Abstract
Long non-coding (lnc) RNAs can regulate gene expression and protein functions. However, the proportion of lncRNAs with biological activities among the thousands expressed in mammalian cells is controversial. We studied Lockd (LncRNA downstream of Cdkn1b), a 434 nt polyadenylated lncRNA originating 4 kilobases (kb) 3′ to the Cdkn1b gene. Deletion of the 25 kb Lockd locus reduced Cdkn1b transcription by approximately 70% in an erythroid cell line. In contrast, homozygous insertion of a polyadenylation cassette 80 bp downstream of the Lockd transcription start site reduced the entire lncRNA transcript level by > 90%, with no effect on Cdkn1b transcription. The Lockd promoter contains a DNase hypersensitive site, binds numerous transcription factors, and physically associates with the Cdkn1b promoter in chromosomal conformation capture studies. Thus, the Lockd gene positively regulates Cdkn1b transcription through an enhancer-like cis element, while the lncRNA itself is dispensable, which may be the case for other lncRNAs.
eTOC blurb
Whether loci encoding lncRNAs function via their lncRNA transcripts or DNA elements is often unclear. Paralkar et al. provide a model for dissecting these contributions and show that the 5′ region of the Lockd lncRNA gene contains an enhancer for the neighboring Cdkn1b gene, while Lockd lncRNA is dispensable for Cdkn1b expression.
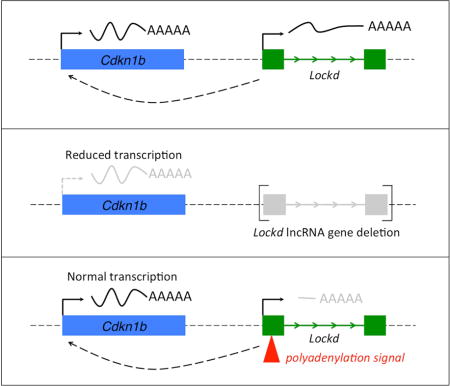
Introduction
Long noncoding RNAs (lncRNAs) are transcripts more than 200 nucleotides in length that do not code for a protein. Dozens of lncRNAs are reported to regulate normal and pathological tissue development through multiple mechanisms(Fatica and Bozzoni, 2014). However, virtually all mammalian cell types express thousands of uncharacterized lncRNAs, and their overall biological impact is debated. On one hand, the limited evolutionary conservation of most lncRNAs raises questions about their biological activities. But on the other hand, some lncRNAs may act through short conserved regions that are not detected by standard sequence alignment algorithms, or through conserved folding structures that are independent of nucleotide sequence similarities.
Gene ablation studies are a standard approach to assess the functions of protein coding and lncRNA genes in vivo. Deletion of entire lncRNA loci (Hotair(Li et al., 2013), Firre(Hacisuleyman et al., 2014)), lncRNA promoters (LincRNA-p21(Dimitrova et al., 2014)), and intron-exon regions (Mdgt, Peril and others(Sauvageau et al., 2013)) produced phenotypes in mice or cell lines. However, lncRNA transcripts often arise from DNA segments that harbor known or candidate regulatory regions for protein coding genes(Bassett et al., 2014). Thus, phenotypes caused by ablation of lncRNA genes could result from disruption of DNA elements therein. In order to prove that a lncRNA is functional, it is necessary to reduce the transcript without eliminating its underlying genomic sequences.
Results and Discussion
Previously, we identified Lockd (AK012387) as one of hundreds of mouse erythroblast lncRNAs(Paralkar et al., 2014). Lockd is a 434 nt polyadenylated lncRNA encoded by two exons (Figure S1). Although Lockd is most abundant in erythroid cells, it is expressed in many other tissues as well (Figure 1A). Consistent with its widespread expression, the 5′ region of the Lockd gene binds generally expressed transcription factors (TFs) and exhibits typical marks of an active promoter in multiple cell types (Figures 1B and S2A–S2C). These active marks include DNase hypersensitivity, prominent H3K4me3 histone signal, low H3K4me1 signal and RNA polymerase II occupancy. The Lockd RNA has no evidence of coding potential(Paralkar et al., 2014), as indicated by its absence in protein databases, a low PhyloCSF score(Lin et al., 2011) reflecting lack of codon conservation during evolution, and no open reading frames longer than 300 nucleotides.
Figure 1. The Lockd genomic locus positively regulates Cdkn1b expression.
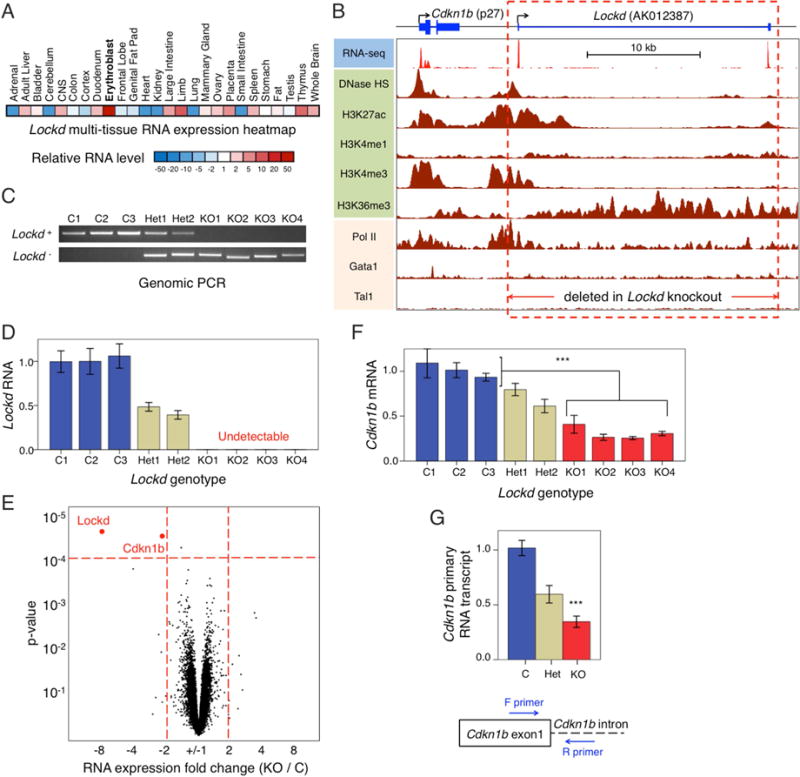
(A) Heatmap showing relative expression levels of Lockd lncRNA in different mouse tissues according to mouse ENCODE RNA-seq datasets. (B) UCSC genome browser image of Lockd and its upstream gene, Cdkn1b. Tracks for RNA-seq studies, DNase hypersensitivity, histone modifications, RNA Polymerase II binding, and TF binding are indicated. The RNA-seq track is from primary mouse fetal liver erythroblasts(Paralkar et al., 2014). All other tracks are from the mouse erythroid cell line G1E and its derivative G1E-ER4(Wu et al., 2011). The red dotted rectangle indicates the 25 kb region deleted using CRISPR/Cas9-guided DNA cleavage. (C) Identification of Lockd intact (Lockd+) or deleted (Lockd−) alleles by PCR (see also Figure S3A). C, Control (homozygous non-deleted); Het, heterozygous deleted; KO, homozygous deleted. (D) Expression of Lockd RNA in C, Het and KO clones, measured by quantitative RT-PCR and normalized to Gapdh and Actb mRNA levels. Data are represented as mean +/− SEM. (E) Microarray transcriptome analysis comparing KO (n=4) and C (n=3) clones. The volcano plot shows fold-change in expression and its significance, with each dot representing an individual gene. Only 2 genes (Lockd and Cdkn1b) passed FDR < 5% cutoff (p-value < 10−4, horizontal dotted red line) with at least 2-fold change (vertical dotted red lines). (F) Expression of Cdkn1b mRNA in Lockd C, Het and KO clones, as measured by quantitative RT-PCR and normalized to Gapdh and Actb mRNA levels. Data are represented as mean +/− SEM. (G) Expression of Cdkn1b primary transcript (pre-mRNA) as measured by quantitative RT-PCR and normalized to Gapdh and Actb mRNA levels. Data are represented as mean +/− SEM.
Attempts to reduce Lockd transcripts by RNA interference were unsuccessful (Figure S2D). Therefore, we used CRISPR-Cas9-mediated genome editing in the mouse erythroid cell line G1E to generate double-strand DNA breaks 1 kb upstream and downstream of the 5′ and 3′ ends (respectively) of the Lockd gene and screened for clones in which the gene locus was deleted by non-homologous end joining of the two DNA breaks (Figures 1B, 1C and S3A). From the same experiment, we also retained 3 clones without Lockd deletion, designated “control” (C) (Figure 1C). Lockd RNA was reduced by about 50% in the heterozygous deleted clones (Het) and was undetectable in the homozygous knockout (KO) clones (Figure 1D).
We performed comparative transcriptome analysis by microarray to determine how excision of the Lockd locus affects gene expression. The most significantly altered transcripts were Lockd and Cdkn1b; these were the only two transcripts whose levels changed more than 2 fold in the KO clones compared to controls at an FDR threshold of < 5% (p-value < 10−4) (Figure 1E and Table S1). In agreement with the transcriptome data, quantitative RT-PCR showed that Cdkn1b mRNA was reduced by approximately 35% and 70% in Het and KO clones, respectively (Figure 1F). The Cdkn1b primary transcript was decreased similarly, indicating that deletion of Lockd reduces Cdkn1b transcription (Figure 1G). The Cdkn1b gene resides 4 kb upstream of Lockd (Figure 1B) and encodes p27, a ubiquitously expressed protein that regulates cell cycle progression(Sherr and Roberts, 1995).
To determine whether normal Cdkn1b transcription requires the Lockd lncRNA transcript itself, we used CRISPR/Cas9-mediated homologous recombination to insert a bovine growth hormone (BGH) polyadenylation (polyA) cassette 80 bp downstream of the Lockd gene transcription start site in G1E cells (Figure 2A). We used genomic PCR and Southern blotting (Figure S3B) to identify four knock-in (KI) clonal lines with homozygous insertions of the polyA cassette into exon 1 of Lockd. From the same experiment, we also expanded 2 wild-type (WT) clones with no insertion or targeted mutation. Insertion of the polyA cassette should cause early termination of the nascent lncRNA transcript while preserving cis elements within the genomic locus. Accordingly, quantitative RT-PCR analysis (Figure 2B) demonstrated that mature Lockd RNA (‘PCR 1’ product) was strongly reduced in all four KI lines (range 6%–13% of WT, mean 10%); similar results were obtained for ‘PCR 3’ and ‘PCR 4’ products. The predicted transcript encoding the first 80 bp of Lockd lncRNA preceding the polyA insertion was also reduced in the KI clones (‘PCR 2’ product), either due to interference with transcription or instability of the truncated RNA. Importantly, Cdkn1b mRNA levels were normal in all four KI clones (Figure 2B, bottom panel), despite strongly reduced Lockd lncRNA. All four KI clones showed low-level residual expression of normal Lockd lncRNA, presumably due to read through of the polyA signal by RNA polymerase (‘PCR 1’, ‘PCR 3’ and ‘PCR 4’ products), but there was no dose relationship between the residual Lockd level and the Cdkn1b mRNA level from clone to clone: KI-B, the clone with reduction of Lockd mRNA to ~ 5%, showed Cdkn1b expression equivalent to WT. These findings are not compatible with a model in which Lockd lncRNA regulates Cdkn1b transcription. More likely, the lncRNA is dispensable for Cdkn1b expression, which may instead either be directly regulated by cis elements such as enhancer(s) within the Lockd locus, or indirectly through changes in local genomic structure as a result of Lockd deletion.
Figure 2. The Lockd lncRNA transcript is dispensable for Cdkn1b expression.
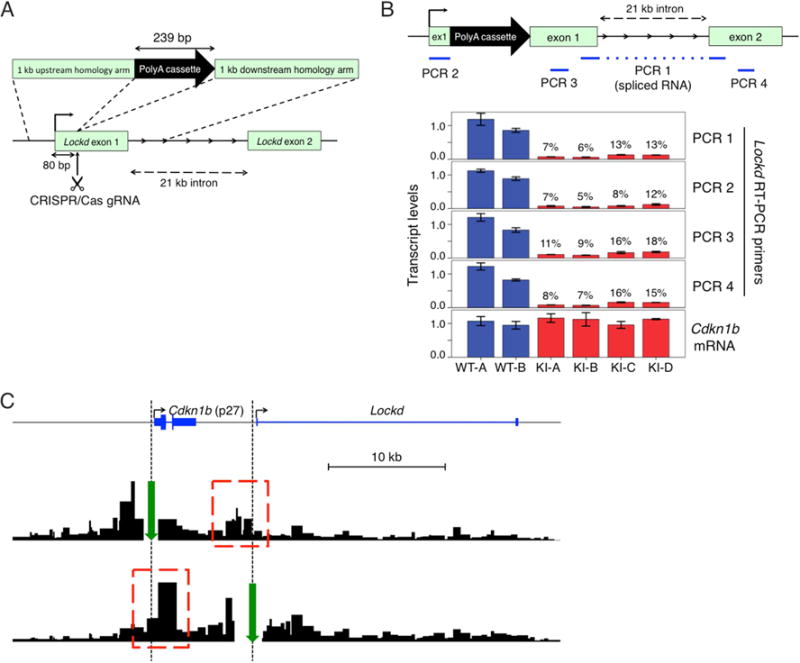
(A) Strategy for premature termination of the Lockd lncRNA transcript. CRISPR/Cas9-mediated homologous recombination was used to insert a 239 bp bovine growth hormone (BGH) polyadenylation (PolyA) cassette into exon 1 of Lockd, 80 bp downstream of the transcription start site (see also Figure S3B). (B) Diagram of the modified Lockd locus is shown on top, with the PCR products used to quantify expression of Lockd lncRNA indicated in blue. The bargraph shows expression of various regions of Lockd lncRNA and of Cdkn1b mRNA, normalized to Gapdh and Actb mRNA levels. Data are represented as mean +/− SEM. (C) Next generation Capture-C tracks showing contacts of anchor regions (green arrows) with adjacent chromosomal regions (red dotted boxes) above background signal.
Enhancers regulate gene transcription through chromosomal contacts with their target gene promoters(Krivega and Dean, 2012). We used the Next-Generation (NG) Capture-C method(Davies et al., 2015) to investigate potential interactions between the Lockd and Cdkn1b genes. We generated a 3C (Chromosomal Conformation Capture) library containing ligated interacting DNA fragments from G1E cells, and used biotinylated oligonucleotide probes to capture segments of DNA that associate with the Cdkn1b or Lockd promoter regions. Deep sequencing of these segments showed reciprocal interaction between these regions (i.e. “looping”), indicating their physical proximity in live cells (Figure 2C). This finding, combined with the effects of Lockd gene deletion, indicate that cis element(s) within the 5′ region of the Lockd locus promote Cdkn1b transcription.
Our findings demonstrate that in order to understand the functions of a lncRNA, its effects must be uncoupled from those of its underlying DNA locus(Bassett et al., 2014). This strategy has demonstrated distinct models for the functions of different lncRNA genes (Figure 3). The Xist genomic locus loops to multiple regions of the X chromosome, potentially promoting spread of the lncRNA(Engreitz et al., 2013). The Xist transcript is required for X-chromosomal inactivation, as evidenced by the ability of antisense oligonucleotides to displace the lncRNA from the X-chromosome and reactivate underlying genes(Beletskii et al., 2001; Sarma et al., 2010). Insertion of PolyA signals across the Airn lncRNA gene showed that its transcription across the promoter of the neighboring antisense Igf2r gene represses expression by interfering with RNA polymerase II recruitment, independent of any intrinsic lncRNA function (Latos et al., 2012). Manipulation of the Haunt lncRNA gene showed that it contains potential cis elements that induce neighboring HoxA genes, while the lncRNA transcript itself appears to repress HoxA expression(Yin et al., 2015). Here we show that the lncRNA Lockd is likely transcribed from an enhancer for Cdkn1b, and that the lncRNA itself is not required for augmenting Cdkn1b transcription. Thus, lncRNA genes act through multiple mechanisms involving the lncRNA transcript, the underlying genomic DNA, transcriptional interference across a nearby locus, or a combination thereof.
Figure 3. LncRNA genes regulate transcription though multiple mechanisms.
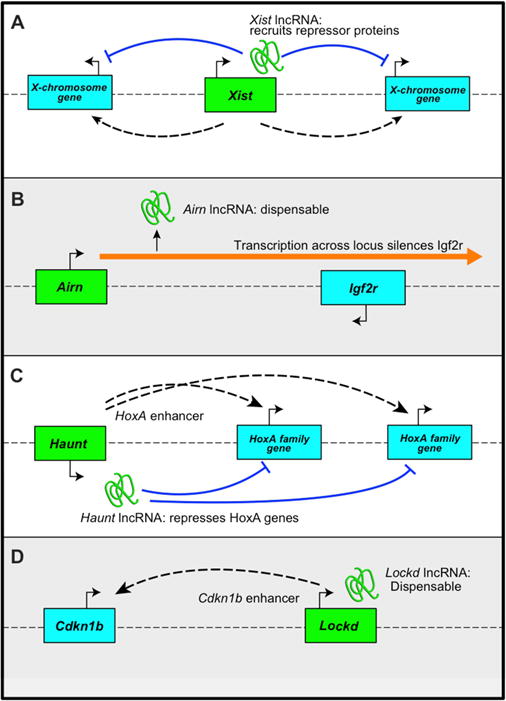
(A) The Xist gene interacts physically with multiple regions of the X chromosome, enhancing spread of Xist lncRNA, which recruits repressor proteins that promote X-inactivation. (B) Airn gene transcription interferes with expression of Igf2r on the antisense strand, while the Airn lncRNA itself is dispensable for Igf2r repression. (C) Haunt genomic element(s) act as enhancer(s) for HoxA family genes, while the Haunt lncRNA represses those genes. (D) The Lockd locus acts as an enhancer for Cdkn1b to promote its transcription. In contrast, the Lockd lncRNA is dispensable for Cdkn1b expression. The dotted lines indicate physical contact or “looping” between lncRNA gene loci and other genes.
Our interest in Lockd initially arose from its high level expression in erythroid cells (Figure 1A). However, Lockd lncRNA is expressed in many cell types, and its locus exhibits open chromatin in multiple tissues, as reflected by DNase hypersensitivity (Figures S2A–S2C). Moreover, the 5′ region of Lockd that contacts Cdkn1b does not bind Gata1 or Tal1, which are usually associated with erythroid-specific enhancers. Rather, it binds more widely distributed TFs including Ets1, Myc, and JunD. Thus, the Lockd enhancer likely regulates Cdkn1b transcription in multiple tissues. Of note, the human genomic region orthologous to the mouse Lockd promoter shows DNase hypersensitivity and binds a similar set of TFs in human K562 erythroleukemia cells, indicating the presence of a functional cis element (Figure S4). RNA-seq studies on the same cells show only low-level transcription of LOCKD exon 1, but no obvious full-length lncRNA. Thus, a human LOCKD-associated enhancer may regulate CDKN1B activity independent of a lncRNA being produced.
In summary, our findings indicate that the Cdkn1b gene is positively regulated by a cis element at the promoter of the adjacent Lockd locus, while the transcribed Lockd lncRNA is dispensable for this function. While the Lockd transcript may have activities not identified by our study, it is also possible that this lncRNA represents an inert by-product arising from its functional cis element. Regardless, our study supports the general paradigm that simply deleting a lncRNA locus is not sufficient for understanding its function; rather, it is necessary to distinguish the activities of the RNA transcript from those of the underlying DNA.
Experimental Procedures
Generation of CRISPR-Cas9 deletion and insertion cell lines
All experiments were performed in G1E cells, cultured as described previously(Weiss et al., 1997; Tsang et al., 1997). Guide RNA (gRNA) sequences for CRISPR were designed using the http://crispr.mit.edu/website and are listed in Table S2. Oligonucleotides with gRNA sequences were cloned into the pX330-U6-Chimeric_BB-CBh-hSpCas9 plasmid(Cong et al., 2013) (a gift from Feng Zhang and Peter Klein; Addgene plasmid # 42230), or the pKLV-U6gRNA(BbsI)-PGKpuro2ABFP plasmid(Koike-Yusa et al., 2014) (a gift from Kosuke Yusa; Addgene plasmid # 50946). For homologous recombination (Figures 2A and S3B), the Bovine Growth Hormone polyadenylation cassette was amplified by PCR from the pKLV-U6gRNA(BbsI)-PGKpuro2ABFP plasmid and cloned along with flanking homology arms using the In-Fusion cloning kit. For all CRISPR-Cas9 mediated genome editing experiments, G1E cells were electroporated in bulk with the appropriate plasmids using an Amaxa electroporator. Fluorescent cells were sorted 24 hours later into 96-well plates at one cell per well. Clonal lines were genotyped using PCR, Southern blotting and Sanger sequencing (Figures 1B, 1C, 2A, S3, and Table S2). To avoid any differences between experiments that could be attributable to clonal bias, identical techniques of single-cell-sorting, clone expansion, and screening were used to pick the Lockd deletion and Lockd truncation clones.
Gene expression
RNA was extracted from cultured cells using the RNeasy-Mini kit (Qiagen), cDNA generated using the iScript kit (Bio-Rad), and RT-PCR done using the SYBR Green PCR Master Mix (Thermo-Fisher). An average of Gapdh and Actb expression was used as RT-PCR control. All RT-PCR primers are listed in Table S2. Microarray profiling was done on the Affymetrix Mouse Gene 2.0ST chip. Raw array data was preprocessed and normalized by RMA algorithm(Bolstad et al., 2003) with “rma-gene-full” option. Significance of differential expression was estimated using SAM algorithm(Zhang, 2007) and significance threshold was set at FDR<5%. The volcano plot was created using fold change and expression values for probes targeting known genes. The microarray data are submitted to GEO database (http://ncbi.nlm.nih.gov/geo/) and can be downloaded using accession number GSE75881.
Next-generation (NG) Capture-C
NG Capture-C was performed as per published methods(Davies et al., 2015). Briefly, 10 million G1E cells were crosslinked with 1.5% formaldehyde at room temperature for 10 minutes. Chromatin was digested with the restriction enzyme DpnII, the digested chromatin was ligated using the in-situ method with T4 DNA ligase(Rao et al., 2014). The 3C libraries were sonicated to 100–300 bp fragment lengths, and sequencing adapters were ligated to generate pre-capture sequencing libraries. Biotinylated DNA oligonucleotides (sequences in Table S2) corresponding to the promoters of Cdkn1b and Lockd (anchors) were separately hybridized with the libraries, and streptavidin beads were used to capture and enrich for ligated fragments corresponding to the promoters. To increase the specificity of capture, a double capture was performed with the same probes. The enriched libraries were amplified by PCR and sequenced on an Illumina Nextseq with paired-end sequencing. FASTQ files were merged and split at DpnII sites, then mapped to the mm9 mouse genome using Bowtie, and customized bioinformatic scripts (details in published methods paper(Davies et al., 2015)) were used to discard PCR duplicate reads and determine frequency of interaction of each restriction fragment with the restriction fragment enriched by anchor probes. NG Capture-C was performed in two replicates and data were pooled for analysis.
Genome Browser images
Published RNA-seq and ChIP-seq datasets were used in conjunction with the UCSC genome browser to generate browser tracks(Kent et al., 2002; Paralkar et al., 2014; Wu et al., 2011; Euskirchen et al., 2007; John et al., 2011; Lara-Astiaso et al., 2014; Ram et al., 2011).
Supplementary Material
Highlights.
-
-
The 5′ region of the Lockd lncRNA gene “loops” to the adjacent Cdkn1b gene promoter
-
-
Deletion of the Lockd gene impairs Cdkn1b transcription
-
-
Lockd RNA was truncated by insertion of a premature polyA signal
-
-
Lockd truncation has no effect on Cdkn1b transcription
Acknowledgments
We thank Peter Klein, Feng Zhang and Kosuke Yusa for kindly providing us with CRISPR/Cas9 plasmids. We thank Chuck Sherr and Chunliang Lee for helpful discussions. This work was supported by the ASH Scholar Award (V.R.P.), the University of Pennsylvania Measey Fellowship Award (V.R.P.), the NIDDK T32 training grant (V.R.P.), the NIDDK K08 1K08DK102533-01A1 award (V.R.P.), the NIDDK R01 DK092318 (M.J.W), the NIDDK R56 DK065806 award (R.C.H), the Wellcome Trust Clinical Research Training Fellowship (ref 098931/Z/12/Z) (J.O.J.D.), the Wellcome Trust Strategic Award (reference 106130/Z/14/Z) (J.R.H.), and the Medical Research Council (MRC Core Funding and Centenary Award reference 4050189188) (J.R.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers
The microarray data are submitted to GEO database (http://ncbi.nlm.nih.gov/geo/) and can be downloaded using accession number GSE75881. The NG Capture-C data are submitted to GEO database (http://ncbi.nlm.nih.gov/geo/) and can be downloaded using accession number GSE75881.
Author contributions
V.R.P. and M.J.W. planned the experiments and wrote the manuscript with contributions from all authors. R.H and G.A.B. assisted in experiment planning and data interpretation. V.R.P. and C.C.T. performed the CRISPR/Cas9 experiments, with technical contributions by Y.Y, R.P, and J.L. A.V.K. analyzed microarray data. P.H. performed Capture-C experiments under the guidance by G.A.B., J.O.J.D and J.R.H.
References
- Bassett AR, Akhtar A, Barlow DP, Bird AP, Brockdorff N, Duboule D, Ephrussi A, Ferguson-Smith AC, Gingeras TR, Haerty W, Higgs DR, Miska EA, Ponting CP. Considerations when investigating lncRNA function in vivo. Elife. 2014;3:e03058. doi: 10.7554/eLife.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletskii A, Hong YK, Pehrson J, Egholm M, Strauss WM. PNA interference mapping demonstrates functional domains in the noncoding RNA Xist. Proc Natl Acad Sci U S A. 2001;98:9215–9220. doi: 10.1073/pnas.161173098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JO, Telenius JM, McGowan SJ, Roberts NA, Taylor S, Higgs DR, Hughes JR. Multiplexed analysis of chromosome conformation at vastly improved sensitivity. Nat Methods. 2015 doi: 10.1038/nmeth.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, Zamudio JR, Jong RM, Soukup D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA, Jacks T. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol Cell. 2014;54:777–790. doi: 10.1016/j.molcel.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, Plath K, Guttman M. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euskirchen GM, Rozowsky JS, Wei CL, Lee WH, Zhang ZD, Hartman S, Emanuelsson O, Stolc V, Weissman S, Gerstein MB, Ruan Y, Snyder M. Mapping of transcription factor binding regions in mammalian cells by ChIP: comparison of array- and sequencing-based technologies. Genome Res. 2007;17:898–909. doi: 10.1101/gr.5583007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- Hacisuleyman E, Goff LA, Trapnell C, Williams A, Henao-Mejia J, Sun L, McClanahan P, Hendrickson DG, Sauvageau M, Kelley DR, Morse M, Engreitz J, Lander ES, Guttman M, Lodish HF, Flavell R, Raj A, Rinn JL. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21:198–206. doi: 10.1038/nsmb.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43:264–268. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera MC, Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol. 2014;32:267–273. doi: 10.1038/nbt.2800. [DOI] [PubMed] [Google Scholar]
- Krivega I, Dean A. Enhancer and promoter interactions-long distance calls. Curr Opin Genet Dev. 2012;22:79–85. doi: 10.1016/j.gde.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, Zaretsky I, Jaitin DA, David E, Keren-Shaul H, Mildner A, Winter D, Jung S, Friedman N, Amit I. Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latos PA, Pauler FM, Koerner MV, Senergin HB, Hudson QJ, Stocsits RR, Allhoff W, Stricker SH, Klement RM, Warczok KE, Aumayr K, Pasierbek P, Barlow DP. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–1472. doi: 10.1126/science.1228110. [DOI] [PubMed] [Google Scholar]
- Li L, Liu B, Wapinski OL, Tsai MC, Qu K, Zhang J, Carlson JC, Lin M, Fang F, Gupta RA, Helms JA, Chang HY. Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Rep. 2013;5:3–12. doi: 10.1016/j.celrep.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MF, Jungreis I, Kellis M. PhyloCSF: a comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics. 2011;27:i275–i282. doi: 10.1093/bioinformatics/btr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VR, Mishra T, Luan J, Yao Y, Kossenkov AV, Anderson SM, Dunagin M, Pimkin M, Gore M, Sun D, Konuthula N, Raj A, An X, Mohandas N, Bodine DM, Hardison RC, Weiss MJ. Lineage and species-specific long noncoding RNAs during erythro-megakaryocytic development. Blood. 2014;123:1927–1937. doi: 10.1182/blood-2013-12-544494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram O, Goren A, Amit I, Shoresh N, Yosef N, Ernst J, Kellis M, Gymrek M, Issner R, Coyne M, Durham T, Zhang X, Donaghey J, Epstein CB, Regev A, Bernstein BE. Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell. 2011;147:1628–1639. doi: 10.1016/j.cell.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma K, Levasseur P, Aristarkhov A, Lee JT. Locked nucleic acids (LNAs) reveal sequence requirements and kinetics of Xist RNA localization to the X chromosome. Proc Natl Acad Sci U S A. 2010;107:22196–22201. doi: 10.1073/pnas.1009785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF, Gerhardinger C, Sanchez-Gomez DB, Hacisuleyman E, Li E, Spence M, Liapis SC, Mallard W, Morse M, Swerdel MR, D’Ecclessis MF, Moore JC, Lai V, Gong G, Yancopoulos GD, Frendewey D, Kellis M, Hart RP, Valenzuela DM, Arlotta P, Rinn JL. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife. 2013;2:e01749. doi: 10.7554/eLife.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Tsang AP, Visvader JE, Turner CA, Fujiwara Y, Yu C, Weiss MJ, Crossley M, Orkin SH. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- Weiss MJ, Yu C, Orkin SH. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol Cell Biol. 1997;17:1642–1651. doi: 10.1128/mcb.17.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Cheng Y, Keller CA, Ernst J, Kumar SA, Mishra T, Morrissey C, Dorman CM, Chen KB, Drautz D, Giardine B, Shibata Y, Song L, Pimkin M, Crawford GE, Furey TS, Kellis M, Miller W, Taylor J, Schuster SC, Zhang Y, Chiaromonte F, Blobel GA, Weiss MJ, Hardison RC. Dynamics of the epigenetic landscape during erythroid differentiation after GATA1 restoration. Genome Res. 2011;21:1659–1671. doi: 10.1101/gr.125088.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yan P, Lu J, Song G, Zhu Y, Li Z, Zhao Y, Shen B, Huang X, Zhu H, Orkin SH, Shen X. Opposing Roles for the lncRNA Haunt and Its Genomic Locus in Regulating HOXA Gene Activation during Embryonic Stem Cell Differentiation. Cell Stem Cell. 2015;16:504–516. doi: 10.1016/j.stem.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang S. A comprehensive evaluation of SAM, the SAM R-package and a simple modification to improve its performance. BMC Bioinformatics. 2007;8:230. doi: 10.1186/1471-2105-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


