Abstract
AIM: To determine the feasibility and safety of transgastric direct endoscopic necrosectomy (DEN) in patients with walled-off necrosis (WON) and gastric varices.
METHODS: A single center retrospective study of consecutive DEN for WON was performed from 2012 to 2015. All DEN cases with gastric fundal varices noted on endoscopy, computed tomography (CT) or magnetic resonance imaging (MRI) during the admission for DEN were collected for analysis. In all cases, external urethral sphincter (EUS) with doppler was used to exclude the presence of intervening gastric varices or other vascular structures prior to 19 gauge fine-needle aspiration (FNA) needle access into the cavity. The tract was serially dilated to 20 mm and was entered with an endoscope for DEN. Pigtail stents were placed to facilitate drainage of the cavity. Procedure details were recorded. Comprehensive chart review was performed to evaluate for complications and WON recurrence.
RESULTS: Fifteen patients who underwent DEN for WON had gastric varices at the time of their procedure. All patients had an INR < 1.5 and platelets > 50. Of these patients, 11 had splenic vein thrombosis and 2 had portal vein thrombosis. Two patients had isolated gastric varices, type 1 and the remaining 13 had > 5 mm gastric submucosal varices on imaging by CT, MRI or EUS. No procedures were terminated without completing the DEN for any reason. One patient had self-limited intraprocedural bleeding related to balloon dilation of the tract. Two patients experienced delayed bleeding at 2 and 5 d post-op respectively. One required no therapy or intervention and the other received 1 unit transfusion and had an EGD which revealed no active bleeding. Resolution rate of WON was 100% (after up to 2 additional DEN in one patient) and no patients required interventional radiology or surgical interventions.
CONCLUSION: In patients with WON and gastric varices, DEN using EUS and doppler guidance may be performed safely. Successful resolution of WON does not appear to be compromised by the presence of gastric varices, with similar rates of resolution and only minor bleeding events. Experienced centers should not consider gastric varices a contraindication to DEN.
Keywords: Necrosectomy, Pancreatic necrosis, Endoscopy, Necrotizing pancreatitis, Gastric varices, Varices, Walled off necrosis, Walled-off necrosis, Gastrointestinal hemorrhage, Endoscopic ultrasound
Core tip: In this retrospective cohort, 15 out of 90 patients (16.7%) presenting for endoscopic necrosectomy had gastric varices. When performed with best practice technique, direct endoscopic necrosectomy may be safely performed in patients with gastric varices. The best practice technique, from Thompson et al. Pancreatology, 2015 includes: (1) EUS evaluation with doppler to confirm absence of intervening vessels; (2) injection of contrast to distend collection and create wall tension for access; (3) stiff guidewire looped in cavity to mark access site for duration of the case; (4) entry into the cavity with stiff balloon catheter dilated to 4-8 mm, then 20 mm; (5) exchange for a large-channel endoscope for lavage and debridement of necrosis; (6) placement of pigtail catheters for ongoing drainage of the cavity; and (7) avoid proton pump inhibitor to encourage ongoing digestion of necrotic material.
INTRODUCTION
Pancreatic walled-off necrosis (WON) may result from acute necrotizing pancreatitis. Direct endoscopic necrosectomy (DEN) has emerged as the treatment of choice supported by high resolution and low complication rates for WON[1-4]. In the patient with WON resulting from acute necrotizing pancreatitis, the presence of gastric varices must be carefully considered, as they may contribute to significant complications including intraprocedural and postprocedural hemorrhage. The prevalence of gastric varices in patients presenting for DEN is unknown, however bleeding is the most common serious adverse event associated with the procedure[1-3]. Gastric varices may be present in this patient population for at least two reasons, (1) local inflammation from necrotizing pancreatitis may result in splenic vein thrombosis and/or portal vein thrombosis leading to gastric variceal formation; or (2) a patient with alcoholic pancreatitis may have concomitant alcoholic cirrhosis leading to portal hypertension and development of gastric varices. Portal vein, splenic vein and mesenteric venous thrombosis is reported to occur in up to 53% of patients with severe acute necrotizing pancreatitis[5,6]. It is therefore possible that the presence and associated procedural risk of gastric varices is underappreciated in this patient population.
Computed tomography (CT) is often used to evaluate the complications of acute pancreatitis and is also used in the pre-procedural evaluation for DEN. CT has been reported to be extremely sensitive at detection of submucosal gastric varices at up to 100%, with good interobserver variability (κ = 0.90) for both variceal diameter and location[7]. While endoscopic evaluation outperforms external urethral sphincter (EUS) in detection of esophageal varices, data supports the opposite for detection of gastric varices, where EUS clearly outperforms the eye of the endoscopist[8].
Non-endoscopic therapies for WON include open and minimally invasive surgical drainage, as well as percutaneous interventional radiology drainage. One randomized control trial comparing endoscopic to surgical necrosectomy found that composite clinical endpoints and inflammatory markers were improved with DEN over surgical drainage[3]. Complications of surgical drainage may include intra-abdominal hemorrhage, which has been reported in 16%-44% of patients in surgical case series[9-11]. Percutaneous catheter drainage, with the poorest clinical success rates among the interventional treatment modalities, has reported bleeding complications ranging from 2%-4%[12,13].
As performance of DEN gains increasing popularity among gastroenterologists managing patients with symptomatic WON, it is important to determine relative and absolute contraindications to the procedure. The aim of this study is to determine the feasibility and safety of transgastric DEN in patients with WON and gastric varices, as this data is previously lacking.
MATERIALS AND METHODS
Population and outcomes
A single center retrospective study of consecutive DEN for WON was performed from 2012 to 2015. Patients were considered for DEN if they met radiographic criteria of a walled-off fluid collection along with presence of symptoms secondary to the collection, including; sepsis, abdominal pain, early satiety, intolerance of full oral diet, nausea and vomiting. All DEN cases with gastric varices noted on endoscopy, CT or magnetic resonance imaging (MRI) during the admission for DEN were collected for analysis. Procedure characteristics including patient demographics, procedure characteristics, acute and delayed adverse events and clinical success were recorded. Clinical success was defined as complete resolution of the primary WON symptom leading to DEN, along with absence of any abdominal pain, early satiety, nausea, vomiting, markers of systemic inflammatory response (fever or hypothermia, leukocytosis or severe leukopenia, tachypnea, tachycardia) and bacteremia.
Direct endoscopic necrosectomy
In all cases, patients received general anesthesia and were intubated with endotracheal tube for mechanical ventilation and to provide airway protection. A linear EUS scope with color doppler (GIF-UC240P, Olympus, Tokyo, Japan) was used to exclude the presence of intervening gastric varices or other vascular structures prior to 19 gauge fine-needle aspiration (FNA) needle (Cook, Winston-Salem, NC) access into the cavity (Figure 1). Necrotic fluid was aspirated and sent for culture and gram stain. The cavity was injected with contrast for fluoroscopic visualization and to expand the cavity to compensate for the fluid previously removed. A stiff wire was advanced and coiled into the cavity and the needle was removed. The tract was serially dilated starting with a 4-mm Hurricane balloon (Boston Scientific, Natick, MA) continuing up to 20 mm with a radially expanding through-the-scope balloon (Boston Scientific). The echoendoscope was then exchanged for a larger channel therapeutic endoscope (GIF XTQ-160 or GIF 2T-160, Olympus) that was used to perform the remaining maneuvers for DEN. This larger channel scope was used to suction out all fluid from the cavity, and then immediate attention was turned to physical debridement of the necrotic material along the cavity walls using various tools including endoscopic retrieval net, forceps and snares until all loose debris was removed (Figure 2). Next 1 to 2L of warmed bacitracin-laden saline solution (25000 UI/L) was used to lavage the cavity. Finally, two to three, 10 French double-pigtail stents (Cook) were placed at the end of the procedure to facilitate ongoing drainage of the cavity (Figure 3). All patients were given two to four weeks of systemic oral antibiotic prophylaxis. Stents, by protocol, were removed at 6-8 wk after placement if they did not spontaneously migrate in that period of time. Follow up procedures for delayed bleeding, repeat DEN or stent retrieval were performed as indicated. Repeat DEN was performed only if patient-reported symptoms of an ongoing fluid collection were present, at which time repeat imaging was used to confirm continued presence of a fluid collection prior to repeating the procedure. Procedure details were recorded retrospectively and comprehensive chart review was performed to evaluate for delayed complications and any recurrence of symptomatic WON occurring after the interval episode of pancreatitis.
Figure 1.
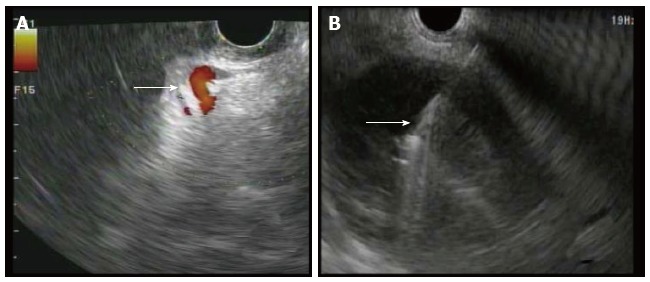
Endoscopic ultrasound of walled off necrosis. A: Doppler used to visualize any interventing vessels (arrow) including varices; B: FNA needle (arrow) seen entering necrotic cyst under EUS guidance. EUS: External urethral sphincter; FNA: Fine-needle aspiration.
Figure 2.
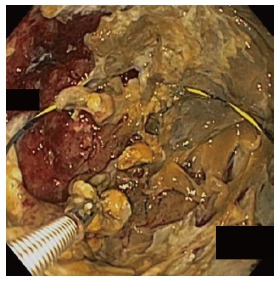
Endoscopic necrosectomy performed with debridement of the cyst cavity. Wire is seen coiled within the cyst to maintain access through the procedure.
Figure 3.
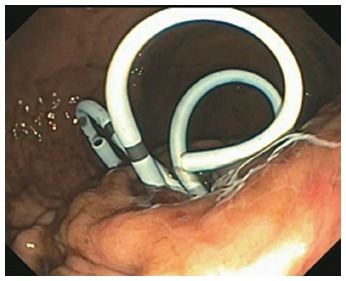
Pigtail stents left in place at the end of endoscopic necrosectomy to encourage ongoing drainage.
RESULTS
Patient characteristics
Out of 90 patients undergoing DEN for WON between 2012 and 2015, a total of 15 patients (16.7%) were determined to have gastric varices at the time of their procedure (Table 1). Mean age was 47.1 years (range 27-62) and six patients (40%) were female. Etiology of pancreatitis leading to WON was alcohol in six patients (40%), gallstone disease in 5 patients (33%) and other/unspecified in four patients (27%). All patients had an INR less than 1.5 (mean 1.16) and platelets greater than 50000/μL (mean 237000/μL). Of these patients, 11 (73%) had splenic vein thrombosis, 2 (13%) had portal vein thrombosis, and two had no notable thrombosis on imaging. Large endoscopically visualized isolated gastric varices, type 1 were present in two patients (13%) and the remaining 13 (87%) had 5 mm or greater gastric submucosal varices identified on imaging by CT, MRI or EUS (Figure 4). No procedures were terminated early without fully completing the DEN.
Table 1.
Patient characteristics
| Patient | Age | Sex | Etiology of pancreatitis | Presence of PVT/SVT | Platelet count (normal range 150-450) | INR (normal range 0.9-1.1) | Gastric varices type | Intraprocedural bleeding? | Postprocedural bleeding? | Any variceal bleeding reported? | Repeat therapy required? | Resolution of varices?1 |
| 1 | 37 | F | Gallstone | SVT | 185 | 1.0 | IGV-1 | - | - | - | DEN × 2 | Unknown |
| 2 | 44 | F | Alcohol | SVT | 235 | 1.3 | SMV | - | - | - | DEN × 1 | No (24 mo) |
| 3 | 45 | M | Alcohol | - | 131 | 1.1 | SMV | - | - | - | - | Unknown |
| 4 | 39 | M | Alcohol | PVT | 256 | 1.4 | SMV | - | - | - | DEN × 1 | No (14 mo) |
| 5 | 42 | M | Gallstone | SVT | 130 | 1.0 | IGV-1 | - | - | - | - | Unknown |
| 6 | 60 | F | Unknown | SVT | 167 | 1.2 | SMV | Minimal | - | - | - | Yes (36 mo) |
| 7 | 27 | M | Alcohol | SVT | 248 | 1.0 | SMV | - | - | - | - | No (32 mo) |
| 8 | 82 | F | Unknown | SVT | 145 | 1.1 | SMV | - | - | - | - | Yes (19 mo) |
| 9 | 41 | F | Gallstone | SVT | 224 | 1.0 | SMV | - | - | - | DEN × 1 | Unknown |
| 10 | 58 | M | Unknown | SVT | 252 | 1.2 | SMV | - | Self-limited (seen in cyst on CT 5d later), no transfusion, no EGD | - | - | Unknown |
| 11 | 62 | F | Unknown | SVT | 199 | 1.3 | SMV | - | - | - | - | Unknown |
| 12 | 42 | M | Gallstone | SVT | 151 | 1.2 | SMV | - | - | - | - | Unknown |
| 13 | 50 | M | Gallstone | SVT | 604 | 1.2 | SMV | - | - | - | DEN × 1 | Unknown |
| 14 | 43 | M | Alcohol | - | 356 | 1.0 | SMV | - | - | - | - | Unknown |
| 15 | 35 | M | Alcohol | PVT | 276 | 1.4 | SMV | - | Self-limited, 1u pRBC given. EGD: Clots on pigtail catheters no active bleeding | - | - | Unknown |
Time interval between procedure and last noted presence of varices. PVT: Portal vein thrombosis; SVT: Splenic vein thrombosis; IGV: Isolated gastric varices; SMV: Submucosal varices; DEN: Direct endoscopic necrosectomy; Male; F: Female.
Figure 4.
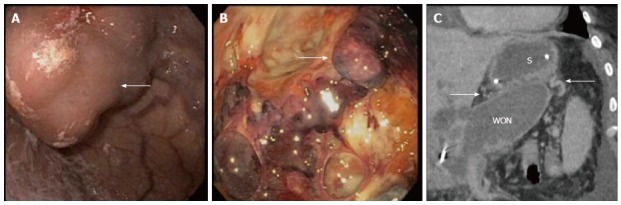
Varices identified through various methods. A: Large gastric varix (arrow) seen endoscopically; B: Peri-gastric varix (arrow) seen within the cyst cavity during endoscopic necrosectomy; C: Computed tomography scan showing gastric varices (arrows) in close proximity to the stomach (S) and walled off necrosis (WON).
Adverse events
One patient had self-limited intraprocedural bleeding noted upon balloon dilation of the necrosectomy tract (Figure 5). Two patients experienced delayed bleeding at two and five days post-procedure, respectively. One, diagnosed incidentally on the basis of blood seen on CT within the cyst required no therapy or intervention. The other, diagnosed on the basis of hemoglobin and hematocrit drop, received one unit transfusion of packed red blood cells and underwent EGD, which revealed no active bleeding. Some clot material was seen at the entrance to the necrosectomy cavity, suggesting that the source of resolved hemorrhage was within the cavity or emanating from the wall of the endoscopic necrosectomy tract.
Figure 5.
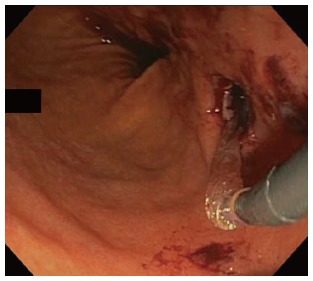
Status-post balloon dilation of the necrosectomy tract, shown with self-limited bleeding.
Clinical resolution
Clinical success and resolution rate of WON in this patient cohort was 100% after up to two additional DEN procedures. One patient required two additional DEN procedures and four patients required one additional DEN for complete resolution of symptoms. No patients required interventional radiology or surgical interventions for complications of the procedure, or for management of the pancreatic necrosis. No patients required adjunctive endoscopic therapies including nasocystic irrigation or pancreatic duct stenting. A total of five patients underwent follow-up imaging after clinical resolution of WON with thrombosis and varices noted to have dissipated in two out of five patients (40%) over a range of 19-36 mo.
DISCUSSION
Gastric varices are common in patients referred for management of WON. Over 16% of our cohort undergoing DEN had gastric varices[1,3,10]. The outcomes in this cohort with gastric varices included similarly high clinical resolution rates and similarly low adverse event rates in line with previously reported DEN cohorts. This study suggests that patients with WON and known or suspected gastric varices may safely undergo DEN guided by EUS with doppler. In our cohort, successful resolution of WON using DEN does not appear to be compromised by the presence of gastric varices. A previously reported cohort of 60 patients undergoing DEN at our institution showed a clinical success rate of nearly 90%, with 3.3% major complication rate[1]. In this cohort of patients undergoing DEN with gastric varices, only minor bleeding events were seen, which did not meet criteria to be listed as a major complication. Importantly, no bleeding events involved puncture or trauma to a gastric varix, likely given the use of EUS doppler guidance when choosing the location of the necrosectomy tract. Furthermore, DEN may be the preferred treatment modality for WON in a population with gastric varices given the ability of EUS to detect submucosal varices, which are not seen when “blindly” accessing the cavity via a surgical or percutaneous route.
Limitations
The retrospective nature of this cohort study is a limitation and the fact that the study was performed in a multidisciplinary center of excellence could limit generalizability. We advocate performing this procedure at a center with DEN-trained endoscopists, and with capable surgical and/or interventional radiology services to manage any procedural complications or therapeutic failures should the need arise. Our single center experience with DEN is relatively robust in numbers, however larger numbers of pooled data would be helpful in making statistically powered clinical observations.
Another limitation to this study population includes the issue that patients should not be subjected to repeat imaging, including the inherent radiation exposure associated with CT, in the absence of symptoms. Because of this, our patients who were asymptomatic on follow up from their initial DEN did not undergo routine repeat imaging, which limited our ability to comment with confidence on variceal resolution rate as well as radiographic resolution rate of the fluid collections. Instead, resolution of symptoms was used to define clinical success.
Future studies
In our study, 40% of patients who had follow up imaging after DEN had resolution of thrombosis and gastric varices. What role DEN may play in affecting recanalization rates of splanchnic venous thrombosis resulting in portal hypertension and gastric varices is unknown, and is an interesting question. Theoretically, this highly clinically effective procedure, with previously mentioned reductions in inflammatory markers as compared to other treatment modalities, may result in timely reduction of inflammation resulting in reabsorption of thrombosis and vessel recanalization. It is also possible that earlier DEN may reduce thrombotic sequelae of acute pancreatitis. This question should be studied in a larger patient population undergoing DEN.
In conclusion, use of EUS guidance appears to allow the endoscopist to safely avoid intervening gastric varices and bleeding complications, a necessity which both surgical and percutaneous interventional radiology techniques lack. As such, reduction in bleeding complications may be considered one advantage to an endoscopic approach to necrosectomy over other techniques. Experienced centers should not consider gastric varices a contraindication to DEN.
COMMENTS
Background
Increasingly minimally invasive techniques, including both percutaneous and endoscopic, have replaced surgery in the management of infected and symptomatic pancreatic necrosis. Pancreatitis may be associated with portal and splenic thrombosis leading to gastric varices, and is an important consideration in the bleeding risk when performing drainage procedures.
Research frontiers
The role of endoscopic management of pancreatic fluid collections has increased significantly over the past 10 years. The American Society for Gastrointestinal Endoscopy has recently published the first guideline statement regarding the flexible endoscopic management of inflammatory pancreatic fluid collections, available on the web at: http://www.asge.org/uploadedFiles/Publications_(public)/Practice_guidelines/Inflammatory_pancreatic_fluid_collections.pdf.
Innovations and breakthroughs
This is the first report suggesting a reasonably high prevalence of gastric varices (16.7%) in patients presenting to a tertiary care facility for endoscopic management of walled off pancreatic necrosis. This may have implications regarding the safety and best approach to resolution of these fluid collections in this patient population.
Applications
This study suggests a need for increased awareness of the relevance of gastric varices in the patient with pancreatic necrosis. The presence of varices should be considered when determining the best approach to managing these patients. Endoscopic ultrasound-guided access, with protocol driven debridement appears to be safe and feasible in this patient population.
Terminology
Walled-off necrosis (WON) is an inflammatory collection of debris and fluid that may form and persist after an episode of acute necrotizing pancreatitis. This collection may become infected, leading to sepsis and bacteremia, or may cause symptoms including abdominal pain, early satiety, anorexia, nausea and/or vomiting; direct endoscopic necrosectomy (DEN) is a per-oral procedure using flexible endoscopes to enter WON and provide debridement of non-viable and infected tissue to aid in resolution of the fluid collection and its associated symptoms.
Peer-review
The purpose of this paper is to determine the feasibility and safety of transgastric DEN in patients with WON and gastric varices. The results are feasible, safe and effective.
Footnotes
Institutional review board statement: Internal approval for data collection pertinent to this study was obtained.
Informed consent statement: Retrospectively collected data made informed consent infeasible.
Conflict-of-interest statement: Dr. Storm reports no conflicts of interest, Dr. Thompson is a consultant to Cook, Olympus and Boston Scientific.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 9, 2016
First decision: March 9, 2016
Article in press: April 6, 2016
P- Reviewer: Liu QD, Teoh AYB, Tham T, Yan SL S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Thompson CC, Kumar N, Slattery J, Clancy TE, Ryan MB, Ryou M, Swanson RS, Banks PA, Conwell DL. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology. 2016;16:66–72. doi: 10.1016/j.pan.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar N, Conwell DL, Thompson CC. Direct endoscopic necrosectomy versus step-up approach for walled-off pancreatic necrosis: comparison of clinical outcome and health care utilization. Pancreas. 2014;43:1334–1339. doi: 10.1097/MPA.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053–1061. doi: 10.1001/jama.2012.276. [DOI] [PubMed] [Google Scholar]
- 4.Muthusamy VR, Chandrasekhara V, Acosta RD, Bruining DH, Chathadi KV, Eloubeidi MA, Faulx AL, Fonkalsrud L, Gurudu SR, Khashab MA, et al. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest Endosc. 2016;83:481–488. doi: 10.1016/j.gie.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Easler J, Muddana V, Furlan A, Dasyam A, Vipperla K, Slivka A, Whitcomb DC, Papachristou GI, Yadav D. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12:854–862. doi: 10.1016/j.cgh.2013.09.068. [DOI] [PubMed] [Google Scholar]
- 6.Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: a single-center experience. Pancreas. 2013;42:1251–1254. doi: 10.1097/MPA.0b013e3182968ff5. [DOI] [PubMed] [Google Scholar]
- 7.Willmann JK, Weishaupt D, Böhm T, Pfammatter T, Seifert B, Marincek B, Bauerfeind P. Detection of submucosal gastric fundal varices with multi-detector row CT angiography. Gut. 2003;52:886–892. doi: 10.1136/gut.52.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boustière C, Dumas O, Jouffre C, Letard JC, Patouillard B, Etaix JP, Barthélémy C, Audigier JC. Endoscopic ultrasonography classification of gastric varices in patients with cirrhosis. Comparison with endoscopic findings. J Hepatol. 1993;19:268–272. doi: 10.1016/s0168-8278(05)80581-1. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Gou S, Wang C, Wu H, Xiong J, Zhao G, Zhou F, Tao J, Yang Z, Yin T, et al. [Surgical treatment of necrotizing pancreatitis: 10-year experience at a single center] Zhonghua Waik Zazhi. 2015;53:672–675. [PubMed] [Google Scholar]
- 10.Busse MJ, Ainsworth AP. Ten years of experience with transgastric necrosectomy for walled-off necrosis in acute pancreatitis. Dan Med J. 2015;62:pii: A5131. [PubMed] [Google Scholar]
- 11.Pupelis G, Fokin V, Zeiza K, Plaudis H, Suhova A, Drozdova N, Boka V. Focused open necrosectomy in necrotizing pancreatitis. HPB (Oxford) 2013;15:535–540. doi: 10.1111/hpb.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudin G, Chassang M, Gelsi E, Novellas S, Bernardin G, Hébuterne X, Chevallier P. CT-guided percutaneous catheter drainage of acute infectious necrotizing pancreatitis: assessment of effectiveness and safety. AJR Am J Roentgenol. 2012;199:192–199. doi: 10.2214/AJR.11.6984. [DOI] [PubMed] [Google Scholar]
- 13.Mortelé KJ, Girshman J, Szejnfeld D, Ashley SW, Erturk SM, Banks PA, Silverman SG. CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. AJR Am J Roentgenol. 2009;192:110–116. doi: 10.2214/AJR.08.1116. [DOI] [PubMed] [Google Scholar]


