outweigh its disadvantageous effects to fish intestines.
To optimize the use of this plant protein, there is an ongoing search to find dietary additives that could protect the intestine from the effects of soybean meal. Traditionally, additives have been incorporated into fish diets to control diseases, increase health, and improve the stress response [1, 15–19]. However, little focus has been given to the use of additives for controlling intestinal inflammation. At present, there are only two studies that evaluate the effects of additives, specifically mannan-oligosaccharide and β-glucans, on soybean meal-triggered intestinal inflammation in farmed fish [1, 17]. These investigations indicate that only mannan-oligosaccharide is able to decrease, to varying degrees, the altered intestinal histology observed in fish fed with diets including low amounts of soybean meal [1, 17].
Lactoferrin (LF) is an abundant iron-binding glycoprotein secreted by epithelial cells and contributes to the composition of bodily fluids in mammals, such as milk, tears, saliva, bile, and pancreatic fluid [20, 21]. Specific LF receptors are present on the surface of different tissues and cell types, including the gastrointestinal tract, lungs, neutrophils, and eosinophils [22, 23]. Previous research has demonstrated that this glycoprotein has antimicrobial activity and can stimulate cytokine production, enhance cell proliferation, and regulate mucosal immunity [20, 24–26]. Due to these properties, LF has been used in prophylactic treatments for fish against different infectious diseases [25, 27–29]. Furthermore, LF exerts a potent anti-inflammatory effect in different tissues, mainly by reducing immune cell recruitment to inflammatory sites. During influenza virus infection, LF reduces the number of infiltrating leukocytes in bronchoalveolar lavage fluid in humans [30]. Likewise, LF can reduce eosinophil infiltration to the pigs small intestine in a mechanism independent of cytokine [31, 32]. Moreover, an in vivo study in rats and mice demonstrated that orally administered LF prevents intestinal injury triggered by nonsteroidal anti-inflammatory drugs. The authors related this effect to the attenuation of neutrophil migration to the intestine [33]. Despite these various related studies, there are currently no reports on the possible role of LF as an intestinal protector in fish and less so on the possible protective effects of LF against soybean meal-induced intestinal inflammation in fish.
Factors contributing to this lack of information are the high costs and long-term assays involved in evaluating the use of additives in the aquaculture industry, which is in addition to the challenge of working with minimal precedents on the cellular and molecular processes in many farmed fish species. Therefore, an alternative research strategy is to perform preliminary studies in a model fish in which many diets can be assessed in a short period and at low costs. Moreover, the selected fish model should facilitate understanding the biological processes triggered by different diets. Considering the extensive biological literature and powerful biotechnological tools available for zebrafish (D. rerio), this teleost fish is an ideal organism for immune-nutrition research [34].
One key advantage of zebrafish is the availability of transgenic lines with certain fluorescently-labeled innate immune cells, such as neutrophils [35]. Since the hallmark of inflammation is neutrophil migration, these cells can be used as inflammatory markers. This strategy has been used before by Hedrera et al. [9], who demonstrated that soybean meal consumption by zebrafish results in inflammatory side effects similar to those observed in commercially farmed fish. A primary advantage of using transgenic, fluorescently labeled zebrafish is that the inflammatory process can be very quickly observed, even before histological effects become recognizable.
This study evaluated the effects of LF on intestinal inflammation induced by a soybean meal-based diet in zebrafish. By using the Tg(BACmpo:GFP)i114 transgenic zebrafish line, neutrophil migration as part of the intestinal inflammatory process was monitored in vivo. To complement this data, the transcriptional levels of different markers related to mucosal barrier functions as matrix metallopeptidase 9, mucin 2.2, and beta-defensin 1 (mmp9, muc2.2, and β-def-1) and lipid absorption like the fatty-acid-binding proteins 2 and 6 (fabp2 and fabp6) were evaluated. Finally, the influence of intestinal inflammation on the immune response to Edwardsiella tarda infection was assessed.
2. Material and Methods
2.1. Zebrafish Strains and Maintenance
Zebrafish were maintained and raised at the Laboratory of Developmental Biology, Universidad Andrés Bello, Chile, according to standard protocols [37]. The Tg(BACmpo:GFP)i114 transgenic zebrafish line was used [35]. All embryos were collected through natural spawning according to Kimmel et al. [38]. Eggs were incubated in petri dishes at 28°C for three days in the E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4, with methylene blue, equilibrated to pH 7.0) [39]. Embryonic and larval stages are expressed in hours postfertilization (hpf) or days postfertilization (dpf). All animal-handling procedures were approved by the Committee of Animal Bioethics of the Universidad Andrés Bello.
2.2. Experimental Diets
Five diets were formulated and prepared (Table 1). The positive control diet contained 50% soybean meal (50SBM), while the negative control diet contained fishmeal as the primary protein source (100FM) [9]. Additionally, a diet normally used for zebrafish larvae (ZFP, sera Micron®, Heinsberg, Germany) was used as a second negative control. To evaluate the intestinal protective effect of LF, three batches of the 50SBM diet were supplemented with bovine LF obtained from milk (Lactoferrin 100% S60, Natural Healthy Concepts, Appleton, WI, USA) in concentrations of 0.5, 1.0, or 1.5 g/kg (50SBM+LF0.5; 50SBM+LF1.0; and 50SBM+LF1.5, resp.) [40]. Likewise, one batch of the 100FM diet was supplemented with 1.5 g/kg of bovine LF (100FM+LF1.5). All diets were supplemented with a standard vitamin and mineral premix and formulated to be isoenergetic, isonitrogenous, and isolipidic. Each feed diet was prepared by cooking-extrusion in a twin screw extruder (CLEXTRAL BC-21, Clextral, Firminy, France) with a 2 mm diameter. The resulting moist pellets were oven-dried at 60°C for approximately eight hours and then coated with fish oil, according to the formulation for each diet, using a laboratory vacuum coater (Dinnissen Model VC10, Sevenum, Netherlands). The pellets were subsequently crumbled, screened to the appropriate particle size (75 μm), and stored at −20°C until use in the feeding trials.
Table 1.
Ingredients and nutrient composition of experimental diets.
| 100FM | 50SBM | Different doses of LF incorporated into diets | ||||
|---|---|---|---|---|---|---|
| 50SBM+LF0.5 | 50SBM+LF1.0 | 50SBM+LF1.5 | FM+LF1.5 | |||
| Ingredients g/kg | ||||||
| Fishmeal | 610 | 250 | 250 | 250 | 250 | 610 |
| Soybean meal | 0 | 500 | 500 | 500 | 500 | 0 |
| Wheat grain meal | 255 | 115 | 115 | 115 | 115 | 255 |
| Starch | 45 | 45 | 45 | 45 | 45 | 45 |
| Fish oil | 30 | 60 | 59.5 | 59 | 58.5 | 28.5 |
| Vitamineral mix1 | 30 | 30 | 30 | 30 | 30 | 30 |
| Cellulose | 30 | 0 | 0 | 0 | 0 | 30 |
| Lactoferrin | 0 | 0 | 0.5 | 1 | 1.5 | 1.5 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
|
| ||||||
| Analytical composition (dry base, %) | ||||||
| Dry matter | 95.3 | 93.5 | 94.0 | 94.0 | 93.04 | 94.27 |
| Total proteins | 46.4 | 43.5 | 43.4 | 43.4 | 43.46 | 44.40 |
| Total lipids | 7.8 | 8.4 | 7.6 | 7.6 | 6.78 | 6.39 |
| Ash | 12.6 | 9.7 | 9.8 | 9.8 | 8.25 | 9.76 |
| Gross energy (MJ/kg) | 20.0 | 20.3 | 20.2 | 20.2 | 20.2 | 20.0 |
1As recommended by the National Research Council, 1993 [36].
2.3. Experimental Feeding Period
Experimental feeding was performed as previously described by Hedrera et al. [9]. Briefly, 45 larvae were fed two times daily from 5 to 8 dpf. The last feed was given at least 14 h before larval fixing to promote intestine emptying.
2.4. Immunohistochemistry and Sudan Black B Staining
Immunohistochemistry was performed as previously described by Feijoo et al. [41]. The following antibodies were used: rabbit anti-Green Fluorescent Protein (anti-GFP) (Cat. number A11122, Invitrogen, Carlsbad, CA, USA) and anti-rabbit peroxidase (Cat. number A8275, Sigma, St. Louis, MO, USA). Additionally, Sudan Black B staining was performed following the manufacturer's protocol (Cat. number 3801, Sigma-Aldrich, St. Louis, MO, USA). The neutrophils present in the intestine were quantified within a defined area that included the mid and posterior intestine. At least 28 larvae were analyzed per diet in three independent experiments.
2.5. Quantitative Polymerase Chain Reaction (qPCR)
Larvae fed with the control and experimental diets were sampled at the end of each treatment for total RNA extraction. Total RNA was extracted from a pool of ~60 larval intestines per diet. The whole intestine was dissected from larvae anesthetized in tricaine methanesulfonate using sterile instruments. Samples were stored in the RNAlater solution and then homogenized in the TRIzol Reagent (Cat. number 15596-026, Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The corresponding cDNA were synthesized from 2.5 μg of total RNA using SuperScript II Reverse Transcriptase (Cat. number 100004925, Invitrogen, Carlsbad, CA, USA) and Oligo-dt primers. Primer sequences and the efficiencies are shown in Table 2. qPCR was performed with the ABI 7300 Real-Time PCR system using the Maxim SYBR Green/ROX qPCR Master Mix (2x) (Fermentas, Waltham, MA, USA) following the manufacturer's instructions. A 15 μL reaction volume was used, containing 1 μL of 10-fold diluted cDNA. The PCR was run with a ten-minute activation and denaturation step at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 57–60°C, and 30 s at 72°C. Reaction specificity was verified using melting curve analysis and the absence of primer dimmers. Standard curves were obtained for each pair of primers by plotting Ct values against the log10 of five different dilutions of a cDNA mix solution for all analyzed samples. Real-time PCR efficiency (E) was calculated from a standard curve according to the equation E = 10(−1/slope) [42]. Relative expression was calculated with the Pfaffl method [42], and the 50SBM diet was used as a calibrator.
Table 2.
Primer sequences used for amplification of specific genes through RT-qPCR.
| Gene | Forward primer | Reverse primer | Amplicon (bp) | Gene ID | Efficiency |
|---|---|---|---|---|---|
| muc2.2 | ACACGCTCAAGTAATCGCACAGTC | TCAGCGAGTGTTTGGCTCACTT | 137 | XM_002667543 | 1.82 |
| mmp9 | CATTAAAGATGCCCTGATGTATCCC | AGTGGTGGTCCGTGGTTGAG | 142 | NM_213123.1 | 1.89 |
| β-def-1 | CTCCTTGTCGTACTAGCATTGCAC | ACACACTCCTTGTCTGCAAACACC | 99 | NM_001081553.1 | 1.86 |
| fabp2 | TCAACGGGACCTGGAAAGTC | CCCATTTGTTCCATGAACTTCTC | 61 | NM_131431.1 | 1.86 |
| fabp6 | CTCCGCTCAATCAACACCAA | TGAGATTCGGTTTCCCACTTG | 59 | NM_001002076.1 | 1.93 |
| β-actin | TTCTGGTCGTACTACTGGTATTGTG | ATCTTCATCAGGTAGTCTGTCAGGT | 144 | NM_131031.1 | 1.99 |
| rpl13α | TCTGGAGGACTGTAAGAGGTATGC | AGACGCACAATCTTGAGAGCAG | 148 | NM_212784.1 | 1.94 |
Primers for mmp9, muc2.2, β-def-1, fapb2, and fabp6 were designed using AmplifX v1.4.0. Two reference genes, β-actin and ribosomal protein L13a (rpl13α), were used [10].
2.6. cDNA Cloning, Probe Synthesis, and Whole-Mount In Situ Hybridization
The fabp2 gene was cloned using the following primers: F-5′-CGACCGCAATGAGAACTACGAGAA-3′ and R-5′-CTCACAGGTGCAAATGACACGA-3′ (gene ID: NM_131431.1) from a 9 dpf cDNA library. A 529 base pair cDNA fragment was cloned into the pGEM-T easy vector (Promega, Madison, WI, USA), which was digested with the ApaI restriction enzyme. The anti-sense riboprobe was then synthesized using the Sp6 RNA polymerase. The fabp6 clone was kindly provided by Oehlers et al. [43]. In situ hybridization was performed as previously described by Jowett and Lettice [44].
2.7. Edwardsiella tarda Challenge
Edwardsiella tarda FL60 was kindly provided by Dr. Phillip Klesius (USDA, Agricultural Research Service, Aquatic Animal Health Research Unit). The E. tarda culture was grown as previously described by Harvie et al. [45] and Van Soest et al. [46], with some modifications. Briefly, E. tarda was grown overnight at 28°C in a trypticase soy broth medium with agitation. The overnight culture was diluted to 1 : 100 in fresh trypticase soy broth medium and incubated at 28°C to reach 108 CFU/mL. The culture was pelleted by centrifugation at 1500 g for five minutes, washed with water from the aquarium, and repelleted to recover the bacteria. The clean E. tarda was suspended in water from the aquarium to reach 108 CFU/mL. After four days of feeding, a group of 30 larvae were challenged for 5 h in 200 mL of the E. tarda water media and were subsequently transferred to a tank with new, clean water. Each respective diet was resumed for the remainder of the trial period, and larvae mortality was monitored every 12 h for four days.
2.8. Statistical Analysis and Imaging
The data were analyzed using a nonparametric, Kruskal-Wallis, one-way ANOVA, and Dunn multiple comparisons test. The data were normally distributed (the D'Agostino and Pearson normality test), but variance was not homogenous (the Brown-Forsythe test). Survival data were analyzed using Kaplan-Meier and group differences were analyzed by the log-rank test, using the Bonferroni correction for multiple comparisons. All analyses were performed using Prism 4 (GraphPad Software, La Jolla, CA, USA). Significance was established for all analyses at P < 0.05. Lateral view photographs of larvae were taken in an Olympus SZX16 stereoscope with a QImaging MicroPublisher 5.0 RVT camera. Images were processed with Photoshop CS4 or ImageJ v1.44.
3. Results
3.1. Effect of Soybean Meal Diet Supplemented with Lactoferrin on Intestinal Inflammation
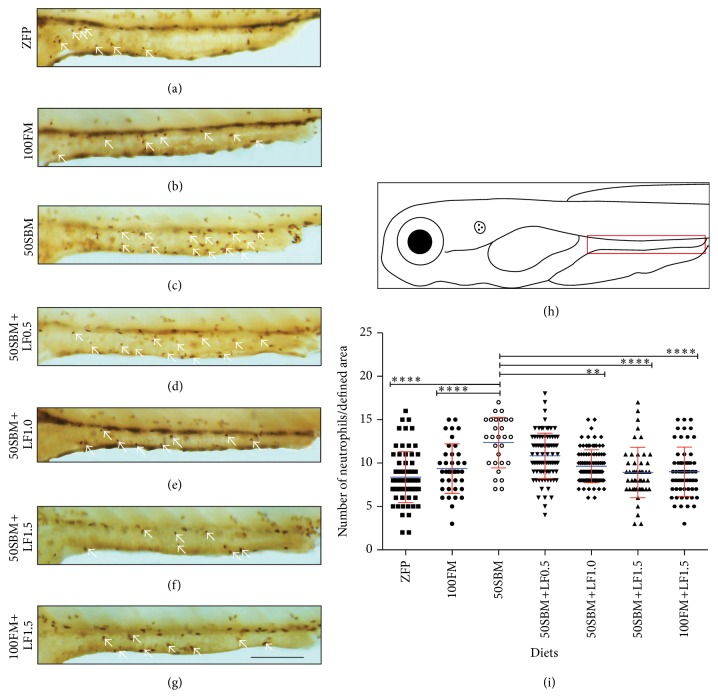
To determine if LF exerted an intestinal protector effect, by preventing or decreasing inflammation, this additive was incorporated to the soybean meal-based diet (50SBM), thereby generating three experimental diets (50SBM+LF0.5, 50SBM+LF1.0, and 50SBM+LF1.5). The 50SBM diet was used as a positive control that triggers inflammation, while the 100FM and ZFP diets were used as negative controls [9]. To determine the extent of intestinal inflammation, the amount of neutrophils present in the intestine was quantified (Figure 1).
Figure 1.
Effect of different lactoferrin doses on neutrophil migration to the intestine. (a–g) Lateral view of mid and posterior intestine of Tg(BACmpo:GFP)i114 larvae of 9 dpf after four days of feeding with different diets (ZFP, 100FM, 50SBM, 50SBM+LF0.5, 50SBM+LF1.0, 50SBM+LF1.5, and 100FM+LF1.5). Neutrophils were quantified through immunohistochemistry against GFP (white arrows). (h) Larva scheme with the intestinal region of interest demarcated with a red rectangle. (i) The experiments were conducted with at least 28 larvae per treatment in three different assays. Statistical analysis was performed by comparing data sets with the 50SBM diet through one-way ANOVA. The graph is a representation of three different results. ∗∗ P < 0.01; ∗∗∗∗ P < 0.0001. Bar scale = 200 μm.
Confirming previously published data, the results indicated a clear increase in the number of neutrophils situated in the intestine of larvae fed with a 50SBM diet compared to larvae fed with the ZFP and 100FM diets (Figures 1(a)–1(c) and 1(i)). In larvae fed with the 50SBM+LF0.5 diet, the number of neutrophils located in the intestine showed no significant differences compared with 50SBM larvae (Figures 1(d), 1(c), and 1(i)). On the other hand, larvae fed with the 50SBM+LF1.0 and 50SBM+LF1.5 diets presented a decreased number of neutrophils in the intestine compared to the 50SBM group and, more importantly, these larvae were indistinguishable from those fed the 100FM and ZFP diets (Figures 1(e), 1(f), and 1(i)). Of significance, the amount of intestine-located neutrophils in larvae fed the 50SBM+LF1.5 diet was similar to larvae fed the 100FM+LF1.5 diet (Figures 1(f), 1(g), and 1(i)).
To corroborate immunohistochemistry data, Sudan Black B staining was performed to specifically label leukocytes. Total concordance was found between the results obtained with the two techniques (Supplementary Figure 1, in Supplementary Material available online at http://dx.doi.org/10.1155/2016/1639720).
Since LF concentrations of 1.0 and 1.5 g/kg exerted similar effects, subsequent analyses were performed using the 50SBM diet supplemented by 1.5 g/kg of LF (i.e., 50SBM+LF1.5).
3.2. Effect of Soybean Meal Diet Supplemented with Lactoferrin on Genes Related to Intestinal Mucosal Function
To evaluate the effect of LF on genes involved in intestinal mucosal function, the transcripts of muc2.2, β-def-1, and mmp9 were determined through qPCR. The relative expressions of these genes in response to the different diets were compared against expressions in larvae fed with the 50SBM diet (Figure 2, dotted line). As expected, in larvae fed with the ZFP and 100FM control diets, the transcriptional levels of muc2.2, β-def-1 and mmp9 were significantly lower than in larvae fed with the 50SBM diet (P < 0.001). This same result was observed in larvae fed the 100FM+LF1.5 diet. Notably, the mRNA levels in larvae fed with the 50SBM+LF1.5 diet were comparable to those observed in the ZFP and 100FM groups and were significantly lower than those in larvae fed with the 50SBM diet (P < 0.001).
Figure 2.
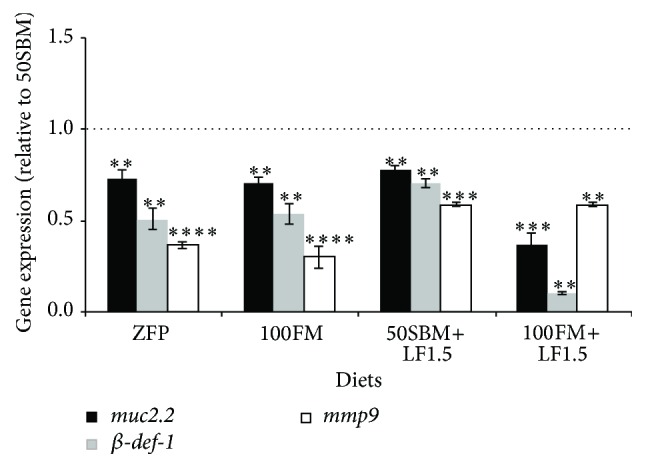
Effect of lactoferrin on transcriptional levels of mucosal barrier functional markers. Transcription levels of muc2.2, β-def-1, and mmp9 were quantified by qPCR. Quantification was performed from a pool of ~60 intestines of larvae of 9 dpf after four days of feeding with different diets (ZFP, 100FM, 50SBM, 50SBM+LF1.5, and 100FM+LF1.5). All data were normalized with β-actin and rpl13α. The data from the different diets were compared to the 50SBM diet (dotted lines). ∗∗ P < 0.01; ∗∗∗ P < 0.001; ∗∗∗∗ P < 0.0001.
3.3. Effect of Soybean Meal Diets Supplemented with Lactoferrin on Genes Related to Intestinal Lipid Absorption
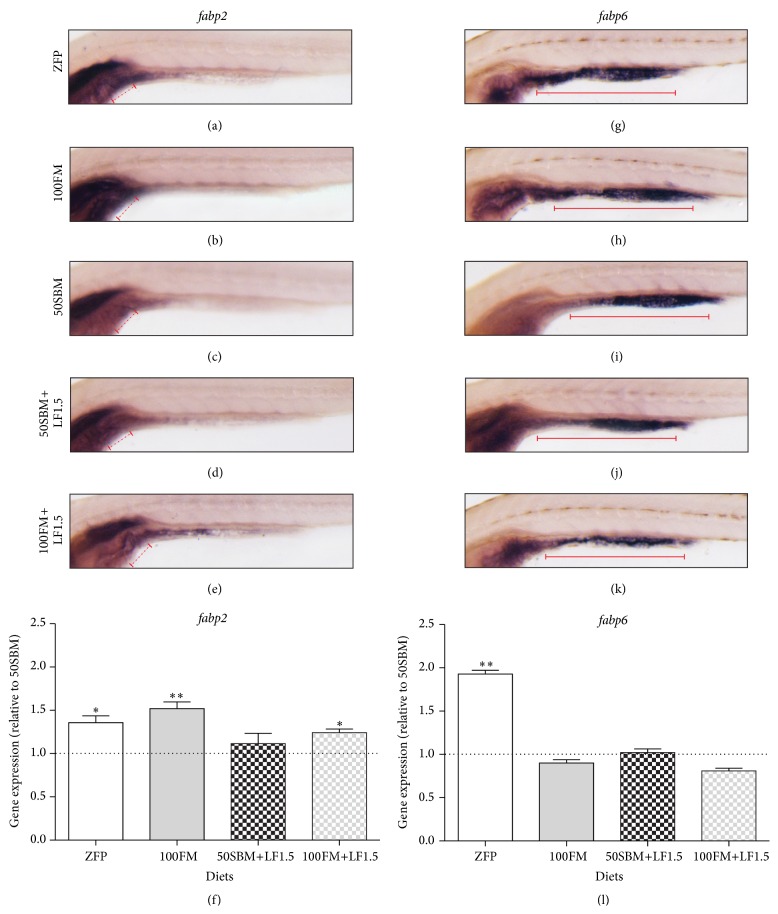
To evaluate the effect of a 50SBM diet and LF supplementation on the expression of genes related to the lipid absorption process in the intestine, in situ hybridization and qPCR were performed for the markers fabp2 and fabp6 at 9 dpf. The fabp2 expression was restricted to the anterior intestine, with a reduction towards the mid and posterior intestine (Figures 3(a)–3(e), red dotted line). In contrast, fabp6 was expressed at the mid and posterior intestine (Figures 3(g)–3(k)). The expression of fabp6 in the defined area was similar between larvae fed with the different diets (Figures 3(g)–3(k), red continued line). Likewise, qPCR analysis revealed a significant upregulation in the expression of fabp2 in control diets (ZFP, 100FM, and 100FM+LF1.5) in comparison to those fed with 50SBM (Figure 3(f)). The transcriptional expression of fabp6 between control and experimental larvae did not vary, except in larvae fed the ZFP diet, where significant upregulation was observed (P < 0.01) (Figure 3(l)).
Figure 3.
Effect of lactoferrin on lipid absorption markers. (a–e) fabp2 and (g–k) fabp6 mRNA expression pattern was analyzed by whole-mount in situ hybridization. Lateral view of larvae of 9 dpf after four days of feeding with different diets (ZFP, 100FM, 50SBM, 50SBM+LF1.5, and 100FM+LF1.5). (a–e) fabp2 expression was restricted to anterior intestine (red dotted line). (g–k) fabp6 expression was observed in the whole intestine, with a stronger expression in the mid and posterior gut (red continued line). (f and l) The transcriptional levels of fabp2 and fabp6 were quantified by qPCR. Data were normalized with β-actin and rpl13α and compared to 50SBM diet (dotted line). ∗ P < 0.05; ∗∗ P < 0.01.
3.4. Effect of Intestinal Inflammation on Immune Performance against Edwardsiella tarda
To determine if intestinal inflammation affected the immune performance of the different larvae groups, a challenge assay was performed using the enterobacteria E. tarda (Figure 4). The challenged larvae fed with the 50SBM diet presented significantly higher mortality rates than those fed with the 100FM or ZFP diets (P < 0.01). However, larvae fed the 50SBM+LF1.5 diet showed a drastic decrease in accumulated mortality at the end of the experiment compared to 50SBM (P < 0.01), and mortality rates were even lower in the 50SBM+LF1.5 group than in larvae fed with the 100FM or ZFP diets. Similarly, larvae fed with the 100FM+LF1.5 diet also showed reduced accumulated mortality compared to the 100FM diet (P < 0.01). The mortality of larvae fed with the 50SBM+LF1.5 or 100FM+LF1.5 diets was comparable, and no significant differences existed between these groups.
Figure 4.
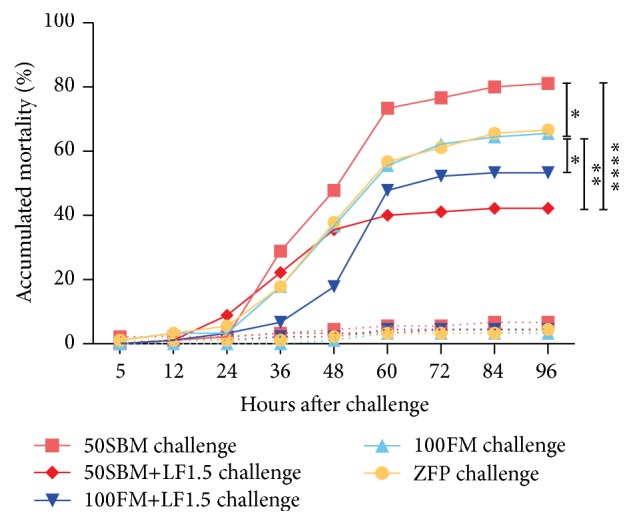
Effect of lactoferrin on fish mortality after pathogen challenge. Tg(BACmpo:GFP)i114 larvae were challenged with Edwardsiella tarda after four days of feeding with different diets at 9 dpf (ZFP, 100FM, 50SBM, 50SBM+LF1.5, and 100FM+LF1.5). Mortality was monitored immediately after the challenge and every 12 h over four days until 13 dpf. Statistical analysis was performed using survival curve analysis with the log-rank test against the 100FM and 50SBM diets. ∗ P < 0.05; ∗∗ P < 0.01; ∗∗∗∗ P < 0.0001. Continuous lines represent challenged larvae while dotted lines represent control larvae.
4. Discussion
This is the first study to provide evidence that orally administered LF protects the fish intestine from the inflammatory effect induced by soybean meal. This observation widens the opportunity for using this plant protein in the fish nutrition industry.
The obtained results suggest that LF reduces neutrophil recruitment to the intestine and that this effect is dose-dependent. In line with this attenuated neutrophil migration, there was a downregulation in the transcription of mmp9, an enzyme that degrades the basement membrane to facilitate cell migration and infiltration to affected tissue [47]. Moreover, neutrophils are the major contributors of MMP-9 during intestinal inflammation in mammals [48, 49].
The effect of LF as an intestinal protector has been previously reported in rodents. Dial et al. [33] found that LF protects the intestine of rats and mice from the effects of nonsteroidal anti-inflammatory drugs. The authors speculated that LF could modulate neutrophil function, attenuating neutrophil migration to the intestine. However, the mechanism by which LF inhibits leukocyte migration is still unknown. There is evidence that this protein reduces the integrin-dependent adherence of eosinophils [50] and inhibits the expression of adhesion molecules, such as E-selectin and ICAM-1, in the vascular endothelium [51]. Considering that the presence of these adhesion molecules on the surface of the endothelium is a key step during leukocyte recruitment to affected sites, the absence or low levels of adhesion molecules could possibly explain the effect observed in the present study on neutrophil migration. On the other hand, LF can also regulate cytokine production in mice. Therefore, another possible scenario is that, by modulating cytokine levels, LF decreases neutrophil migration to the intestine. Data supporting this hypothesis indicate that LF can suppress the proinflammatory cytokines tumor necrosis factor alfa [52] and IL-1 [53], in addition to promoting the anti-inflammatory cytokine IL-18 in the gut [54]. Moreover, it is possible that the decreased neutrophil migration triggered by LF is partly a consequence of the inhibition of different proinflammatory cytokines.
Regarding the effect of intestinal inflammation on survival rate, the challenge assay with E. tarda results clearly indicated that an inflammatory process significantly affects the immune response against pathogens. The present results showed that the survival rate of larvae fed with soybean meal was almost half of that observed in larvae fed with fishmeal (19% and 35%, resp.). Similar results have been reported in adult zebrafish specimens. Specifically, oxazolone-induced intestinal inflammation resulted in treated zebrafish being more susceptible to E. tarda infection than healthy zebrafish [55]. Therefore, it is not the soybean meal per se that affects the immune response against pathogens, but rather intestinal inflammation. Moreover, in a mouse colitis model with concomitant intestinal inflammation, infection with Salmonella enterica was facilitated [56].
The present results further suggest that the effect of LF on the intestine is not limited to preventing intestinal inflammation but that LF can also influence larvae performance against pathogen. This was evidenced in the challenge assay when comparing the survival rates of larvae fed diets with or without LF. In the case of larvae fed with fishmeal that did not have intestinal inflammation, the survival rate increased from 35% to 47% when LF was incorporated. Interestingly, transcriptional analyses of muc2.2 and β-def-1 in larvae fed with LF were indistinguishable from control larvae. These results indicate that LF did not improve the mucosal barrier function of the host immune response. Defensins, including β-def-1, are crucial antimicrobial peptides that protect the intestine against infection as a result of antibacterial and immunomodulatory properties [55, 57, 58]. Likewise, mucins such as muc2.2 form part of the mucosal defense system present in the mucous gel layer that covers the luminal surface of the gastrointestinal tract. This viscoelastic protective barrier forms the first line of defense to the external environment [59]. Additionally, in a chemically induced zebrafish model of intestinal inflammation, the mucus layer increases [60].
Therefore, the presently observed increase in survival rate could be the result of LF directly acting against the invading bacteria. Indeed, LF has an antimicrobial effect against a broad spectrum of bacteria, mainly Gram-negative bacteria present in the gut [61]. By inhibiting the overgrowth and/or colonization of bacterial pathogens, LF could promote a healthy condition. This inhibitory action of LF could be achieved by the following three events: (1) chelating ferric iron necessary for bacterial growth; (2) destabilizing microbial membranes; or (3) modifying microbial adherence to host cells independent of the microbe's iron-binding properties [62]. Obviously, the antimicrobial activities of LF are not absolute and permit the growth of commensal bacteria [63].
5. Conclusions
The present study provides novel and relevant data regarding the effects of LF on fish physiology, especially in relation to intestinal inflammation. In light of the obtained results, the supplementation of fish diets with LF appears to be a plausible alternative to cope, at least in part, with two major problems currently affecting the aquaculture industry-soybean meal-triggered enteritis and pathogenic infections.
Supplementary Material
The supplementary Figure 1 shows the Sudan Black B staining in order to label leukocytes in the intestine of 9dpf larvae fed with the different diets. Total concordance between these result and those obtained from the immunohistochemistry analysis were found.
Acknowledgments
This work was supported by the Fondo Nacional de Desarrollo Cientifico y Tecnologico 3130664 awarded to Pilar E. Ulloa and Fondo Nacional de Desarrollo Cientifico y Tecnologico 1140297, Direccion de Investigacion UNAB DI-483-14/R, and Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias 15110027 awarded to Carmen G. Feijóo. The authors also thank Alex Oporto and Alex Cabrera for manufacturing the experimental diets.
Competing Interests
The authors declare no competing interests in relation to the research and authorship.
References
- 1.Refstie S., Baeverfjord G., Seim R. R., Elvebø O. Effects of dietary yeast cell wall β-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture. 2010;305(1–4):109–116. doi: 10.1016/j.aquaculture.2010.04.005. [DOI] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations. El Estado Mundial de la Pesca y la Acuicultura. Rome, Italy: FAO; 2012. [Google Scholar]
- 3.Naylor R. L., Hardy R. W., Bureau D. P., et al. Feeding aquaculture in an era of finite resources. Proceedings of the National Academy of Sciences of the United States. 2009;106(36):15103–15110. doi: 10.1073/pnas.0905235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundheim H., Aksnes A., Hope B. Growth, feed efficiency and digestibility in salmon (Salmo salar L.) fed different dietary proportions of vegetable protein sources in combination with two fish meal qualities. Aquaculture. 2004;237(1–4):315–331. doi: 10.1016/j.aquaculture.2004.03.011. [DOI] [Google Scholar]
- 5.Médale F., Boujard T., Vallée F., et al. Voluntary feed intake, nitrogen and phosphorus losses in rainbow trout (Oncorhynchus mykiss) fed increasing dietary levels of soy protein concentrate. Aquatic Living Resources. 1998;11(4):239–246. doi: 10.1016/s0990-7440(98)89006-2. [DOI] [Google Scholar]
- 6.Pongmaneerat J., Watanabe T., Takeuchi T., Satoh S. Use of different protein meals as partial or total substitution for fish meal in carp diets. Nippon Suisan Gakkaishi. 1993;59(7):1249–1257. doi: 10.2331/suisan.59.1249. [DOI] [Google Scholar]
- 7.Fontaínhas-Fernandes A., Gomes E., Reis-Henriques M. A., Coimbra J. Replacement of fish meal by plant proteins in the diet of Nile tilapia: digestibility and growth performance. Aquaculture International. 1999;7(1):57–67. doi: 10.1023/a:1009296818443. [DOI] [Google Scholar]
- 8.Gómez-Requeni P., Mingarro M., Calduch-Giner J. A., et al. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata) Aquaculture. 2004;232(1–4):493–510. doi: 10.1016/s0044-8486(03)00532-5. [DOI] [Google Scholar]
- 9.Hedrera M. I., Galdames J. A., Jimenez-Reyes M. F., et al. Soybean meal induces intestinal inflammation in zebrafish larvae. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069983.e69983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulloa P. E., Peña A. A., Lizama C. D., et al. Growth response and expression of muscle growth-related candidate genes in adult zebrafish fed plant and fishmeal protein-based diets. Zebrafish. 2013;10(1):99–109. doi: 10.1089/zeb.2012.0823. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen D., Urán P., Arnous A., Koppe W., Frøkiær H. Saponin-containing subfractions of soybean molasses induce enteritis in the distal intestine of Atlantic salmon. Journal of Agricultural and Food Chemistry. 2007;55(6):2261–2267. doi: 10.1021/jf0626967. [DOI] [PubMed] [Google Scholar]
- 12.Urán P. A., Gonçalves A. A., Taverne-Thiele J. J., Schrama J. W., Verreth J. A. J., Rombout J. H. W. M. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.) Fish and Shellfish Immunology. 2008;25(6):751–760. doi: 10.1016/j.fsi.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Merrifield D. L., Dimitroglou A., Bradley G., Baker R. T. M., Davies S. J. Soybean meal alters autochthonous microbial populations, microvilli morphology and compromises intestinal enterocyte integrity of rainbow trout, Oncorhynchus mykiss (Walbaum) Journal of Fish Diseases. 2009;32(9):755–766. doi: 10.1111/j.1365-2761.2009.01052.x. [DOI] [PubMed] [Google Scholar]
- 14.Montero D., Mathlouthi F., Tort L., et al. Replacement of dietary fish oil by vegetable oils affects humoral immunity and expression of pro-inflammatory cytokines genes in gilthead sea bream Sparus aurata . Fish and Shellfish Immunology. 2010;29(6):1073–1081. doi: 10.1016/j.fsi.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Staykov Y., Spring P., Denev S., Sweetman J. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss) Aquaculture International. 2007;15(2):153–161. doi: 10.1007/s10499-007-9096-z. [DOI] [Google Scholar]
- 16.Piaget N., Vega A., Toledo A. S. Effect of the application of β-glucans and mannan-oligosaccharides (βG MOS) in an intensive larval rearing system of Paralichthys adspersus (Paralichthydae) Investigaciones Marinas. 2007;35(2):35–43. [Google Scholar]
- 17.Dimitroglou A., Merrifield D. L., Spring P., Sweetman J., Moate R., Davies S. J. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata) Aquaculture. 2010;300(1–4):182–188. doi: 10.1016/j.aquaculture.2010.01.015. [DOI] [Google Scholar]
- 18.Tahmasebi-Kohyani A., Keyvanshokooh S., Nematollahi A., Mahmoudi N., Pasha-Zanoosi H. Effects of dietary nucleotides supplementation on rainbow trout (Oncorhynchus mykiss) performance and acute stress response. Fish Physiology and Biochemistry. 2012;38(2):431–440. doi: 10.1007/s10695-011-9524-x. [DOI] [PubMed] [Google Scholar]
- 19.Esteban M. A., Rodríguez A., Cuesta A., Meseguer J. Effects of lactoferrin on non-specific immune responses of gilthead seabream (Sparus auratus L.) Fish and Shellfish Immunology. 2005;18(2):109–124. doi: 10.1016/j.fsi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Larkins N. Potential implications of lactoferrin as a therapeutic agent. American Journal of Veterinary Research. 2005;66(4):739–742. doi: 10.2460/ajvr.2005.66.739. [DOI] [PubMed] [Google Scholar]
- 21.Legrand D., Mazurier J. A critical review of the roles of host lactoferrin in immunity. BioMetals. 2010;23(3):365–376. doi: 10.1007/s10534-010-9297-1. [DOI] [PubMed] [Google Scholar]
- 22.Elfinger M., Maucksch C., Rudolph C. Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells. Biomaterials. 2007;28(23):3448–3455. doi: 10.1016/j.biomaterials.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y. A., Lopez V., Lönnerdal B. Mammalian lactoferrin receptors: structure and function. Cellular and Molecular Life Sciences. 2005;62(22):2560–2575. doi: 10.1007/s00018-005-5371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legrand D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochemistry and Cell Biology. 2012;90(3):252–268. doi: 10.1139/o11-056. [DOI] [PubMed] [Google Scholar]
- 25.Sakai M., Otubo T., Atsuta S., et al. Enhancement of resistance to bacterial infection in rainbow trout Oncorhynchus mykiss (Walbaum) by oral administration of bovine lactoferrin. Journal of Fish Diseases. 1993;16(3):239–247. doi: 10.1111/j.1365-2761.1993.tb01253.x. [DOI] [Google Scholar]
- 26.Ward P. P., Uribe-Luna S., Conneely O. M. Lactoferrin and host defense. Biochemistry and Cell Biology. 2002;80(1):95–102. doi: 10.1139/o01-214. [DOI] [PubMed] [Google Scholar]
- 27.Kakuta I., Kurokura H. Defensive effect of orally administered bovine lactoferrin against Cryptocaryon irritans infection of Red Sea Bream. Fish Pathology. 1995;4:289–290. [Google Scholar]
- 28.Kumari J., Swain T., Sahoo P. K. Dietary bovine lactoferrin induces changes in immunity level and disease resistance in Asian catfish, Clarias batrachus . Veterinary Immunology and Immunopathology. 2003;94:1–9. doi: 10.1016/s0165-2427(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 29.Lygren B., Sveier H., Hjeltness B., Waagbø R. Examination of the immunomodulatory properties and the effect on disease resistance of dietary bovine lactoferrin and vitamin C fed to atlantic salmon (Salmo salar) for a short-term period. Fish and Shellfish Immunology. 1999;9(2):95–107. doi: 10.1006/fsim.1998.0179. [DOI] [Google Scholar]
- 30.Yamauchi K., Wakabayashi H., Shin K., Takase M. Bovine lactoferrin: benefits and mechanism of action against infections. Biochemistry and Cell Biology. 2006;84(3):291–296. doi: 10.1139/o06-054. [DOI] [PubMed] [Google Scholar]
- 31.Cooper C., Nonnecke E., Lönnerdal B., Murray J. The lactoferrin receptor may mediate the reduction of eosinophils in the duodenum of pigs consuming milk containing recombinant human lactoferrin. BioMetals. 2014;27(5):1031–1038. doi: 10.1007/s10534-014-9778-8. [DOI] [PubMed] [Google Scholar]
- 32.Bournazou I., Mackenzie K. J., Duffin R., Rossi A. G., Gregory C. D. Inhibition of eosinophil migration by lactoferrin. Immunology and Cell Biology. 2010;88(2):220–223. doi: 10.1038/icb.2009.86. [DOI] [PubMed] [Google Scholar]
- 33.Dial E. J., Dohrman A. J., Romero J. J., Lichtenberger L. M. Recombinant human lactoferrin prevents NSAID-induced intestinal bleeding in rodents. Journal of Pharmacy and Pharmacology. 2005;57(1):93–99. doi: 10.1211/0022357055191. [DOI] [PubMed] [Google Scholar]
- 34.Ulloa P. E., Medrano J. F., Feijo C. G. Zebrafish as animal model for aquaculture nutrition research. Frontiers in Genetics. 2014;5, article 313 doi: 10.3389/fgene.2014.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renshaw S. A., Loynes C. A., Trushell D. M., et al. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108(13):3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- 36.National Research Council. Nutritional Requirements of Fish. Washington, DC, USA: National Academies Press; 1993. [Google Scholar]
- 37.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of the Zebrafish (Danio rerio) 5th. Eugene, Ore, USA: University of Oregon; 1994. [Google Scholar]
- 38.Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 39.Brand M., Granato M., Nusslein-Volhard C. Keeping and raising zebrafish. In: Nüsslein-Volhard C., Dahm R., editors. Zebrafish: A Practical Approach. New York, NY, USA: Oxford University Press; 2002. pp. 7–37. [Google Scholar]
- 40.Welker T. L., Lim Ch., Yildirim-Aksoy M., Klesius P. H. Growth, immune function, and disease and stress resistance of juvenile Nile tilapia (Oreochromis niloticus) fed graded levels of bovine lactoferrin. Aquaculture. 2007;262(1):156–162. doi: 10.1016/j.aquaculture.2006.09.036. [DOI] [Google Scholar]
- 41.Feijoo C. G., Sarrazin A., Allende M. L., et al. Cysteine-serinerich nuclear protein 1, Axud1/Csrnp1, is essential for cephalic neural progenitor proliferation and survival in zebrafish. Developmental Dynamics. 2009;238:2034–2043. doi: 10.1002/dvdy.22006. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9, article e45) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oehlers S. H., Flores M. V., Okuda K. S., Hall C. J., Crosier K. E., Crosier P. S. A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Developmental Dynamics. 2011;240(1):288–298. doi: 10.1002/dvdy.22519. [DOI] [PubMed] [Google Scholar]
- 44.Jowett T., Lettice L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends in Genetics. 1994;10(3):73–74. doi: 10.1016/0168-9525(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 45.Harvie E. A., Green J. M., Neely M. N., Huttenlocher A. Innate immune response to Streptococcus iniae infection in zebrafish larvae. Infection and Immunity. 2013;81(1):110–121. doi: 10.1128/iai.00642-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Soest J. J., Stockhammer O. W., Ordas A., Bloemberg G. V., Spaink H. P., Meijer A. H. Comparison of static immersion and intravenous injection systems for exposure of zebrafish embryos to the natural pathogen Edwardsiella tarda . BMC Immunology. 2011;12, article 58 doi: 10.1186/1471-2172-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Sullivan S., Gilmer J. F., Medina C. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators of Inflammation. 2015;2015:19. doi: 10.1155/2015/964131.964131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koelink P. J., Overbeek S. A., Braber S., et al. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut. 2014;63(4):578–587. doi: 10.1136/gutjnl-2012-303252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandooren J., Van den Steen P. E., Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Critical Reviews in Biochemistry and Molecular Biology. 2013;48(3):222–272. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 50.Curran C. S., Bertics P. J. Lactoferrin regulates an axis involving CD11b and CD49d integrins and the chemokines MIP-1α and MCP-1 in GM-CSF-treated human primary eosinophils. Journal of Interferon and Cytokine Research. 2012;32(10):450–461. doi: 10.1089/jir.2011.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baveye S., Elass E., Fernig D. G., Blanquart C., Mazurier J., Legrand D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infection and Immunity. 2000;68(12):6519–6525. doi: 10.1128/iai.68.12.6519-6525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machnicki M., Zimecki M., Zagulski T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. International Journal of Experimental Pathology. 1993;5:433–439. [PMC free article] [PubMed] [Google Scholar]
- 53.Zucali J. R., Broxmeyer H. E., Levy D., Morse C. Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interleukin-1. Blood. 1989;74(5):1531–1536. [PubMed] [Google Scholar]
- 54.Kuhara T., Iigo M., Itoh T., et al. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutrition and Cancer. 2000;38(2):192–199. doi: 10.1207/s15327914nc382_8. [DOI] [PubMed] [Google Scholar]
- 55.Liu X., Chang X., Wu H., Xiao J., Gao Y., Zhang Y. Role of intestinal inflammation in predisposition of Edwardsiella tarda infection in zebrafish (Danio rerio) Fish and Shellfish Immunology. 2014;41(2):271–278. doi: 10.1016/j.fsi.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Stecher B., Robbiani R., Walker A. W., et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biology. 2007;5(10):2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cederlund A., Gudmundsson G. H., Agerberth B. Antimicrobial peptides important in innate immunity. FEBS Journal. 2011;278(20):3942–3951. doi: 10.1111/j.1742-4658.2011.08302.x. [DOI] [PubMed] [Google Scholar]
- 58.García-Valtanen P., Martinez-Lopez A., Ortega-Villaizan M., Perez L., Coll J. M., Estepa A. In addition to its antiviral and immunomodulatory properties, the zebrafish β-defensin 2 (zfBD2) is a potent viral DNA vaccine molecular adjuvant. Antiviral Research. 2014;101(1):136–147. doi: 10.1016/j.antiviral.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Corfield A. P., Myerscough N., Longman R., Sylvester P., Arul S., Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47(4):589–594. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oehlers S. H., Flores M. V., Hall C. J., Crosier K. E., Crosier P. S. Retinoic acid suppresses intestinal mucus production and exacerbates experimental enterocolitis. Disease Models & Mechanisms. 2012;5(4):457–467. doi: 10.1242/dmm.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.García-Montoya I. A., Cendón T. S., Arévalo-Gallegos S., Rascón-Cruz Q. Lactoferrin a multiple bioactive protein: an overview. Biochimica et Biophysica Acta—General Subjects. 2012;1820(3):226–236. doi: 10.1016/j.bbagen.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valenti P., Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cellular and Molecular Life Sciences. 2005;62(22):2576–2587. doi: 10.1007/s00018-005-5372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jenssen H., Hancock R. E. W. Antimicrobial properties of lactoferrin. Biochimie. 2009;91(1):19–29. doi: 10.1016/j.biochi.2008.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary Figure 1 shows the Sudan Black B staining in order to label leukocytes in the intestine of 9dpf larvae fed with the different diets. Total concordance between these result and those obtained from the immunohistochemistry analysis were found.