Abstract
Understanding the development of a skull deformity requires an understanding of the normal morphogenesis of the cranium. Craniosynostosis is the premature, pathologic ossification of one or more cranial sutures leading to skull deformities. A review of the English medical literature using textbooks and standard search engines was performed to gather information about the prenatal development and growth of the cranial vault of the neurocranium. A process of morphogenic sequencing begins during prenatal development and growth, continues postnatally, and contributes to the basis for the differential manner of growth of cranial vault bones. This improved knowledge might facilitate comprehension of the pathophysiology of craniosynostosis.
Keywords: Cranial sutures, Craniosynostosis, Embryonic development, Growth, Skull
INTRODUCTION
The cranium (or skull) is a composite structure made up of the neurocranium, which surrounds and protects the brain, and the viscerocranium, which forms the skeleton of the face. The neurocranium can be subdivided into the cartilaginous part, which form base of the skull (or chondrocranium), and the membranous part, which forms cranial vault (or calvarium). The cranial vault consists mainly of the flat bones : paired frontal and parietal bones; the squamous parts of the temporal bone; and interparietal part of occipital bone. All of these bones are formed by intramembranous (IM) ossification. At birth, the bones of the cranial vault are unilaminar tables and, thereafter, the intervening diploe appears about the fourth year. Morphogenesis of the bones of the cranial vault is a lengthy developmental process initiated during early embryogenesis and completed during adulthood.
This review will primarily focus on prenatal development and growth of the human cranial vaults. Described below are the sections into which this review is divided.
First is the embryonic phase which is the first 8 weeks of pregnancy. In this phase, formation of the cranial vault is preceded by the formation of mesenchymal cells by epithelial-mesenchymal transformation (EMT) via the mesenchymatous or precondensation. And development of the cranial bones begins with condensation of mesenchymal cells3).
Second is the fetal phase which is the interval from the end of the embryonic phase to birth. In this phase, IM ossification for the primitive membranous skull formation begins. And cranial sutures are formed and it plays a critical role as IM bone growth sites. Also, skull bones growth through displacement and bone remodeling.
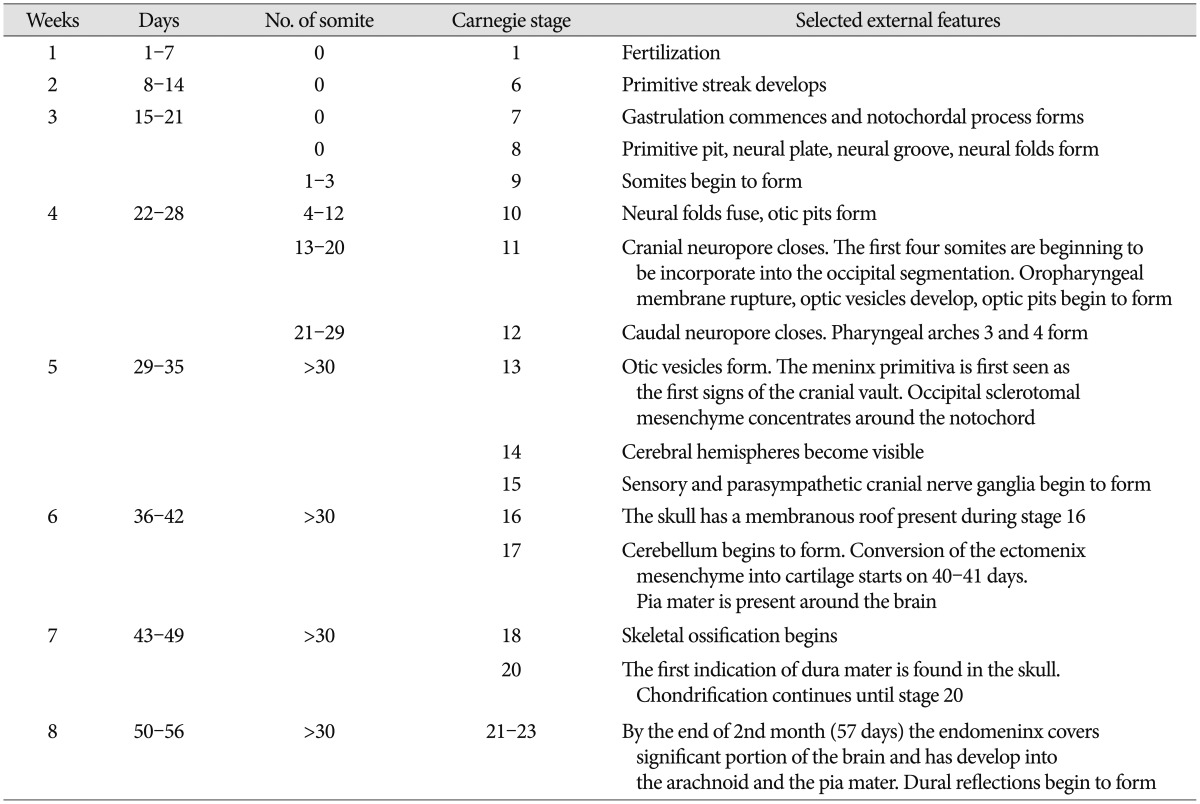
Throughout this review, we refer to human developmental stages in terms of gestation or postfertilization day or week and the embryonic Carnegie stages according to the references9). Timing of some events and stage can vary by up to 7 days during the embryonic period. Characteristic features associated with the development of neurocranium in the embryonic period are collected in the Table 1 for comparative reference.
Table 1. Stages of human embryonic developments.
Timing of some events and stage can vary by up to 4–5 days during stages 10–23
EMBRYONIC PHASE
Formation of mesenchymal cells by EMT
All bones of the skull pass first through a mesenchymatous phase or precondensation phase. Within the first 4 weeks of gestation, the fetal head has mesenchyme originating from two sources, mainly unsegmented paraxial mesoderm and the cranial neural crest by way of a process known as EMT. This process can be defined as a process by which epithelial cells become mesenchymal cells6,11).
At the end of gastrulation (third week), mesenchymal cells produced by epithelial cells have migrated through the primitive streak to organize into a third germ layer–the embryonic mesoderm. The mesoderm on each side of the notochord and neural tube proliferate to form longitudinal columns of paraxial mesoderm. Toward the end of the third week, the paraxial mesoderm differentiates and begins to form somites which differentiate to become sclerotome. The somites first appear in the future occipital region of the embryo. Rostral to the first somite, the head mesoderm forms seven cranial somitomeres, which represent the most cephalic mesodermal contribution from the primitive streak and do not condense to form somites4). The mesoderm becomes more dispersed with development to loosely fill the developing head as the head mesenchyme.
Later, the head mesenchyme becomes supplemented with cranial neural crest cells. Neural crest cells are specialized, multipotential migratory cells which are also generated by EMT involving the epithelial cells of the neuroectoderm. Formation of the cranial neural crest begins during elevation of the neural folds before they fuse and continue from the closed neural tube well and after the neural folds have fuses. They are spread within the mesenchyme8,11). Thus, the head mesenchyme is derived from both head mesoderm and neural crest.
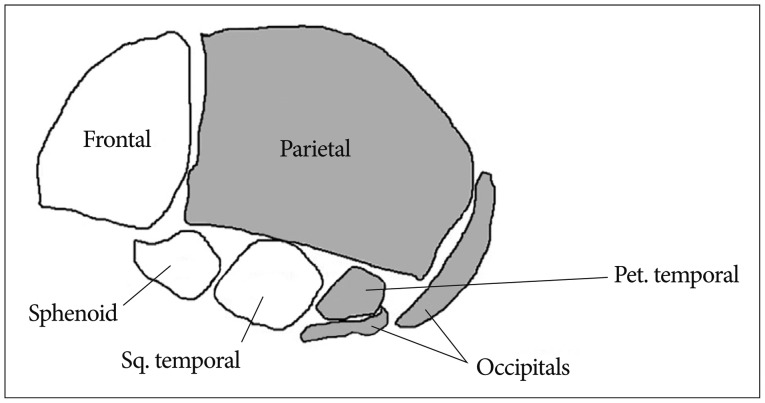
The neurocranium develops from the paraxial mesenchyme in the head, i.e., the first five somites and the unsegmented somitomeres rostral to the first somite, and from ectoderm via the neural crest11). The viscerocranium is derived exclusively from neural crest mesenchyme11). The neural crest provides the mesenchyme forming the frontal, sphenoid, squamous temporal bones as well as facial bone14). Paraxial mesoderm (somites and somitomeres) play a direct role in skeletogenesis of the parietal, petrous temporal and occipital bones (Fig. 1). However, at the space of two parietal bones, the neural crest also plays a significant role. A small line of the neural crest is derived from mesenchyme and remains between the two parietal bones and contributes to the signaling system that governs growth of the cranial vault at the sutures and to the development of the under lying meninges11).
Fig. 1. Skeletal structure of skull : mesenchyme for these structures is derived from neural crest (white) and paraxial mesoderm (gray). Sq : squamous, Pet : petrous.
Condensation of mesenchymal cells
Development of the cranial bones begins with condensation of mesenchymal cells that surround the developing brain and contain osteoprogenitor cells. At the site where bone is to be formed, the mesenchymal cells become closely packed to form a mesenchyme condensation. As the region becomes highly vascular, a membrane is formed. Condensation of mesenchyme also marks the beginning of selective genes specific for either chondro- or IM osteogensis. However, this is beyond the scope of this review.
During the 4th week of gestation, the head mesenchyme develops to form the base of the ectomeningeal capsules (membrane), which is the earliest evidence of skull formation13). At the same time, the occipital sclerotomal mesenchyme concentrates around the notochord underlying the developing hindbrain. From this region, the mesenchymal condensation extends cephalically, forming a floor for the brain. The base of the mesenchymatous capsule (i.e., primordium of the cranial base) becomes the thickest area of the capsule7).
During the 5th week of gestation, the mesenchyme (mensenchymal tissue) that gives rise to the membranous neurocranium (calvaria) is first arranged as a capsular membrane around the developing brain12). The membrane is called the meninx primitiva or primary meninx1,4). This is the first sign of the cranial vault which appears around the 30th day of gestation. They consist of curved plates of mesenchyme at the sides of the skull and gradually extend cranially to blend with each other; they also extend towards and reach the base of the skull, which will become part of the chondrocranium.
The meninx primitiva can be subdivided into two layers : the endomeninx (or secondary meninx), which contribute to the formation of the leptomeninx (pia and arachnoid); and the ectomeninx, which contributes to the formation of the inner dura mater and an outer superficial membrane (periosteal or endosteal layer) with osteogenic and chondrogenic properties1,14).
By the end of stage 16 (37 days post ovulation), the first phase of the period of membrane formation is completed3).
At this time, the brain expands dramatically and the capsule is totally responsive to the expanding spatial demands. Growth of the brain and surrounding mesenchyme occurs simultaneously in all three dimensions; biodynamic differentiation, however, cause some area of the developing brain to grow more rapidly than others.
FETAL PHASE
Ossification (osteogenesis)
The process of bone formation is called ossification. There are two processes resulting in the formation of normal, healthy bone tissue2) : IM ossification is the direct laying down of bone into the primitive connective tissue (mesenchyme); and endochondral ossification, which involves cartilage as a precursor.
Osteogenesis of the ectomeninx occurs as IM bone formation forming the cranial vault, whereas the ectomeninx forming the floor of the brain chondrifies as the chondrocranium.
IM ossification (osteogenesis) starts by developing the ossification centers in the outer layer of the ectomeninx to form individual bones. Ossification centers first appear in areas corresponding to the future eminences as early as 7th and 8th week post conception (PC) and with bone formation spread centrifugally. The next step is calcification which begins a few days after the deposit of organic bone substance (or osteoid) by osteoblasts. With ongoing condensation of these centers, ossification proceeds to form bones that are characterized bone spicules. These spicules progressively radiate from primary ossification centers toward the periphery. The osteoid calcifies leading to the formation of the primitive trabecular bone. The type of the first-formed bone in the embryo is called woven bone, which is an immature type of bone with almost random orientation of collagen fibers. At approximately the time of birth, this woven bone is gradually replaced by the more mature lamellar bone. During fetal and postnatal growth, flat bones enlarge by apposition of new layers on the outer surface and by simultaneous osteoclastic resorption from the inside5,12).
The embryological development of the individual calvarial bones is summarized as follow13).
Two frontal bones appear from a primary ossification center for each bone forming at position of the frontal eminence at the 8th week. Three pairs of secondary centers appear later in the zygomatic processes; nasal spine, and trochlear fossae and fuse together by 6 to 7 months. The frontal bones remain separated until after birth by the frontal (metopic) suture; synostotic fusion of this suture takes place during the second postnatal year and unites into a single frontal bone by 7 years of age. Frontal sinuses do not develop until the first postnatal year14,15).
The two parietal bones arise from two primary ossification centers for each bone which appear at the parietal eminence in the 8th week PC and fuse in the 4th month. Ossification progresses radially from the central focus toward the periphery of the bone. By 14 weeks, there is extensive ossification of both parietal bones and that continues along all margins throughout fetal life. Nonetheless, at term, the cranial sutures adjacent to the parietal bones are relatively broad, particularly in the parietotemporal region14,15).
The interparietal portion of the occipital bone ossifies intramembranously from two centers, one on each side, appearing in the 8th week PC. The rest of the occipital bone ossifies endochondrally14,15).
The squamous portion and the tympanic ring of the temporal bone ossify intramembranously from a single and four centers appearing at the 8th week PC and in the 3rd month PC, respectively. The two membranous bone portions of the temporal bone fuse at birth. The rest of the temporal bone ossifies endochondrally14,15).
In the floor of the brain, in contrast to the cranial vault, the bones of the cranial base are formed initially in the cartilage and are later transformed by endochondral ossification into bone. Conversion of the ectomeninx mesenchyme into cartilage constitutes the beginning of the chondrocranium, which commences from the 40th day of gestation and onwards13). By 8 weeks, through endochondral ossification, the desmocranium is replaced with cartilage and ossification begins at precisely 12 weeks and 4 days of gestation, initially within the chondrocranium, forming part of the occipital bone7).
Development of suture
Sutures are formed during embryonic development at the sites of approximation of the membranous bones of the cranial bones and as a flexible fibrous tissue uniting the adjacent bones10).
The site of suture formation corresponds to the location of major dural reflections. Dural reflexions are double folding of the meningeal dura which firmly attach the skull base at the crista galli, the cribriform plate, the lesser wings of the sphenoid and the petrous temporal crests5). These reflections act as partitions of the cranial cavity under the calvarium, adopting a course that follows the main direction of the sutures. In conjunction with falx cerebri and the tentorium cerebelli, these come to define the zones where bone growth slows down and the coronal, lambdoid, and sagittal sutures develop. Without the dural bands, the brain would expand as a perfect sphere5). By 16 weeks, the radiating centers of ossification have almost reached the sites of reflective bands in the dura. These latter sites remain unossified as regions of connective tissue between the outspreading islands of membranous bone15).
For sutures to function as IM bone growth sites, they need to remain in an unossified state, yet allow new bone to be formed at the edges of the overlapping bone fronts. This process relies on the production of sufficient new bone cells to be recruited into the bone fronts, while ensuring that the cells remaining in the suture remain undifferentiated. So sutures allow spatial separation of the bones during growth10).
Once sutures are formed, a second phase of development occurs, in which rapid growth of the cranial bone takes place via the regulated proliferation and differentiation of osteoprogenitor at the periphery of each bone field, which is called the osteogenic front.
While the sutures are developing, the growing and expanding bone fronts both invade and recruit the intervening mesenchymal tissue into the advancing edges of the bone fronts. In this process, the mesenchyme is separated with an outer ectoperiosteal layer and an inner dura mater by the intervening bones.
Growth : remodeling and displacement
Growth is a differential developmental process. A process of morphogenic sequencing begins during prenatal growth, continues postnatally, and contributes to the basis for the differential manner of growth.
Postnatal growth of the calvarial bone is a combination of 1) sutural growth, 2) remodeling, and 3) centrifugal displacement by the expanding brain :
1) Sutural growth : In cranial vault, the flat bones are embedded within the connective tissue membranes of the brain on one side and the integument of the other. As the brain grows, the calvarial bones are drawn outward, in part, by the expanding meninges, the outer part of which are osteogenic and are responsible for production of the inner table of each flat bone. As the bones are drawn apart by their displacement movements, the osteogenic sutural membranes produce membranous bone in amounts equal to the extents of displacement, thereby enlarging the circumference of each bone and sustaining constant articular contact.
2) Remodeling takes place through patterns of deposit and reabsorption throughout all of the regional parts of any given bone by direct osteoblastic and osteoclastic activities. Remodeling enlarges a whole bone with reshaping and resizing each level within a growing bone.
3) Displacement movements occur for all bones and have a separating effect at the articular junctions among them. Primary displacement occurs in conjunction with bone's own growth. Secondary displacement is caused by enlargement of adjacent or remote bones or soft tissues, but not of the bone itself. During primary displacement the moving bone and other skeletal parts are growing simultaneously, while in secondary displacement the displacement of a bone is not directly related to its own enlargement.
Growth in size of the bone can occur by deposits to the bone on the edges adjoining sutures. Growth in thickness and size of the bone also occurs when the overlying periosteum forms bone by the process of IM ossification over the outer surface of the bone. Simultaneously, there is removal of bone from the inner surface. In this way, as the bone grows in size, there is simultaneous increase in the size of the cranial cavity.
The growing brain does not actually push the bones outward. Rather, each flat bone is suspended, with the existent traction forces, within a widespread sling of the collagenous fibers of the enlarging inner (meningeal) and out (cutaneous) periosteal layers. As these membranes grow in an ectocranial direction ahead of the expanding brain, the bones are displaced with them. This draws all of them apart, and the tensile physiological forces thus created are believed to be the stimulus that triggers the bone producing response.
The stimulus arises primarily from the expanding brain, sending signals by means of the dura mater2). As the brain expands and the cranial base synchondroses (cartilaginous bone growth plates) lengthen, the sutures respond by adding IM bone at the edges of the bone fronts, such that the sutures remain approximately the same width and the cranial vault increases in size to accommodate the enlarging brain.
At birth, the flat bones of the skull are rather widely separated by the sutures. These open spaces, the fontanelles, allow a considerable amount of deformation of the skull at birth–a fact which is important in allowing the relatively large head to pass through the birth canal. After birth, apposition of bone along the edges of the fontanelles eliminates these open spaces fairly quickly, but the bones remain separated by a thin periosteum line suture form many years, eventually fusing in adult life. In the calvaria, marked displacement of the frontal, parietal, and occipital bones, with concomitant osseous deposition at their sutural margins, is characteristic for the infant and young child with rapid brain growth. The displacement type of growth depends on the presence of normally functioning sutures acting as areas of growth and adjustment, whereas endocranial and ectocranial remodeling serve to adjust for necessary changes in the curvature of individual bones. The growth of the calvarial sutures follows the natural growth curve (i.e., the growth rate is extremely rapid during the first few years of life but slows down dramatically by the age of six to seven years). Growth of the cranial vault takes place in the following way : 1) increases in width primarily through fill in ossification of the proliferating connective tissue in the interparietal, lambdoidal, parietosphenoidal and parietotemporal sutures. 2) Increase in length may be primarily due to the growth of the cranial base with active response at the coronal suture. 3) Increase in height is due to the activity of the parietal sutures along with the occipital, temporal, and sphenoidal contiguous osseous structures13).
Premature osseous obliteration of sutures (craniosynostosis) by fusion of bone fronts across the suture site prevents further bone formation at this site. The loss of the sutural growth sites causes an inability to accommodate rapid, expansive growth of the neurocranium, leading to abnormal compensatory morphogenesis throughout the head and typically results in craniofacial dysmorphology10).
References
- 1.Adeeb N, Mortazavi MM, Tubbs RS, Cohen-Gadol AA. The cranial dura mater : a review of its history, embryology, and anatomy. Childs Nerv Syst. 2012;28:827–837. doi: 10.1007/s00381-012-1744-6. [DOI] [PubMed] [Google Scholar]
- 2.Caetano-Lopes J, Canhão H, Fonseca JE. Osteoblasts and bone formation. Acta Reumatol Port. 2007;32:103–110. [PubMed] [Google Scholar]
- 3.Cohen MM, MacLean RE. Craniosynostosis : Diagnosis, Evaluation, and Management. ed 2. New York: Oxford University Press; 2000. pp. 105–107. [Google Scholar]
- 4.Dixon AD, Hoyte DA, Ronning O. Fundamentals of Craniofacial Growth. New York: CRC Press; 1997. pp. 101–102. [Google Scholar]
- 5.Glorieux FH, Pettifor JM, Juppner H. Pediatric bone : Biology & Diseases. San Diego: Academic Press; 2003. pp. 77–103. [Google Scholar]
- 6.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 7.Liem T. Cranial Osteopathy. Edinburgh, UK: Churchill Livingstone; 2005. pp. 41–48. [Google Scholar]
- 8.Moore KL, Persaud TVN, Torchia MG. The Developing Human : Clinically Oriented Embryology. ed 10. Philadelphia: Elsevier Health Sciences; 2015. pp. 389–390. [Google Scholar]
- 9.O'Rahilly R, Müller F. Developmental Stages in Human Embryos. Washington D.C.: Carnegie Institution of Washington; 1987. pp. 1–3. [Google Scholar]
- 10.Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Rodeck CH, Whittle MJ. Fetal medicine : Basic Science and Clinical Practice. ed 2. London: Churchill Livingstone; 2009. pp. 39–41. [Google Scholar]
- 12.Sadler TW. Langman's Medical Embryology. ed 10. Baltimore, MD: Lippincott Williams & Wilkins; 2011. pp. 125–127. [Google Scholar]
- 13.Singh G. Textbook of Orthodontics. ed 3. New Delhi: JP Medical Ltd; 2015. pp. 44–45. [Google Scholar]
- 14.Sperber GH, Sperber SM, Guttmann GD. Craniofacial Embryogenetics and Development. ed 2. Shelton, CT: PMPH-USA; 2010. pp. 95–118. [Google Scholar]
- 15.Tubbs RS, Bosmia AN, Cohen-Gadol AA. The human calvaria : a review of embryology, anatomy, pathology, and molecular development. Childs Nerv Syst. 2012;28:23–31. doi: 10.1007/s00381-011-1637-0. [DOI] [PubMed] [Google Scholar]