Abstract
Pelvic organ prolapse (POP) occurs when the pelvic organs (bladder, bowel or uterus) herniate into the vagina, causing incontinence, voiding, bowel and sexual dysfunction, negatively impacting upon a woman’s quality of life. POP affects 25% of all women and results from childbirth injury. For 19% of all women, surgical reconstructive surgery is required for treatment, often augmented with surgical mesh. The surgical treatment fails in up to 30% of cases or results in adverse effects, such as pain and mesh erosion into the bladder, bowel or vagina. Due to these complications the Food and Drug Administration cautioned against the use of vaginal mesh and several major brands have been recently been withdrawn from market. In this review we will discuss new cell-based approaches being developed for the treatment of POP. Several cell types have been investigated in animal models, including a new source of mesenchymal stem/stromal cells (MSC) derived from human endometrium. The unique characteristics of endometrial MSC, methods for their isolation and purification and steps towards their development for good manufacturing practice production will be described. Animal models that could be used to examine the potential for this approach will also be discussed as will a rodent model showing promise in developing an endometrial MSC-based therapy for POP. The development of a preclinical large animal model for assessing tissue engineering constructs for treating POP will also be mentioned.
Keywords: Endometrium, Mesenchymal stem cells, Endometrial mesenchymal stem cells, Pelvic organ prolapse, Mesh, Tissue engineering, Regenerative medicine
Core tip: Pelvic organ prolapse is the herniation of pelvic organs into the vaginal cavity and affects approximately 25% of all women. Traditional mesh-augmented surgical treatments cause complications such as pain and mesh erosion. A tissue engineering approach using endometrial mesenchymal stem cells seeded on new composite mesh show promise in animal models through their modulation of the chronic inflammatory response and promotion of physiological and biomechanically compliant neotissue.
INTRODUCTION
The repair of damage to tissues and organs constitutes almost half of all medical expenses[1]. In the early 1990s in the United States alone, $400 billion was spent per annum treating conditions linked with tissue and organ failure[1]. Despite both this enormous cost and high demand for tissue and organ repair, therapies currently available are unable to fully restore tissues and organs. With an ageing population and increasing demand for organ and tissue replacement the emerging field of tissue engineering and regenerative medicine offers hope for a possible solution for many intractable clinical problems[2].
TISSUE ENGINEERING
Tissue engineering combines both biological sciences and engineering to develop treatments that restore, maintain or improve tissue function[1,3,4]. Though similar to regenerative medicine, an important distinction resides in the potential use of synthetic and semisynthetic materials in tissue engineering[4-6]. This separation can be better understood by considering the three major components of tissue engineering: Metabolically active cells[7], polymeric micro-carriers or scaffolds[8] and bioreactors to produce the tissue engineered construct for implantation[9].
The application of stem cells to tissue engineering applications has been a major recent advance in the field. Although a variety of stem cell types exist, including human embryonic cells and induced pluripotent stem cells, this review will focus on mesenchymal stem/stromal cells (MSCs). The potential for using MSCs for clinical purposes is an expanding area, for both their relative ease of acquisition and their versatility although many utilize their immunomodulatory and anti-inflammatory properties rather than generating new tissue[10-12]. Polymeric micro-carriers, hydrogels and scaffolds are essential components for supporting the reconstitution of damaged tissue. Seeding a scaffold with viable adult stem cells enables their differentiation into the cells desired when implanted into the body[13]. One key question in the tissue engineering field is the choice of polymer, particularly whether to use synthetic or biodegradable polymers. Bioreactors are generally defined as devices in which biological and/or biochemical processes for generating the tissue engineering construct are developed under closely monitored and tightly controlled environmental and operating conditions, i.e., Good Manufacturing Practice[14]. In modern tissue engineering, bioreactors are powerful tools to support and direct in vitro development of stem cell populations into functional tissues by simulating an appropriate biological, physical and mechanical environment. In essence, bioreactors are the means by which the desired tissue is generated in vitro and directed in its development for transplanting into the patient.
PELVIC ORGAN PROLAPSE
Pelvic organ prolapse (POP) is the herniation of pelvic organs into the vagina (Figure 1)[15,16]. Symptoms of POP include bowel and urinary incontinence, pain, voiding, bowel and sexual dysfunction, severely affecting the quality of life of affected women[17]. POP is a common condition, affecting approximately 25% of all women in the United States and Western countries, and is particularly prevalent in post-menopausal women. The main risk factor is vaginal birth and age. However, obesity is also a contributing factor, particularly in regard to POP recurrence[18]. Though not as well understood, a genetic predisposition to POP is a factor in some cases, particularly in genes regulating collagen and elastin synthesis in the pelvic floor and vaginal walls[19-21]. Given that the United States, Europe and Australia face increasing obesity rates and an aging population, the prevalence and severity of POP will only increase over the coming years. The economic and healthcare costs are considerable, approximating US$1 billion each year[22].
Figure 1.
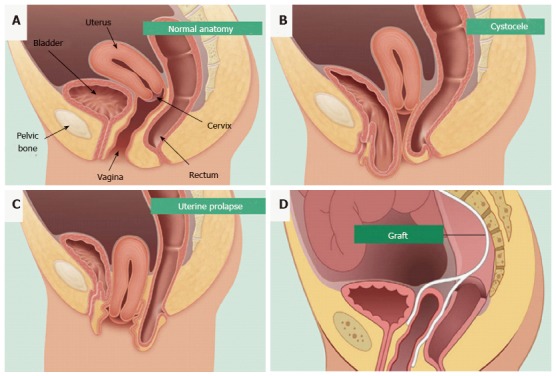
Pelvic organ prolapse mesh treatment. Normal pelvic anatomy (A) and herniation of the bladder (B) and uterus into the vagina (C). Synthetic mesh augmentation of vaginal walls as a colporrhaphy treatment for pelvic organ prolapse (D). Hysterectomies are also used to treat uterine prolapse (reproduced with permission from BARD medical).
Surgical reconstruction for treatment of POP
Currently the standard treatment for POP is native tissue repair conducted transvaginally (colporrhaphy) or abdominally (sacral colpopexy). This surgical treatment has a high failure rate with 30% of patients requiring one or more further surgeries due to recurrence of POP[23]. Additionally, reconstructive procedures in older women have complication rates from 15.5% to 33%, with the majority related to urinary tract infections, febrile morbidity and blood loss requiring transfusion[24]. Indeed, the mortality from urogynecological surgery increases with each decade of life, with the most common complications occurring in women 80 years or older[25].
The first generation of augmented treatments for POP involved the implantation of polypropylene mesh into the vaginal walls to alleviate the herniation and support the pelvic organs (Figure 1D)[26]. Mesh has been available since the 1950s for the repair of abdominal hernias[26]. Though successful for many women, up to 30% will require subsequent surgery while also enduring other complications such as fibrosis, mesh erosion into the vagina, bladder or bowel, chronic inflammation and mesh shrinkage[24,26,27]. This resulted in worldwide recalls of many of the leading brands of meshes for vaginal surgery, leaving women with fewer options for treatment once again.
CANDIDATE CELLS FOR TISSUE ENGINEERING APPLICATIONS FOR POP
Skeletal muscle derived stem cells
Skeletal muscle has been identified previously as a potential source of progenitor stem cells capable of differentiating into myogenic and osteogenic cell lineages in rat models[28-33]. The use of skeletal muscle stem cells to deliver gene therapy is being explored for treating muscular dystrophy and stress urinary incontinence, another pelvic floor disorder involving the urethra[28]. In addition, they are being used to regenerate both skeletal and cardiac muscle, bone and cartilage. As a potential source of cells for treating POP, muscle-derived stem cells (MDSC) are particularly attractive as they can now be isolated from human skeletal muscles and differentiated into skeletal myotubes, in vitro and in vivo[33]. In rat models MDSC have been used to treat fibrosis. The ability of MDSC to promote vaginal epithelial regeneration and vaginal wall repair in a rat model makes them candidates for treating POP[34]. However to avoid the risk of immune rejection from allogeneic sources, MDSC are better derived from the patient’s own muscle tissue. Such an autologous procedure is expensive and invasive, causing significant pain and morbidity for the patient. An alternative source of cells for POP treatment could prove more beneficial and practical for the patient.
Fibroblasts and myofibroblasts
As major producers of collagen and an essential cell for the formation of connective tissue, fibroblasts have also been suggested as an alternative cell source for POP treatment[35]. Vaginal myofibroblasts from nulliparous women have higher contractile strength compared to those from parous women, suggesting that vaginal delivery and overstretching of the vaginal wall affects myofibroblast function[36]. However, the use of autologous vaginal fibroblasts from patients for treating their pelvic floor disorders raises concerns about the quality of cells utilised. Other studies have observed that vaginal fibroblasts derived from prolapsed tissues have impaired function, such as delayed fibroblast-mediated collagen contraction and lower production of collagen synthesising enzymes[21]. This could be avoided if women have a vaginal biopsy to collect and cryopreserve fibroblasts before childbirth in order to obtain better quality cells, however long-term planning and storage facilities are not available to most women. The invasive method of acquiring human vaginal fibroblasts and subsequent morbidity is unfortunately an obstacle in their use as the main source of cells for a tissue engineering-based approach to treating POP.
Buccal mucosal fibroblasts (BMF), however offer a readily available and plentiful source of cells and could prove an alternative to human vaginal fibroblasts. BMF are harvested from the inside of the cheek lining and express the typical MSC/fibroblast surface markers but do not function as MSC[37]. They produce important components of the extracellular matrix, collagen I and elastin, both of which are required for strengthening the vaginal walls to alleviate and prevent herniation[35,38]. The interaction of BMF with various biodegradable scaffolds has been examined in vitro for potential treatment of PFDs including POP[38]. Although BMF offer a potential candidate for the treatment of POP, they currently remain untested for this purpose in animal models and their ultimate suitability remains unknown.
MSCs
MSC have been extensively used as cell-based therapies predominantly for their anti-inflammatory and immunomodulatory non-stem cell properties[39,40]. However they also have potential for tissue engineering purposes for regenerating new tissues or promoting the activity of endogenous stem cells[10,13,41]. MSC populations have the capacity for self-renewal, high proliferative potential and differentiate into a variety of mesodermal and other lineages[42]. Recent advances in cellular identification using more specific markers has shown that MSC can be extracted from most tissues including bone marrow, umbilical cord, placenta, adipose tissue and endometrium, although not all of these sources have demonstrated clonogenicity for their MSC populations[43-47]. Typically, MSC actively respond to stress or injury in a similar manner to the way cells of the innate immune system respond to pathogen exposure. When supplied systemically, exogenous MSCs home to sites of injury in response to inflammation[48]. Here MSCs operate in a paracrine manner secreting large amounts of diverse proteins, growth factors, cytokines and chemokines that promote a variety of effects including neo-angiogenesis, tissue regeneration and remodelling, immune cell activation, suppression of inflammation and cellular recruitment[13,41,49-51].
The potential of MSC as a cell-based therapy has recently been explored in numerous clinical applications. The ability to direct bone marrow MSC differentiation into other cell types and lineages has shown that these cells maintain a phenotype lacking tissue-specific characteristics until exposed to signals in damaged tissues[52]. MSC obtained from dental pulp have been used to repair related tissues such as periodontal ligament, dental papilla and dental follicle[53]. The ability of adipose tissue and bone marrow MSC to act as precursor cells has also been exploited by directing their differentiation toward the chondrogenic lineage in order to produce cartilage-synthesising chondrocytes[54]. Although MSC show promise as cell-based therapies, more understanding of their mechanism of action and utilising their potential is needed. Early use of MSC has not always met expectations, often producing inconsistent results[55]. This may be due to lesser refined methods of isolating and cultivating MSC resulting in the administration of fibroblasts and myofibroblasts rather than undifferentiated MSC[56]. Until recently, production of significant numbers of MSCs posed a challenge, as the regenerative potential of MSC declined during culture expansion[57,58], which is required due to the small numbers of perivascular MSC present within tissues[59]. For tissue engineering applications and tissue repair following ischaemia (e.g., cardiac muscle), local rather than systemic delivery is desirable and will likely result in greater local concentration of MSC at the desired tissue site, even when the mechanism of action is paracrine[60]. A further consideration is allogeneic vs autologous. Seeding MSC onto scaffolds, such as polyamide/gelatin (PA + G) for POP or poly-lactic-co-glycolic acid nano-fibers appears to produce better outcomes in preclinical studies[57,61]. MSCs are a versatile and promising stem/stromal cell which can be used for a variety of regenerative medicine applications. Additionally, MSC have greater capacity to regenerate tissues from which they are derived[39]. With this in mind, MSC obtained from the lining of the uterus could be useful in the development of treatments for other regions of the female reproductive tract, e.g., vaginal wall tissue in cases of POP.
ENDOMETRIUM AS A NOVEL SOURCE OF MSC
Regenerative potential of endometrium
The endometrial lining of the uterus serves as the site of embryo implantation, placentation and the development of the embryo and foetus during pregnancy[62]. The upper functional layer of the human endometrium undergoes extensive growth, differentiation and shedding each menstrual cycle under the influence of sex steroid hormone fluctuations[63]. Following menstruation, the remaining basal layer regenerates the new functional layer, which undergoes rapid cellular proliferation followed by differentiation (Figure 2). If an embryo does not implant, the terminally differentiated epithelium and stroma is shed during menstruation[64]. Much like the continuously renewing small intestinal mucosa, the endometrial mucosa undergoes many cycles of regeneration during a woman’s lifetime, indicative of its highly dynamic and regenerative capacity.
Figure 2.
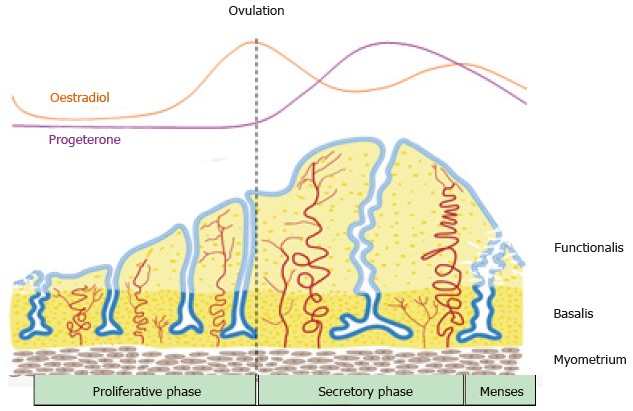
Schematic of changes in the human endometrium during the menstrual cycle, illustrating the growth, differentiation and shedding of the functionalis layer. The functionalis layer regenerates 4-10 mm during the proliferative phase (10 d) as cells proliferate in response to rising circulating estrogen levels. During the secretory phase, progesterone induces differentiation of the epithelium and stroma to generate an endometrium receptive to implantation of an embryo. This entire process occurs over 400 times during a woman’s reproductive life indicating the regenerative potential of human endometrium (reproduced from ref.[63] with permission).
Endometrial MSC
The existence of stem/progenitor cells within the endometrium and their role as progenitor cells for regenerating endometrial tissue has only recently been reported. Endometrial MSC (eMSC) are clonogenic, multipotent, differentiating into four mesodermal lineages: Osteoblasts, chondrocytes, smooth muscle cells and adipocytes in vitro (Figure 3) and expressing the typical pattern of MSC surface markers[44,65,66]. Endometrial side population (SP cells) also demonstrate MSC properties[67,68]. Serial clonal culture shows that clonogenic eMSC undergo self-renewal in vitro and have high proliferative potential[44]. The population of clonogenic eMSC within human endometrium is small approximating 1.3%, necessitating the identification of specific surface markers to allow their prospective isolation and enrichment from endometrial biopsies[69,70].
Figure 3.
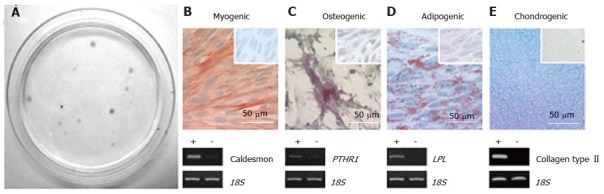
Endometrial mesenchymal stem cells. Clonogenic (A); and differentiate into 4 mesodermal lineages from a single clonogenic cell (B-E); myocytes (B); osteocytes (C); adipocytes (D); chondrocytes (reproduced from ref. [44] with permission) (E). PTHR1: Parathyroid hormone 1 receptor; LPL: Lipoprotein lipase.
Prospective isolation of eMSC
In order to exploit the regenerative ability of eMSC, they must first be isolated from the heterogeneous population of cells obtained from dissociated endometrial tissue. Ideally this requires the identification of unique surface markers on eMSC that will identify their in vivo niche and separate them from undesired stromal fibroblasts and other cells. Indeed several sets of specific surface markers have been identified on eMSC[70-73]. Almost all clonogenic human endometrial stromal cells with MSC properties are found in the CD140b+CD146+ population, comprising 1.5% of the stromal fraction[70]. These markers revealed a perivascular niche for eMSC adjacent to endothelial cells suggesting they are pericytes (Figure 4). The transcriptome of the co-expressing CD140b+CD146+ cells indicates they are distinct from CD140b-CD146+ endothelial cells, but more similar to endometrial CD140b+CD146- stromal fibroblasts[73]. To obtain these co-expressing cells, a flow cytometry sorter must be used, which limits the utility of this marker set, given the damaging effects of automated cell sorting on cell viability[70]. To overcome this problem a single perivascular marker was sought for isolating eMSC. The W5C5 antibody identified a population of perivascular endometrial stromal cells with typical MSC properties that also reconstituted stromal tissue in vivo when transplanted beneath the kidney capsule[72]. The W5C5+ cells comprised 4.4% of endometrial stromal cells. The epitope recognised by the W5C5 antibody is the Sushi Domain-containing 2 (SUSD2) adhesion molecule[74]. A single marker enables magnetic bead sorting, a gentler protocol than using a cell sorter as evidenced by increased clonogencity of SUSD2+ cells compared to CD140b+CD146+ cells[72]. TNAP (tissue non-specific alkaline phosphatase) is another single marker that identifies eMSC, but has less utility as the epitope is also expressed by endometrial epithelial cells[75]. Another perivascular marker (AOC3) identified by RNA sequencing SUSD2+ and SUSD2- cells may have utility for isolating eMSC[76], but the common bone marrow MSC marker Stro-1 does not enrich for endometrial stromal cells with MSC properties[69]. All these markers revealed that the perivascular eMSC were found in both the functionalis and basalis layers of human endometrium, indicating that eMSC will be found in menstrual blood and can be isolated from biopsies and curettage as well as hysterectomies[56,77].
Figure 4.
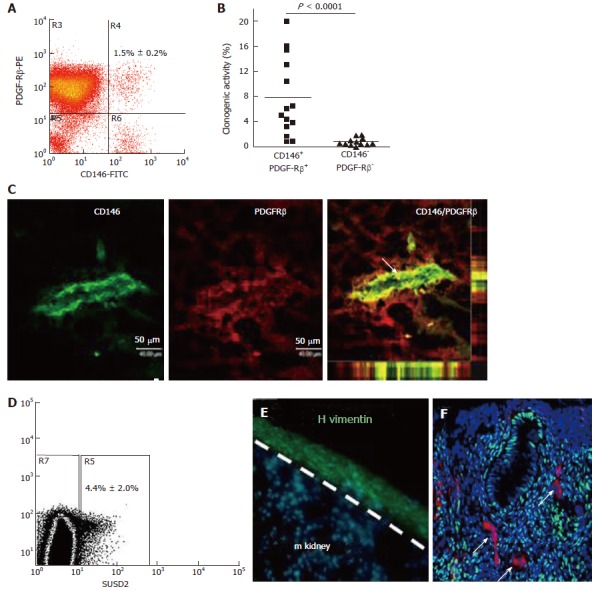
Specific enriching for endometrial mesenchymal stem cells. Flow cytometry plot of CD146+PDGFRB+ fraction (A) which contains most of the clonogenic stromal cells (B) and reveals their pericyte identity in vivo (C); SUSD2+ cells in endometrial cell suspensions (D) which E reconstitute human vimentin+ stromal tissue when transplanted under the kidney capsule of NSG mice, and F have a perivascular location in human endometrium. SUSD2+ cells (red) do not express estrogen receptor-α (green), but endometrial stromal cells do (DNA blue). The white arrow indicates perivascular SUSD2+ cells (reproduced ref. [70,72,78] with permission).
EMSC can also be obtained from post-menopausal women following short term (8 wk) estrogen replacement which regenerates their atrophic endometrial tissue[78]. Collection of menstrual blood or an endometrial biopsy are convenient sources not requiring anaesthesia, with the latter available as a simple office based procedure. Such tissue sources are ideal for cell-based therapies (Figure 5). Despite their great promise, eMSC and menstrual blood MSC have yet to be significantly explored as therapeutic agents for stem cells therapies. There are certain endometrial disorders where caution maybe required eg endometriosis. However this disorder affects young infertile women who will not have the opportunity to develop POP. Indeed, it will be important to ensure no underlying uterine or other pathology (e.g., malignant tumour) in identifying suitable patients for cell harvesting to treat their POP. For example, should a woman have uterine cancer, it would not be possible to use her eMSC for cell-based therapies. Similarly, it would also be contraindicated to use another source of autologous MSC in case tumour cells have spread to organs such as bone. These important issues should be considered in developing the potential of eMSC as cell-based therapies.
Figure 5.
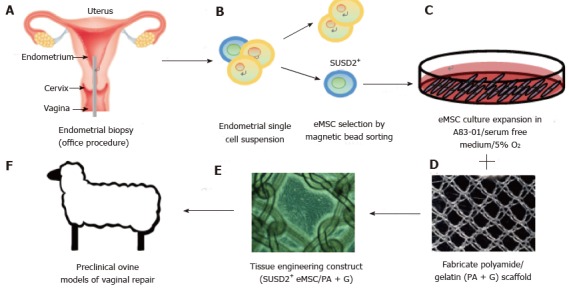
Isolation and application of e-mesenchymal stem cells in pelvic organ prolapse vaginal repair. (A) simple office based endometrial biopsies can be used to obtain patients’ tissues, which are dissociated, then (B) eMSC selected using SUSD2 magnetic bead sorting, followed by (C) culture expansion in A83-01/serum free medium in 5% O2 to generate large numbers of undifferentiated SUSD2+ eMSC (90%-95%) for (D) seeding onto fabricated scaffolds which will create an (E) eMSC/PA-G tissue engineering construct for implantation into (F) a large animal preclinical model to assess their efficacy in vaginal repair of parous ewes with evidence of POP (reproduced with permission from ref.[57,103] with permission). POP: Pelvic organ prolapse; MSC: Mesenchymal stem cells.
Large animal models are usually required to provide data for regulatory bodies prior to translating potential cell-based therapies into the clinic. If autologous applications are being evaluated, it becomes necessary to derive MSC from species such as ovine, porcine, canine, equine and non-human primates[79,80]. Often antibodies used as biomarkers to derive MSC from human or mouse do not cross react with these species. For example, neither CD140b, CD146 nor SUSD2 cross react with ovine endometrial tissue[81]. However, the bone marrow MSC surface marker CD271[82] cross reacts with ovine endometrial stromal cells enriching for eMSC demonstrating clonogenicity, in vitro self-renewal and the ability to differentiate into adipogenic, myogenic, osteogenic and chondrogenic lineages[81]. The CD271+ ovine eMSC were identified in a perivascular niche around arterioles and venules in vivo, but unlike human eMSC which have a pericyte location, ovine CD271+ stromal cells occupied the adventitia in the periphery of these vessels (Figure 6). In human bone marrow and adipose tissue, vascular adventitial cells show similar MSC properties as those located in the pericyte position[83].
Figure 6.
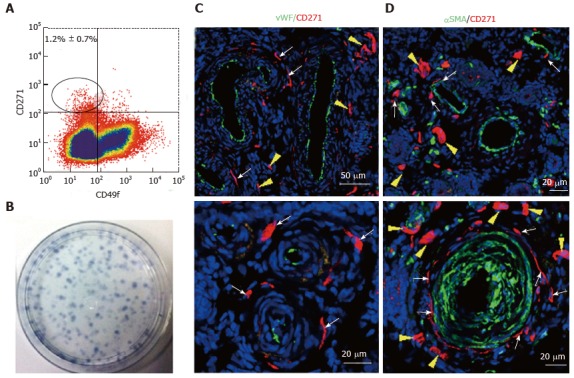
Specific markers for ovine e-mesenchymal stem cells. Flow cytometry plot of ovine endometrial cells immunolabelled with CD271 and CD49f antibodies (A). The CD271+CD49f- population enriches; Clonogenic stromal cells (B); Immunofluorescence images of ovine endometrium stained with CD271 (red) and vascular markers reveals their in vivo perivascular location in the adventitia of veins and arteries (C, D); vWF an endothelial marker (green), showing CD271+ cells are perivascular but not pericytes (C); αSMA, a perivascular marker (green) showing CD271+ cells located adjacent to αSMA+ cells in the adventitia of vessels rather than expressing αSMA themselves (D). White arrows: perivascular CD271+ cells; yellow arrows: CD271+ cells not associated with vessels (reproduced from ref.[81] with permission). vWF: Von Willebrand factor; αSMA: Alpha smooth muscle actin.
eMSC phenotype and gene profile
Cell fate decisions made by somatic stem cells to self-renew or undergo differentiation depends upon the cellular microenvironment or niche from signals emanating from cells and extracellular matrix that comprise this niche[84]. In this context, understanding both the extrinsic and intrinsic gene regulation pathways operating in undifferentiated eMSC and their more differentiated progeny could shed light on their function in endometrial regeneration. Gene expression profiling comparing purified endometrial cell populations of CD140b+CD146+ eMSC, CD140b+CD146- stromal fibroblasts and CD140b-CD146+ endothelial cells showed that eMSC differentially expressed 762 and 1518 genes, respectively[73]. The eMSC gene expression profile was typical of stem cells, showing upregulation of self-renewal genes of the TGFβ, FGF2, WNT, IGF and Hedgehog signalling pathways in comparison with the endometrial stromal fibroblasts and endothelial cells. The expression of SUSD2 was also elevated in the double positive eMSC population. G-protein coupled receptor- and cAMP-mediated signalling were also upregulated in the CD140b+CD146+ population, similar to genes involved in maintaining the undifferentiated state of bone marrow MSC. The CD140b+CD146+ population also showed upregulation of immunomodulatory and immunosuppressive genes[73]. eMSC displayed increased expression of Cyclin D1 (CCND1) which ensures their progression through the G1 phase of the cell cycle[73]. Gene profiling has confirmed human eMSC as pericytes, while RNA sequencing of cultured endometrial SUSD2+ and SUSD2- cells revealed 134 differentially expressed genes, with many of those in the SUSD2+ population characteristic of perivascular cells[76]. The in vivo differentiation pathway for eMSC is to decidualised perivascular cells and decidual cells of the endometrial stroma, a process mediated by the post-ovulation sex steroid hormone, progesterone, via production of cAMP. The perivascular location of eMSC in the spiral arterioles renders them well situated to participate in the regeneration of the uterine lining and formation of the placenta during embryo implantation and subsequent pregnancy[76].
Tissue engineering for POP repair
Given the problem associated with mesh implantation for POP repair, and the need for physical support, a tissue engineering approach may provide a more durable treatment. The ideal treatment for POP would be an implantable autograft that alleviated herniation and regenerated the damaged tissue within the vaginal wall.
In vitro studies
For a cell based treatment to be practical, methods for procuring and expanding the necessary cells need to be developed. Culturing and expanding eMSC in vitro has been optimised in serum-free conditions, showing that fibronectin is the optimal substrate for attachment[85]. Additionally, hypoxic conditions of 5% O2 increased the proliferation rate and yield of eMSC, whilst maintaining multipotency and their expression of CD140b, CD146 and SUSD2. Culturing eMSC on a polyamide/gelatin composite scaffold with exogenous TGFβ1 and PDGF-BB induced their differentiation into smooth muscle cells expressing SM22α and SM-myosin heavy chain[86]. Incubation with connective tissue growth factor induced the eMSC to differentiate into collagen-producing fibroblasts. The differentiated smooth muscle cells and fibroblasts no longer expressed the eMSC marker SUSD2, confirming their differentiation into these desired cell types for POP repair[86]. Although these in vitro studies show promise, it is also essential to confirm smooth muscle and fibroblast differentiation in vivo to gain mechanistic understanding prior to transferring this technology into clinical applications.
Methodology has now been developed for culture expansion of eMSC in serum free medium containing A83-01, a TGFβ1 receptor inhibitor, that maintains eMSC stemness and SUSD2 phenotype[87]. TGFβ1-mediated apoptosis and senescence is prevented and proliferation promoted in A83-01-treated eMSC cultures maintaining the percentage of SUSD2+ cells to more than 90% for all samples. This effect of A83-01 is mediated via Smad2/3 phosphorylation. A83-01 treated eMSC are more clonogenic than untreated control cells and retain their MSC properties[87]. A major advantage of this culture method is that a reproducible percentage of SUSD2+ eMSC is achievable for all patient samples, an important consideration for scale out culture expansion of autologous cells.
In vivo studies
As outlined earlier there are substantial problems with current mesh augmentation of POP surgery. The use of autografts increases morbidity at the donor tissue site, biological materials often fail due to their rapid and unpredictable degradation[16], and the synthetic PP mesh currently used is biomechanically too stiff and often erodes into adjacent organs[56]. A better solution may be to combine the advantages of both the synthetic and biological approaches. This could utilise a synthetic mesh as a scaffold to not only support the prolapsed tissue but also provide a vehicle upon which to seed eMSC for delivery to sites of vaginal damage[26,88]. The eMSC could serve by modulating the inflammatory and immune responses and perhaps more importantly incorporating into the vaginal wall to regenerate the lost or damaged tissue or promoting endogenous stem cell populations to initiate repair which mesh alone cannot do.
Small animal rodent models
Recent efforts to test this possibility show potential utility. A non-degradable, polyamide (PA) mesh with biomechanical properties more closely matching vaginal tissue was coated with gelatin[88] to provide a substrate for seeding with SUSD2+ eMSC. This tissue engineering construct was then implanted into a fascial defect on the dorsum of immunocompromised rats and assessed following necropsy at several time points over 90 d[57]. In the explanted eMSC/PA + G tissue complexes, greater neovascularisation was observed early on at 7 d compared with PA + G controls. Initially there was a greater influx of M1 inflammatory macrophages around the eMSC-seeded mesh. At 60 d these macrophages had changed to a M2 wound healing phenotype and by 90 d there were fewer CD68+ macrophages around the cell-seeded PA + G filaments in comparison to PA + G alone, indicating a milder chronic inflammatory response in the long term. Importantly in these studies the cellular response at the mesh interface was assessed quantitatively in 50 μm increments around individual filaments using image analysis rather than subjective scoring[57,88]. Similar quantities of new collagen were generated around the PA + G mesh filaments, irrespective of the inclusion of eMSC, which was derived from rat fibroblasts rather than derivatives of the implanted human eMSC. However, this new collagen around the eMSC/PA + G mesh filaments showed physiological crimping by scanning electron microscopy, while more scar-like collagen was deposited around the PA + G mesh without eMSC[89]. This deposition of physiological collagen likely contributed to the improved biomechanical properties of the mesh/tissue complexes harvested at 90 d, where a longer toe region and lower stiffness was observed in the stress strain curves of the cell-seeded PA + G mesh compared with PA + G alone (Figure 7)[57]. The improved tissue organisation around the mesh filaments shown by histological staining suggests that eMSC promoted tissue regeneration and improved the biocompatibility of the synthetic PA + G mesh[57,89]. In this xenogeneic model, the eMSC survived a maximum of 14 d indicating that they exerted a paracrine effect in promoting vascularisation and reducing fibrosis similar to MSC effects on many other tissues[13]. However the percentage of SUSD2+ cells in the single sample of passage 6 cells used for the entire study was only 10%. It will be of interest to determine whether more than a paracrine effect will be observed if > 90% of the cells are SUSD2+, now a possibility by culturing them in A83-01-containing medium[87].
Figure 7.
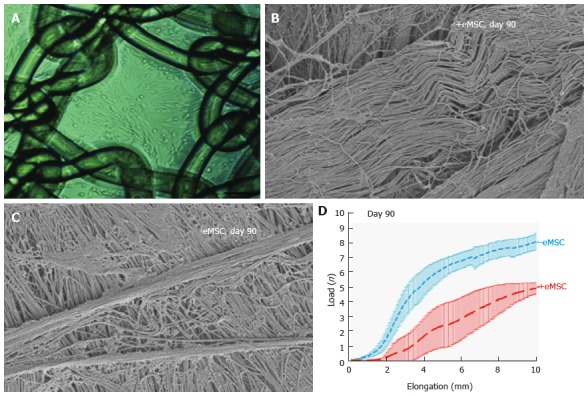
Human e-mesenchymal stem cells improves the biocompatibility of polyamide/gelatin (PA + G) mesh in a fascial wound defect in nude rats. PA + G mesh seeded with 100000 eMSC/cm2 and cultured for 48 h, prior to implantation (A); Physiological crimped collagen deposited around eMSC+/PA + G mesh (B); Scar-like collagen in PA + G mesh alone as observed by SEM (C); Load-elongation curves of explanted meshes with (red) and without (blue) eMSC showing less stiffness (slope) and longer toe region for mesh seeded with eMSC, indicating improved biomechanical properties (reproduced from ref.[57,86,89] with permission) (D). MSC: Mesenchymal stem cells; PA : Polyamide; G: Gelatin.
Despite the significant biological differences between human females and rodents, mouse models have proven invaluable for the investigation of the underlying biochemical mechanisms involved in the development of POP. The use of genetically modified mice has allowed exploration of the genetic underpinnings of POP, such as lysyl oxidase like-1 (LOXL1), an enzyme involved in elastin biosynthesis within vaginal tissue walls, and fibulin 5 (FBLN5) which regulates expression of collagen and elastin. Depletion of either LOXL1 or FBLN5 has been associated with POP[20,90]. The LOXL1 deficient mouse creates a POP-like condition where the mice develop an obvious bulge in the perineal region. It would be of interest to determine if an injectable MSC-based cell therapy alleviates the prolapse symptoms of LOXL-1 deficient mice. While extremely useful for investigating genetic contribution, transgenic mice are limited in their utility as models for exploring tissue engineering based treatments for POP due to the small size of their vagina.
Large animal preclinical models
Of the large animal models available for assessing cell-based therapies for POP, the domestic sheep is the most promising candidate due to their ready availability and physiological similarity to the human female pelvis in size and structure. Ewes also have a similar oestrus cycle of 17 d, a long labour and deliver a foetus with a large head to body ratio that is closer to humans than rodents[91,92]. Like humans, ewes undergo spontaneous POP with similar frequency and predisposing factors, such as parity, age and breeds with a large rump[91,93]. Although the ovine species are quadrupeds with a horizontal rather than vertical pelvic floor subject to differing forces, the overall arrangement of the pelvic organs and the similar vaginal dimensions to women make them a useful model for assessing new mesh and tissue engineering constructs[56]. Additionally, the ovine vagina has a similar histological structure, biochemical and biomechanical properties to that of women. Finally, the most common form of prolapse in sheep involves the bladder (cystocele) as it is for women Sheep have already been vaginally implanted with various POP mesh materials for evaluation of their efficacy and adverse effects in female pelvic reconstructive surgery[16,92,94,95]. The biochemical and biomechanical properties of ovine vaginal tissue has already been examined by quantitative histological imaging, biochemical collagen/GAG/elastin assays and biomechanical analyses, providing a platform for the evaluation of next generation eMSC-seeded mesh in the ovine vaginal repair model[96,97]. It is now possible to evaluate autologous eMSC since methods have been developed for obtaining MSC from the ovine bone marrow[79] and endometrium (Figure 5)[81].
Additional large animal models for assessing cell-based therapies for POP surgery include cows, pigs and non-human primates. Cows develop prolapse with similar predisposition and frequency to humans and sheep[98], however their purchase, handling and agistment costs make them a less practical model. Pigs are a common preclinical model for various clinical conditions but their foetuses do not have the large head-to body-ratio responsible for inducing spontaneous POP in the ovine model, reducing their utility[99]. Non-human primates such as Rhesus macaques and squirrel monkeys offer useful animal models due to a similar pelvic anatomy to humans and their more upright posture[100,101]. Furthermore, the Rhesus species develop spontaneous POP. Non-human primates have been used for assessing new POP meshes and for investigating the mechanism of action of their deleterious effects[27,102]. However ethical limitations, prohibitive cost of handling and necessary specialist expertise limit their availability for many investigators. Despite these limitations, assessment of tissue engineering constructs in the macaque model, particularly in retired breeders with evidence of POP, might provide the ultimate model of postmenopausal women with POP in which to assess a cell-based therapy. However it would be necessary to develop methods for obtaining MSC populations from the macaque species, for both autologous and allogeneic use.
CONCLUSION
POP is a common hidden disease burden for large numbers of women. Compounding this burden is the inadequacies of current surgical treatments with or without mesh. To overcome this clinical challenge, recent advances in cellular phenotyping and gene profiling suggest endometrial MSC as a possible compliment to mesh-based POP treatment. The eMSC capacity for regenerating tissue is exemplified during a woman’s reproductive life, where they regenerate at least one centimetre of endometrial lining each menstrual cycle for over 400 menstrual cycles. By seeding eMSC onto polyamide/gelatin composite mesh and implanting into vaginal walls, it may be possible to favourably modulate the innate immune response and accelerate organised tissue rehabilitation. That the first attempt at combining eMSC and mesh to treat a fascial defect has been successful using rodent models is encouraging, suggesting that further development of this approach using the ovine model is warranted.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 4, 2015
First decision: November 30, 2015
Article in press: February 16, 2016
P- Reviewer: Bussolati B, Sacco E, Tsai EM, Tan BK S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 3.Freed LE, Langer R, Martin I, Pellis NR, Vunjak-Novakovic G. Tissue engineering of cartilage in space. Proc Natl Acad Sci USA. 1997;94:13885–13890. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel G. Tissue engineering. Mending the youngest hearts. Science. 2011;333:1088–1089. doi: 10.1126/science.333.6046.1088. [DOI] [PubMed] [Google Scholar]
- 5.Marx V. Tissue engineering: Organs from the lab. Nature. 2015;522:373–377. doi: 10.1038/522373a. [DOI] [PubMed] [Google Scholar]
- 6.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354 Suppl 1:SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 7.Buehrer BM, Cheatham B. Isolation and characterization of human adipose-derived stem cells for use in tissue engineering. Methods Mol Biol. 2013;1001:1–11. doi: 10.1007/978-1-62703-363-3_1. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Pérez RA, Jin GZ, Choi SJ, Kim HW, Wall IB. Microcarriers designed for cell culture and tissue engineering of bone. Tissue Eng Part B Rev. 2013;19:172–190. doi: 10.1089/ten.TEB.2012.0432. [DOI] [PubMed] [Google Scholar]
- 9.Yeatts AB, Geibel EM, Fears FF, Fisher JP. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol Bioeng. 2012;109:2381–2391. doi: 10.1002/bit.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 11.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 13.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sensebé L, Gadelorge M, Fleury-Cappellesso S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: a review. Stem Cell Res Ther. 2013;4:66. doi: 10.1186/scrt217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLancey JO. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am J Obstet Gynecol. 2005;192:1488–1495. doi: 10.1016/j.ajog.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Deprest J, Zheng F, Konstantinovic M, Spelzini F, Claerhout F, Steensma A, Ozog Y, De Ridder D. The biology behind fascial defects and the use of implants in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17 Suppl 1:S16–S25. doi: 10.1007/s00192-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 17.Claes L. The mechanical and morphological properties of bone beneath internal fixation plates of differing rigidity. J Orthop Res. 1989;7:170–177. doi: 10.1002/jor.1100070203. [DOI] [PubMed] [Google Scholar]
- 18.Diez-Itza I, Aizpitarte I, Becerro A. Risk factors for the recurrence of pelvic organ prolapse after vaginal surgery: a review at 5 years after surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1317–1324. doi: 10.1007/s00192-007-0321-0. [DOI] [PubMed] [Google Scholar]
- 19.Aboushwareb T, McKenzie P, Wezel F, Southgate J, Badlani G. Is tissue engineering and biomaterials the future for lower urinary tract dysfunction (LUTD)/pelvic organ prolapse (POP) Neurourol Urodyn. 2011;30:775–782. doi: 10.1002/nau.21101. [DOI] [PubMed] [Google Scholar]
- 20.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol. 2008;198:590.e1–590.e6. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Zapata AM, Kerkhof MH, Zandieh-Doulabi B, Brölmann HA, Smit TH, Helder MN. Functional characteristics of vaginal fibroblastic cells from premenopausal women with pelvic organ prolapse. Mol Hum Reprod. 2014;20:1135–1143. doi: 10.1093/molehr/gau078. [DOI] [PubMed] [Google Scholar]
- 22.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–651. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 23.Stanford EJ, Cassidenti A, Moen MD. Traditional native tissue versus mesh-augmented pelvic organ prolapse repairs: providing an accurate interpretation of current literature. Int Urogynecol J. 2012;23:19–28. doi: 10.1007/s00192-011-1584-z. [DOI] [PubMed] [Google Scholar]
- 24.Gerten KA, Markland AD, Lloyd LK, Richter HE. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111–2118. doi: 10.1016/j.juro.2008.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung VW, Weitzen S, Sokol ER, Rardin CR, Myers DL. Effect of patient age on increasing morbidity and mortality following urogynecologic surgery. Am J Obstet Gynecol. 2006;194:1411–1417. doi: 10.1016/j.ajog.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Wein AJ. Re: Implications of the FDA statement on transvaginal placement of mesh: the aftermath. J Urol. 2015;193:606–607. doi: 10.1016/j.juro.2014.11.070. [DOI] [PubMed] [Google Scholar]
- 27.Feola A, Abramowitch S, Jallah Z, Stein S, Barone W, Palcsey S, Moalli P. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG. 2013;120:224–232. doi: 10.1111/1471-0528.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr LK, Robert M, Kultgen PL, Herschorn S, Birch C, Murphy M, Chancellor MB. Autologous muscle derived cell therapy for stress urinary incontinence: a prospective, dose ranging study. J Urol. 2013;189:595–601. doi: 10.1016/j.juro.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med. 2015;21:854–862. doi: 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9:642–647. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 31.van der Schaft DW, van Spreeuwel AC, Boonen KJ, Langelaan ML, Bouten CV, Baaijens FP. Engineering skeletal muscle tissues from murine myoblast progenitor cells and application of electrical stimulation. J Vis Exp. 2013;(73):e4267. doi: 10.3791/4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tare RS, Kanczler J, Aarvold A, Jones AM, Dunlop DG, Oreffo RO. Skeletal stem cells and bone regeneration: translational strategies from bench to clinic. Proc Inst Mech Eng H. 2010;224:1455–1470. doi: 10.1243/09544119JEIM750. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Wilschut KJ, Kouklis G, Tian H, Hesse R, Garland C, Sbitany H, Hansen S, Seth R, Knott PD, et al. Human Satellite Cell Transplantation and Regeneration from Diverse Skeletal Muscles. Stem Cell Reports. 2015;5:419–434. doi: 10.1016/j.stemcr.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho MH, Heydarkhan S, Vernet D, Kovanecz I, Ferrini MG, Bhatia NN, Gonzalez-Cadavid NF. Stimulating vaginal repair in rats through skeletal muscle-derived stem cells seeded on small intestinal submucosal scaffolds. Obstet Gynecol. 2009;114:300–309. doi: 10.1097/AOG.0b013e3181af6abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapple C, Osman N, MacNeil S. Developing tissue-engineered solutions for the treatment of extensive urethral strictures. Eur Urol. 2013;63:539–541. doi: 10.1016/j.eururo.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 36.Meyer S, Achtari C, Hohlfeld P, Juillerat-Jeanneret L. The contractile properties of vaginal myofibroblasts: is the myofibroblasts contraction force test a valuable indication of future prolapse development. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1399–1403. doi: 10.1007/s00192-008-0643-6. [DOI] [PubMed] [Google Scholar]
- 37.Roman S, Mangera A, Osman NI, Bullock AJ, Chapple CR, MacNeil S. Developing a tissue engineered repair material for treatment of stress urinary incontinence and pelvic organ prolapse-which cell source. Neurourol Urodyn. 2014;33:531–537. doi: 10.1002/nau.22443. [DOI] [PubMed] [Google Scholar]
- 38.Mangera A, Bullock AJ, Roman S, Chapple CR, MacNeil S. Comparison of candidate scaffolds for tissue engineering for stress urinary incontinence and pelvic organ prolapse repair. BJU Int. 2013;112:674–685. doi: 10.1111/bju.12186. [DOI] [PubMed] [Google Scholar]
- 39.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 41.James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, Nguyen A, Megerdichian S, Asatrian G, Pang S, et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. 2012;1:673–684. doi: 10.5966/sctm.2012-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caplan AI. Why are MSCs therapeutic New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 44.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadekar D, Kale V, Limaye L. Differential ability of MSCs isolated from placenta and cord as feeders for supporting ex vivo expansion of umbilical cord blood derived CD34(+) cells. Stem Cell Res Ther. 2015;6:201. doi: 10.1186/s13287-015-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Rustad KC, Gurtner GC. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv Wound Care (New Rochelle) 2012;1:147–152. doi: 10.1089/wound.2011.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E. Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J. 2011;22:91–98. doi: 10.1590/s0103-64402011000200001. [DOI] [PubMed] [Google Scholar]
- 50.Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy. 2006;8:559–561. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 51.Wong SP, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther. 2015;151:107–120. doi: 10.1016/j.pharmthera.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013;2013:132642. doi: 10.1155/2013/132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther. 2013;13:1387–1400. doi: 10.1517/14712598.2013.826187. [DOI] [PubMed] [Google Scholar]
- 57.Ulrich D, Edwards SL, Su K, Tan KS, White JF, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A. 2014;20:785–798. doi: 10.1089/ten.tea.2013.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 59.Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 60.Katare R, Riu F, Rowlinson J, Lewis A, Holden R, Meloni M, Reni C, Wallrapp C, Emanueli C, Madeddu P. Perivascular delivery of encapsulated mesenchymal stem cells improves postischemic angiogenesis via paracrine activation of VEGF-A. Arterioscler Thromb Vasc Biol. 2013;33:1872–1880. doi: 10.1161/ATVBAHA.113.301217. [DOI] [PubMed] [Google Scholar]
- 61.Isakova IA, Lanclos C, Bruhn J, Kuroda MJ, Baker KC, Krishnappa V, Phinney DG. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS One. 2014;9:e87238. doi: 10.1371/journal.pone.0087238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 63.Gargett CE, Chan RW, Schwab KE. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288:22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 64.Gargett CE. Uterine stem cells: what is the evidence. Hum Reprod Update. 2007;13:87–101. doi: 10.1093/humupd/dml045. [DOI] [PubMed] [Google Scholar]
- 65.Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P, Zlatkov V, Kehayov I, Kyurkchiev S. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135:551–558. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- 66.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 67.Cervelló I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, Critchley HO, Simón C. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6:e21221. doi: 10.1371/journal.pone.0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H, et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One. 2010;5:e10387. doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23:934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 70.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 71.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 72.Masuda H, Anwar SS, Bühring HJ, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012;21:2201–2214. doi: 10.3727/096368911X637362. [DOI] [PubMed] [Google Scholar]
- 73.Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, Meyer M, Tamaresis JS, Hamilton AE, Irwin JC, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sivasubramaniyan K, Harichandan A, Schumann S, Sobiesiak M, Lengerke C, Maurer A, Kalbacher H, Bühring HJ. Prospective isolation of mesenchymal stem cells from human bone marrow using novel antibodies directed against Sushi domain containing 2. Stem Cells Dev. 2013;22:1944–1954. doi: 10.1089/scd.2012.0584. [DOI] [PubMed] [Google Scholar]
- 75.Sobiesiak M, Sivasubramaniyan K, Hermann C, Tan C, Orgel M, Treml S, Cerabona F, de Zwart P, Ochs U, Müller CA, et al. The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev. 2010;19:669–677. doi: 10.1089/scd.2009.0290. [DOI] [PubMed] [Google Scholar]
- 76.Murakami K, Lee YH, Lucas ES, Chan YW, Durairaj RP, Takeda S, Moore JD, Tan BK, Quenby S, Chan JK, et al. Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology. 2014;155:4542–4553. doi: 10.1210/en.2014-1370. [DOI] [PubMed] [Google Scholar]
- 77.Schüring AN, Schulte N, Kelsch R, Röpke A, Kiesel L, Götte M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril. 2011;95:423–426. doi: 10.1016/j.fertnstert.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 78.Ulrich D, Tan KS, Deane J, Schwab K, Cheong A, Rosamilia A, Gargett CE. Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum Reprod. 2014;29:1895–1905. doi: 10.1093/humrep/deu159. [DOI] [PubMed] [Google Scholar]
- 79.Gronthos S, McCarty R, Mrozik K, Fitter S, Paton S, Menicanin D, Itescu S, Bartold PM, Xian C, Zannettino AC. Heat shock protein-90 beta is expressed at the surface of multipotential mesenchymal precursor cells: generation of a novel monoclonal antibody, STRO-4, with specificity for mesenchymal precursor cells from human and ovine tissues. Stem Cells Dev. 2009;18:1253–1262. doi: 10.1089/scd.2008.0400. [DOI] [PubMed] [Google Scholar]
- 80.Rozemuller H, Prins HJ, Naaijkens B, Staal J, Bühring HJ, Martens AC. Prospective isolation of mesenchymal stem cells from multiple mammalian species using cross-reacting anti-human monoclonal antibodies. Stem Cells Dev. 2010;19:1911–1921. doi: 10.1089/scd.2009.0510. [DOI] [PubMed] [Google Scholar]
- 81.Letouzey V, Tan KS, Deane JA, Ulrich D, Gurung S, Ong YR, Gargett CE. Isolation and characterisation of mesenchymal stem/stromal cells in the ovine endometrium. PLoS One. 2015;10:e0127531. doi: 10.1371/journal.pone.0127531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bühring HJ, Treml S, Cerabona F, de Zwart P, Kanz L, Sobiesiak M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann N Y Acad Sci. 2009;1176:124–134. doi: 10.1111/j.1749-6632.2009.04564.x. [DOI] [PubMed] [Google Scholar]
- 83.Crisan M, Corselli M, Chen WC, Péault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 85.Rajaraman G, White J, Tan KS, Ulrich D, Rosamilia A, Werkmeister J, Gargett CE. Optimization and scale-up culture of human endometrial multipotent mesenchymal stromal cells: potential for clinical application. Tissue Eng Part C Methods. 2013;19:80–92. doi: 10.1089/ten.tec.2011.0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su K, Edwards SL, Tan KS, White JF, Kandel S, Ramshaw JA, Gargett CE, Werkmeister JA. Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater. 2014;10:5012–5020. doi: 10.1016/j.actbio.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 87.Gurung S, Werkmeister JA, Gargett CE. Inhibition of Transforming Growth Factor-β Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells. Sci Rep. 2015;5:15042. doi: 10.1038/srep15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ulrich D, Edwards SL, White JF, Supit T, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE. A preclinical evaluation of alternative synthetic biomaterials for fascial defect repair using a rat abdominal hernia model. PLoS One. 2012;7:e50044. doi: 10.1371/journal.pone.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edwards SL, Ulrich D, White JF, Su K, Rosamilia A, Ramshaw JA, Gargett CE, Werkmeister JA. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater. 2015;13:286–294. doi: 10.1016/j.actbio.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 90.Takacs P, Nassiri M, Viciana A, Candiotti K, Fornoni A, Medina CA. Fibulin-5 expression is decreased in women with anterior vaginal wall prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:207–211. doi: 10.1007/s00192-008-0757-x. [DOI] [PubMed] [Google Scholar]
- 91.Couri BM, Lenis AT, Borazjani A, Paraiso MF, Damaser MS. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol. 2012;7:249–260. doi: 10.1586/eog.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krause H, Goh J. Sheep and rabbit genital tracts and abdominal wall as an implantation model for the study of surgical mesh. J Obstet Gynaecol Res. 2009;35:219–224. doi: 10.1111/j.1447-0756.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura A, Osonoi T, Terauchi Y. Relationship between urinary sodium excretion and pioglitazone-induced edema. J Diabetes Investig. 2010;1:208–211. doi: 10.1111/j.2040-1124.2010.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feola A, Endo M, Urbankova I, Vlacil J, Deprest T, Bettin S, Klosterhalfen B, Deprest J. Host reaction to vaginally inserted collagen containing polypropylene implants in sheep. Am J Obstet Gynecol. 2015;212:474.e1–474.e8. doi: 10.1016/j.ajog.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 95.de Tayrac R, Alves A, Thérin M. Collagen-coated vs noncoated low-weight polypropylene meshes in a sheep model for vaginal surgery. A pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:513–520. doi: 10.1007/s00192-006-0176-9. [DOI] [PubMed] [Google Scholar]
- 96.Ulrich D, Edwards SL, Letouzey V, Su K, White JF, Rosamilia A, Gargett CE, Werkmeister JA. Regional variation in tissue composition and biomechanical properties of postmenopausal ovine and human vagina. PLoS One. 2014;9:e104972. doi: 10.1371/journal.pone.0104972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ulrich D, Edwards SL, Alexander DLJ, Rosamilia A, Werkmeister JA, Gargett CE, Letouzey V. Changes in pelvic organ prolapse mesh mechanical properties following implantation in rats. Am J Obstet Gynecol. 2016;214:260.e1–e8. doi: 10.1016/j.ajog.2015.08.071. [DOI] [PubMed] [Google Scholar]
- 98.Gardner IA, Reynolds JP, Risco CA, Hird DW. Patterns of uterine prolapse in dairy cows and prognosis after treatment. J Am Vet Med Assoc. 1990;197:1021–1024. [PubMed] [Google Scholar]
- 99.Finegold AA, Schafer WR, Rine J, Whiteway M, Tamanoi F. Common modifications of trimeric G proteins and ras protein: involvement of polyisoprenylation. Science. 1990;249:165–169. doi: 10.1126/science.1695391. [DOI] [PubMed] [Google Scholar]
- 100.Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144 Suppl 1:S146–S158. doi: 10.1016/j.ejogrb.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 101.Otto LN, Slayden OD, Clark AL, Brenner RM. The rhesus macaque as an animal model for pelvic organ prolapse. Am J Obstet Gynecol. 2002;186:416–421. doi: 10.1067/mob.2002.121723. [DOI] [PubMed] [Google Scholar]
- 102.Liang R, Abramowitch S, Knight K, Palcsey S, Nolfi A, Feola A, Stein S, Moalli PA. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG. 2013;120:233–243. doi: 10.1111/1471-0528.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edwards SL, Werkmeister JA, Rosamilia A, Ramshaw JA, White JF, Gargett CE. Characterisation of clinical and newly fabricated meshes for pelvic organ prolapse repair. J Mech Behav Biomed Mater. 2013;23:53–61. doi: 10.1016/j.jmbbm.2013.04.002. [DOI] [PubMed] [Google Scholar]


