Abstract
We propose the main phenotypic characteristics and the complete genome sequence and annotation of Planococcus massiliensis strain ES2T (= CSUR P1103 = DSM 28915), the type strain of P. massiliensis sp. nov., isolated from a faeces sample collected from a healthy Senegalese man. It is an aerobic, Gram-positive, moderately halophilic, motile and rod-shaped bacterium. The 3 357 017 bp long genome exhibits a G+C content of 46.0% and contains 3357 protein-coding genes and 48 RNA genes.
Keywords: Culturomics, genome, human gut microbiota, Planococcus massiliensis, taxonogenomics
Introduction
Planococcus massiliensis strain ES2T (= CSUR P1103 = DSM 28915) is the type strain of P. massiliensis sp. nov. This bacterium was isolated from a stool sample of a healthy Senegalese man. This isolation is part of a wider culturomics study, with an aim to maximize the culture conditions to explore the human microbiota in depth [1]. For this project, several hundred samples from healthy individuals, antibiotic-treated individuals, or people with, for example, obesity, anorexia nervosa, or malnutrition were analysed by culturomics in order to extend our knowledge of gut microbes [2]. In this case, we created media containing a high salt concentration in order to cultivate halophilic microorganisms [2]. Furthermore, the availability of genomic data for many bacterial species [3] inspired us to propose a new concept for the description of new species of bacteria by adding proteomic information obtained by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) [4] and genomic analyses [5]. This concept changes the current methods of defining a new bacterial species, which are based on the genetic, phenotypic and chemotaxonomic criteria that are not reproducible and cannot be applied to all the bacterial genus [6], [7], [8].
Here we present a summary classification and a set of features for the type strain Planococcus massiliensis sp. nov. strain ES2T, together with the description of the complete genomic sequence and its annotation. These characteristics support the circumscription of the species Planococcus massiliensis. To our knowledge, Planococcus massiliensis is the first representative member of the genus of Planococcus isolated from a human subject. To date, 17 recognized species are representatives of Planococcus (P. alkanoclasticus, P. antarcticus, P. citreus, P. columbae, P. donghaensis, P. halocryophilus, P. kocurii, P. maitriensis, P. maritimus, P. mcmeekini, P. okeanokoites, P. plakortidis, P. psychrophylus, P. rifietoensis, P. salinarum, P. stackebrandtii) (http://www.bacterio.net). All these species are Gram-positive, aerobic cocci that are able to grow at moderately low temperatures and high salt concentrations, and have been predominantly isolated from saline environments [9], [10].
Material and Methods
Sample collection and culture conditions
Signed informed consent was obtained from each person included in the study. The study and the assent procedure were approved by the National Committee of Senegal and the ethics committee of Federative Research Institute 48 (Faculty of Medicine, Marseille, France) under agreement 09-022. The sample was obtained from a native Senegalese man living in Ndiop, a rural village in the Guinean–Sudanese zone in Senegal. The specimen was collected in sterile plastic containers, formed into aliquots and stored at −80°C until use. For each sample, pH and salinity were systematically determined with a pH meter (ThermoFisher Scientific, Saint Aubin, France) and a digital refractometer (ThermoFisher Scientific) before any analysis was performed. Then it was cultured in a liquid Colombia broth culture medium (Sigma-Aldrich, Saint-Quentin Fallavier, France) modified by adding (per litre): 5 g MgCl2 6H2O; 5 g MgSO4 7H2O; 2 g KCl; 1 g CaCl2 2H2O; 0.5 g NaBr; 0.5 g NaHCO3; 2 g glucose; and 100 g NaCl. pH was adjusted to 7.5 with 10 M NaOH.
Strain identification by MALDI-TOF and sequencing of 16S rRNA
MALDI-TOF analysis of proteins was used for the identification of bacteria. Each colony was deposited in duplicate on a MALDI-TOF MSP96 target and covered with 1.5 μL of a matrix solution (saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile, 2.5% trifluoroacetic acid) to allow crystallisation of molecules. MALDI-TOF was performed using the LT Microflex spectrometer (Bruker Daltonics, Leipzig, Germany). All spectra were recorded in positive linear mode for the mass range from 2000 to 20 000 Da (parameters: ion source 1 (ISI), 20 kV; IS2, 18.5 kV lens, 7 kV). The generated spectra were then compared to the Bruker database, which was supplemented with the new species found through the culturomics project. The resulting score enabled the identification or not of tested species: a score of ≥2 with a validly published species enables identification at the species level, a score of ≥1.7 but <2 enables identification at the genus level and a score of <1.7 does not enable any identification.
After three assays, unidentified colonies were sequenced using 16S rRNA for formal identification. Isolated colonies were suspended in 200 μL distilled water for DNA extraction using an EZ1 DNA Tissue Kit (Qiagen, Venlo, Netherlands). The amplification of the 16S rRNA was performed by a standard PCR using the universal primer pair FD1 5′-AGAGTTTGATCCTGGCTCAG-3′ and RP2 5′-ACGGCTACCTTGTTACGACTT-3′ [11]. The PCR product was purified and sequenced using the Big Dye Terminator Sequencing kit v.1.1 (Perkin-Elmer, Courtaboeuf, France) with the following internal primers: 536F, 536R, 800F, 800R, 1050F and 1050R; 16S rRNA amplification and sequencing were carried out as previously described by Steven et al. [12]. The 16S rRNA nucleotide sequence was corrected using Chromas Pro 1.34 software (Technelysium, Tewantin, Australia), and the BLASTn searches were performed by National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov.gate1.inist.fr/Blast.cgi). MEGA6 (Molecular Evolutionary Genetics Analysis) software [13] allowed us to construct a phylogenetic tree. Sequences alignment of the different species was performed by CLUSTALW, and calculation of the evolutionary distance was done with the Kimura two-parameter model [14], [15].
Atmospheric, sporulation and microscopy tests
Growth of the strain ES2T was tested under aerobic atmosphere, in the presence of 5% CO2, and also in anaerobic and microaerophilic atmospheres created using AnaeroGen and CampyGen respectively (ThermoFisher Scientific).
Spore formation was determined by thermal shock and observed under a microscope.
Gram staining and motility were observed by the use of the DM1000 photonic microscope (Leica Microsystems, Nanterre, France). Cell morphology was examined with a Tecnai G20 (FEI, Limeil-Brevannes, France) transmission electron microscope.
Biochemistry and antimicrobial susceptibility
Biochemical tests were realized by using the commercially available API ZYM, API 50CH and API 20 NE strips (bioMérieux, Marcy l’Étoile, France). Oxidase and catalase reactions were determined by using a BD BBL DrySlide (Becton Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer's instructions.
Sensitivity to antibiotics was determined by using Sircan Discs (i2a, Montpellier, France) on Mueller-Hinton agar in a petri dish (bioMérieux). Doxycycline, rifampicin, vancomycin, nitrofurantoin, amoxicillin, erythromycin, ampicillin, ceftriaxone, ciprofloxacin, gentamicin, penicillin, trimethoprim/sulfamethoxazole, imipenem and metronidazole activity were tested on our strain.
Genomic DNA preparation
After 48 hours of culture, the bacteria were resuspended in sterile water and centrifuged at 4°C at 2000 × g for 20 min. Cell pellets were resuspended in 1 mL Tris/EDTA/NaCl solution (10 mM Tris/HCl (pH7.0), 10 mM EDTA (pH8.0) and 300 mM NaCl) and recentrifuged under the same conditions. The pellets were then resuspended in 200 μL of Tris-EDTA (TE) buffer and proteinase K and kept overnight at 37°C for cell lysis. DNA was purified with phenol/chloroform/isoamylalcohol (25:24:1), followed by an overnight precipitation with ethanol at −20°C. It was then resuspended in 205 μL TE buffer and quantified (155 ng/μL) by a Qubit fluorometer using the high-sensitivity kit (ThermoFisher Scientific).
Genome sequencing and assembly
Genomic DNA of Planococcus massiliensis was sequenced on the MiSeq Technology (Illumina, San Diego, CA, USA) with the mate pair strategy. The gDNA was barcoded in order to be mixed with 11 other projects with the Nextera Mate Pair sample prep kit (Illumina). The mate pair library was prepared with 1 μg of gDNA using the Nextera mate pair Illumina guide. The gDNA sample was simultaneously fragmented and tagged with a mate pair junction adapter. The pattern of fragmentation was validated on an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA) with a DNA 7500 lab chip. The DNA fragments ranged in size from 1 to 11 kb, with an optimal size at 4.008 kb. No size selection was performed, and 388.3 ng of tagmented fragments were circularized. The circularized DNA was mechanically sheared to small fragments, with an optimum of 634 bp, on the Covaris device S2 in microtubes (Covaris, Woburn, MA, USA). The library profile was visualized on a High Sensitivity Bioanalyzer LabChip (Agilent Technologies), and the final concentration library was measured at 35.59 nmol/L. The libraries were normalized at 2 nM and pooled. After a denaturation step and dilution at 15 pM, the pool of libraries was loaded onto the reagent cartridge and then onto the instrument along with the flow cell. Automated cluster generation and the sequencing run were performed in a single 39-hour run at a 2 × 251 bp read length. Total information of 10.6 Gb was obtained from a 1326K/mm2 cluster density with a cluster passing quality control filters of 99.1% (24 492 260 clusters). Within this run, the index representation for Planococcus massiliensis was determined to be 7.06%. The 1 481 197 paired reads were filtered according to the read qualities. These reads were trimmed, then assembled using the CLC genomics WB4 software.
Genome annotation and comparison
Open reading frames (ORFs) were predicted using Prodigal [16] with default parameters, but the predicted ORFs were excluded if they spanned a sequencing gap region. The predicted bacterial protein sequences were searched against the GenBank database [17] and the Clusters of Orthologous Groups (COGs) database using BLASTP. The tRNAScanSE tool [18] was used to find tRNA genes, whereas ribosomal RNAs were found using RNAmmer [19] and BLASTn against the GenBank database. Lipoprotein signal peptides and the number of transmembrane helices were predicted using SignalP [20] and TMHMM [21] respectively. ORFans were identified if their BLASTP E value was lower than 1e-03 for alignment length greater than 80 amino acids. If alignment lengths were smaller than 80 amino acids, we used an E value of 1e-05. Such parameter thresholds have already been used in previous works to define ORFans. Artemis [22] was used for data management and DNA Plotter [23] for visualization of genomic features. The Mauve 2.3.1 alignment tool was used for multiple genomic sequence alignment [24]. To estimate the mean level of nucleotide sequence similarity at the genome level, we used the MAGI homemade software to calculate the average genomic identity of gene sequences (AGIOS) among compared genomes. Briefly, this software combines the Proteinortho software [25] for detecting orthologous proteins in pairwise genomic comparisons, then retrieves the corresponding genes and determines the mean percentage of nucleotide sequence identity among orthologous ORFs using the Needleman-Wunsch global alignment algorithm. Genomes from the genus Planococcus and closely related genera were used for the calculation of AGIOS values.
The genome of Planococcus massiliensis strain ES2T (GenBank accession no. CCXS00000000) was compared to the one of Planomicrobium glaciei strain CHR43 (NTS) (GenBank accession no. AUYR00000000), Planococcus halocryophilus strain Or1 (GenBank accession no. ANBV01000000), Planococcus donghaensis strain MPA1U2 (NTS) (GenBank accession no. AEPB01000000) and Bacillus subtilis subsp. spizizenii strain TU-B-10 (GenBank accession no. CP002905).
To estimate the overall similarity between the genomes, the Genome-to-Genome Distance Calculator (GGDC) was used [26], [27]. The system calculates the distances by comparing the genomes to obtain high-scoring segment pairs (HSP) and interfering distances from a set of formulas: 1, HSP length/total length; 2, identities/HSP length; and 3, identities/total length.
Results
Strain identification
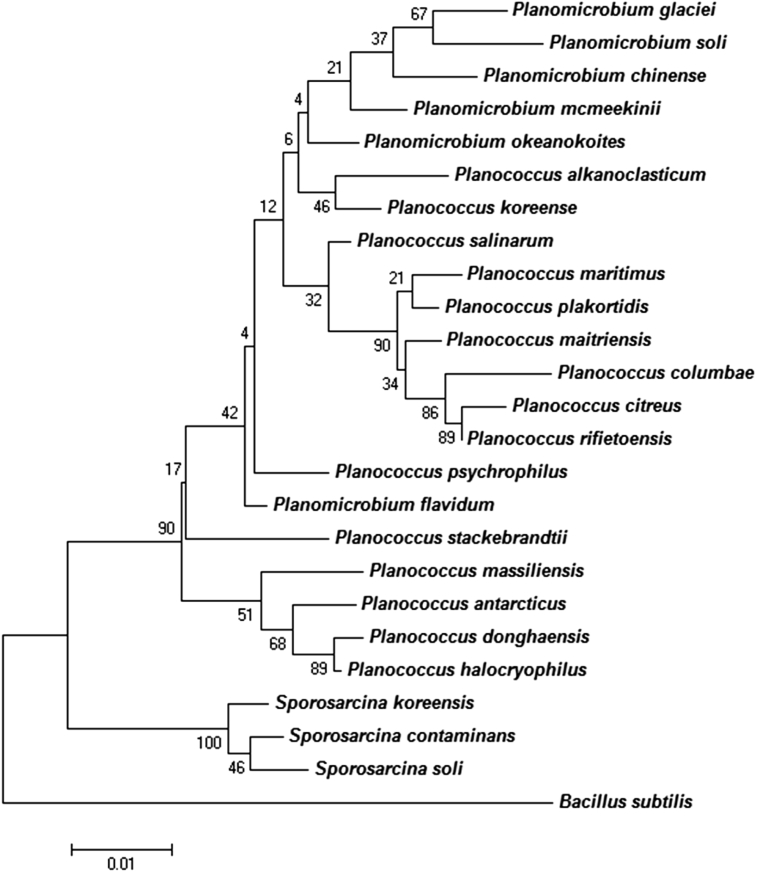
We did not obtain a significant MALDI-TOF score for strain ES2T against the Bruker database, suggesting that our isolate was not a known species. Its spectrum was added to our database (Figure 1). The gel view highlighted the spectral differences with other members of the genus Planococcus (Figure 2). PCR-based identification of the 16S rRNA of our new isolate (GenBank accession no. LK021122) revealed 1516 bp long sequences. This indicated a 97.95% 16S rRNA sequence similarity with Planococcus halocryophilus (GenBank accession no. AJ314745), the phylogenetically closest validated Planococcus species (Figure 3). The other closest species were P. donghaensis (97.72%), Planococcus glaciei (97.06%) and B. subtilis (91.92%). The species P. massiliensis, P. halocryophilus and P. donghaensis shared a single cluster, whereas P. glaciei was present in a distant clade in the phylogenetic tree (Figure 3). This value of similarity remains lower than the 98.7% 16S rRNA gene sequence threshold recommended by Stackebrandt and Ebers to delineate a new species without carrying out DNA-DNA hybridization [6]. Thus, this bacterium was considered to be a new species called Planococcus massiliensis strain ES2T sp. nov.
Fig. 1.
Reference mass spectrum from Planococcus massiliensis strain ES2T.
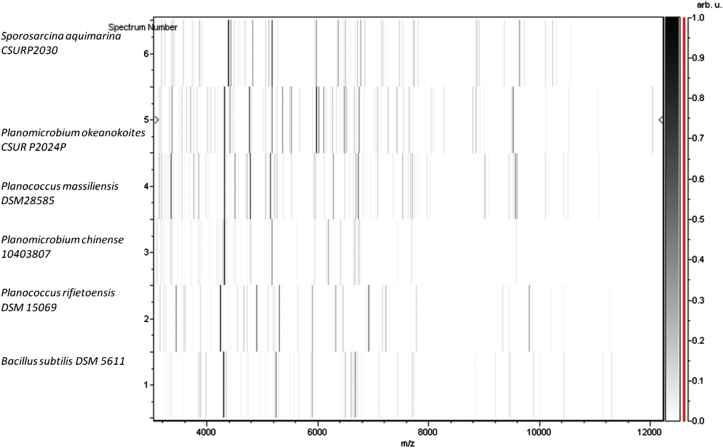
Fig. 2.
Gel view comparing Planococcus massiliensis strain ES2T to other species within genera Planomicrobium, Planococcus and Bacillus. Gel view displays raw spectra of loaded spectrum files arranged in pseudo-gel-like look. x-axis records m/z value. Left y-axis displays running spectrum number originating from subsequent spectra loading. Peak intensity is expressed by greyscale scheme code. Color bar and right y-axis indicate relation between color peak; peak intensity in arbitrary units. Displayed species are indicated on left.
Fig. 3.
Phylogenetic tree highlighting position of Planococcus massiliensis sp. nov. strain ES2T (1536 bp) relative to other type strains within genus. Planomicrobium glaciei strain 0423 (EU036220), Planomicrobium soli strain XN13 (JQ772482), Planomicrobium chinense strain DX3-12 (AJ697862), Planomicrobium mcmeekinii strain S23F2 (AF041791), Planomicrobium okeanokoites strain ATCC 33414 (D55729), Planomicrobium alkanoclasticum strain MAE2 (AF029364), Planomicrobium koreense strain JG07 (AF144750), Planococcus salinarum strain ISL-16 (FJ765415), Planococcus maritimus strain TF-9 (AF500007), Planococcus plakortidis strain MTCC 8491 (JF775504), Planococcus maitriensis strain S1 (AJ544622), Planococcus columbae strain PgEx11 (AJ966515), Planococcus citreus strain ATCC 14404 (X62172), Planococcus rifietoensis strain M8 (AJ493659), Planomicrobium psychrophilus strain CMS 53°r (AJ314746), Planomicrobium flavidum strain ISL-41 (FJ265708), Planococcus stackebrandtii strain K22–03 (AY437845), Planococcus massiliensis (LK021122–1516 bp), Planococcus antarcticus strain CMS 26or (AJ314745), Planococcus donghaensis strain JH1 (EF079063), Planococcus halocryophilus strain Or1 (JF742665), Sporosarcina koreensis strain F73 (DQ073393), Sporosarcina contaminans strain CCUG 53915 (FN298444), Sporosarcina soli strain I80 (DQ073394). GenBank accession numbers are indicated in parentheses. Sequences were aligned using CLUSTALW, and phylogenetic inferences were obtained using maximum likelihood method within MEGA software. Bacillus subtilis subsp. spizizenii strain TU-B-10 (AF074970) was used as outgroup. Scale bar = 0.005% nucleotide sequence divergence.
Physiologic and biochemical characteristics
Strain ES2T is able to grow at temperatures between 25 and 40°C (optimum 37°C) and pH 6–9 (optimum pH 7.0–8.0), and it tolerates NaCl concentrations between 5 to 200 g/L (optimum 75 g/L). We tested the Planococcus massiliensis growth on 5% sheep's blood–enriched Columbia agar (bioMérieux, Marcy l’Étoile, France, at 37°C) but we observed weak growth with colonies measuring about 0.3 to 0.6 mm after 48 hours of growth. It is a motile, non-spore-forming and Gram-positive bacterium (Figure 4). Atmospheric testing demonstrated that Planococcus massiliensis was strictly aerobic and grew in the presence of 5% CO2 but did not grow in an anaerobic atmosphere. Colonies that grow on our homemade culture medium were orange, circular, entire, smooth and convex, and they had a diameter of 1.0 to 2.0 mm after 48 hours. Individual cells exhibited a diameter of 0.6 to 0.9 μm and had a slightly curved form with a flagellum under electron microscopy (Figure 5).
Fig. 4.
Gram staining of Planococcus massiliensis strain ES2T.
Fig. 5.
Transmission electron microscopy of Planococcus massiliensis strain ES2T. Cells are observed on Tecnai G20 transmission electron microscope operated at 200 keV. Scale bar = 500 nm.
Using API galleries, we observed positive reactions for esterase, lipase, trypsin, naphthol-AS-BI-phosphohydrolase, α-glucosidase, d-glucose, d-fructose, d-mannose, d-ribose and d-arabinose. Negative reactions were observed for leucine arylamidase, valine arylamidase, β-galactosidase, alkaline phosphatase, cystine arylamidase, α-chymotrypsin, acid phosphatase, α-galactosidase, β-glucuronidase, β-glucosidase, α-mannosidase, α-fucosidase, arginine dihydrolase, N-acetyl-β-glucosaminidase, nitrate, d-galactose, d-mannitol and urease. The strain was also oxidase positive but catalase negative. Phenotypic characteristics were compared to those of the most closely related species (Table 1).
Table 1.
Differential characteristics of Planococcus massiliensis strain ES2T compared to other closely related Planococcus species
| Property | P. massiliensis | P. okeanokoites | P. koreense | P. mcmeekinii | P. donghaensis | P. halocryophilus | P. glaciei | P. salinarum | P. columbae | P. alkanoclasticum | P. soli |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.6–0.9 | 0.4–0.8 | 0.4–0.8 | 0.6–0.9 | 0.8–1.2 | 0.8–1.2 | 0.4–0.8 | 0.4–0.8 | 0.8–1.0 | 0.4–0.8 | 0.8–1.0 |
| Oxygen requirement | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic | Aerobic |
| Gram stain | + | + to v | + to v | + | + | + | + | + | + | + to v | + |
| Salt requirement | + | + | + | + | + | + | + | − | + | + | + |
| Motility | + | + | + | + | − | + | + | − | + | + | + |
| Endospore formation | − | + | + | + | − | − | + | + | − | + | + |
| Indole | − | − | − | − | − | + | − | NA | − | − | − |
| Production of: | |||||||||||
| Alkaline phosphatase | − | NA | NA | NA | − | NA | + | NA | NA | + | − |
| Catalase | − | + | + | + | NA | NA | + | NA | NA | + | + |
| Oxidase | + | w | − | − | + | + | − | + | − | − | − |
| Nitrate reductase | − | − | − | + | − | − | + | − | − | − | − |
| Urease | − | − | − | − | NA | − | − | NA | NA | NA | NA |
| Arginine dihydrolase | − | NA | NA | NA | NA | − | − | NA | NA | NA | NA |
| Β-Galactosidase | − | NA | NA | NA | + | NA | − | − | NA | − | − |
| N-acetyl-β-glucosaminidase | − | NA | − | NA | + | NA | NA | NA | NA | − | − |
| Acid from: | |||||||||||
| l-Arabinose | − | − | − | − | − | − | − | NA | − | − | NA |
| d-Ribose | + | + | − | − | + | + | + | NA | NA | − | NA |
| d-Mannose | + | − | − | − | − | + | NA | − | NA | − | − |
| d-Mannitol | − | − | − | − | − | + | NA | − | − | − | NA |
| D-Sucrose | − | − | − | − | + | + | NA | − | + | − | − |
| d-Glucose | + | − | w | + | + | + | NA | − | − | + | − |
| d-Fructose | + | + | − | + | − | + | NA | + | + | + | − |
| d-Maltose | − | − | + | w | + | + | NA | − | NA | − | − |
| d-Lactose | − | − | + | − | − | + | NA | − | + | − | − |
| Habitat | Human gut | Fermented seafood | Fermented seafood | Fermented seafood | Sea | Soil | Glacier | Coastal sediment | Coastal sediment | Coastal sediment | Soil |
+, positive result; −, negative result; v, variable result; w, weakly positive result; NA, data not available.
Antimicrobial susceptibility testing demonstrate that strain ES2T was susceptible to doxycycline, rifampicin, vancomycin, nitrofurantoin, amoxicillin, erythromycin, ampicillin, ceftriaxone, ciprofloxacin, gentamycin, penicillin, trimethoprim/sulfamethoxazole and imipenem, but it was resistant to metronidazole.
Genome properties
The GenBank Bioproject number is PRJEB6479 and consists of 192 large contigs. The draft genome of P. massiliensis ES2T consists of six scaffolds with 32 contigs and generated a genome size of 3 357 017 bp with a 46.0% G+C content (Table 2, Figure 6). Of the 3405 predicted genes, 3357 are protein-coding genes and 48 are RNAs (eight genes are 5S rRNA, two are 16S rRNA, three are 23S rRNA and 35 are tRNA). A total of 2601 genes (66.90%) were assigned a putative function. A total of 75 genes (1.93%) were identified as ORFans. The remaining genes were annotated as hypothetical proteins. The properties and statistics of the genome are summarized in Table 2.
Table 2.
Nucleotide content and gene count levels of genome
| Attribute | Value | % of totala |
|---|---|---|
| Size (bp) | 3 357 017 | 100 |
| G+C content (bp) | 1 544 227 | 46.0 |
| Coding region (bp) | 2 972 253 | 88.53 |
| Total genes | 3405 | 100 |
| RNA genes | 48 | 1.40 |
| Protein-coding genes | 3357 | 98.59 |
| Genes with function prediction | 2319 | 68.10 |
| Genes assigned to COGs | 2405 | 70.63 |
| Genes with peptide signals | 188 | 5.52 |
| Genes with transmembrane helices | 776 | 22.79 |
COGs, Clusters of Orthologous Groups database.
Total is based on either size of genome in base pairs or total number of protein-coding genes in annotated genome.
Fig. 6.
Graphical circular map of genome of Planococcus massiliensis strain ES2T. From outside to center: contigs (red/grey), COGs category of genes on forward strand (three circles), genes on forward strand (blue circle), genes on reverse strand (red circle), COGs category on reverse strand (three circles), GC content.
The distribution of genes into COGs functional categories is presented in Table 3.
TABLE 3.
Number of genes associated with 25 general COGs functional categories
| Code | Value | % Value | Description |
|---|---|---|---|
| J | 174 | 5.18 | Translation |
| A | 0 | 0 | RNA processing and modification |
| K | 248 | 7.38 | Transcription |
| L | 133 | 3.96 | Replication, recombination and repair |
| B | 1 | 0.02 | Chromatin structure and dynamics |
| D | 34 | 1.01 | Cell cycle control, mitosis and meiosis |
| Y | 0 | 0 | Nuclear structure |
| V | 64 | 1.90 | Defense mechanisms |
| T | 152 | 4.52 | Signal transduction mechanisms |
| M | 132 | 3.93 | Cell wall/membrane biogenesis |
| N | 41 | 1.22 | Cell motility |
| Z | 1 | 0.02 | Cytoskeleton |
| W | 0 | 0 | Extracellular structures |
| U | 48 | 1.42 | Intracellular trafficking and secretion |
| O | 95 | 2.82 | Posttranslational modification, protein turnover, chaperones |
| X | 14 | 0.41 | Phages, Prophages, Transposable elements, Plasmids |
| C | 163 | 4.85 | Energy production and conversion |
| G | 216 | 6.84 | Carbohydrate transport and metabolism |
| E | 317 | 9.44 | Amino acid transport and metabolism |
| F | 80 | 2.38 | Nucleotide transport and metabolism |
| H | 95 | 2.82 | Coenzyme transport and metabolism |
| I | 131 | 3.90 | Lipid transport and metabolism |
| P | 169 | 5.03 | Inorganic ion transport and metabolism |
| Q | 85 | 2.53 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 487 | 14.50 | General function prediction only |
| S | 269 | 8.01 | Function unknown |
| — | 1183 | 35.23 | Not in COGs |
COGs, Clusters of Orthologous Groups database.
Genome comparison
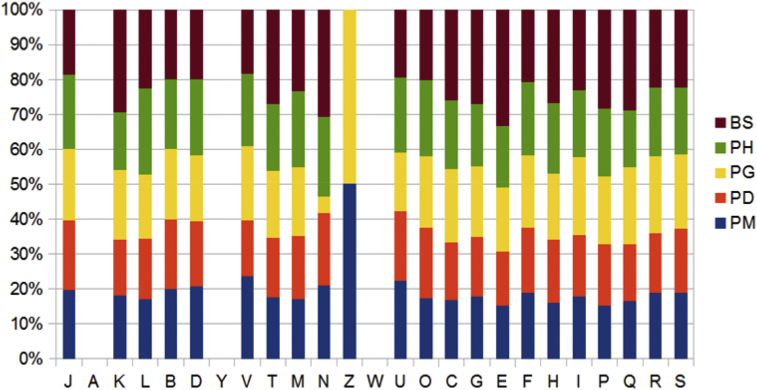
The draft genome of Planococcus massiliensis strain ES2T is smaller than those of Planomicrobium glaciei, Planococcus halocryophilus and Bacillus subtilis subsp. spizizenii (3.35, 3.9, 3.43 and 4.21 Mb respectively) but larger than those of Planococcus donghaensis (3.30 Mb). The G+C content of Planococcus massiliensis is smaller than those of P. glaciei (46.0 and 47.0% respectively) but larger than those of Planococcus halocryophilus, P. donghaensis and Bacillus subtilis (39.9, 39.7 and 43.8% respectively). The gene content of P. massiliensis is smaller than those of P. glaciei, P. halocryophilus and B. subtilis (3405, 3967, 3429 and 4307 respectively) but larger than that of P. donghaensis (3251). The number of rRNA genes varied from four for P. donghaensis, 13 for P. massiliensis, 30 for B. subtilis, 60 for P. halocryophilus and 62 for P. glaciei respectively. A large number of genes assigned to COGs functional categories for amino acid transport and metabolism, transcription, carbohydrate transport and metabolism and translation were identified. Nevertheless, we observed a relative lower number of genes assigned for amino acid transport and metabolism in P. massiliensis compared to other species (Figure 7). The genes for RNA processing and modification, nuclear structure and extracellular structures were absent in all the genomes. Finally, the genes coding for COGs category cytoskeleton were present only in P. massiliensis and P. glaciei (Figure 7). In addition, P. massiliensis shared 3880, 2775, 3146 and 4099 orthologous genes with P. glaciei, P. halocryophilus, P. donghaensis and B. subtilis (Table 4). The average nucleotide sequence identity ranged from 85.84% between P. donghaensis and P. halocryophilus to 56.69% between P. halocryophilus and B. subtilis (Table 4). The genomic similarity level between strain ES2T and closely related Planomicrobium and Planococcus species was also estimated using the GGDC (Table 5). This comparison of the genomes using GGDC revealed that P. massiliensis shows a slightly higher DNA-DNA hybridization (DDH) estimate with P. glaciei compared to those with P. halocryophilus and B. subtilis (Table 5). For B. subtilis, a higher DDH value was estimated with P. halocryophilus but did not vary much to the other genomes. These results are in accordance with the 16S rRNA (Figure 1). However, given the confidence intervals (Table 5), the DDH estimates do not show significant differences.
Fig. 7.
Distribution of functional classes of predicted genes according to clusters of orthologous groups of proteins. BS, Bacillus subtilis; PD, Planococcus donghaensis; PG, Planomicrobium glaciei; PH, Planococcus halocryophilus; PM, Planococcus massiliensis.
TABLE 4.
Numbers of orthologous proteins shared between genomes (upper right) and AGIOS values obtained (lower left)
| PM | PD | PG | PH | BS | |
|---|---|---|---|---|---|
| PM | 3357a | 2255 | 2275 | 2195 | 1533 |
| PD | 70.55 | 3146a | 2318 | 2340 | 1544 |
| PG | 74.92 | 70.50 | 3880a | 2256 | 1562 |
| PH | 67.78 | 85.84 | 67.70 | 2775a | 1497 |
| BS | 59.21 | 58.76 | 59.25 | 56.69 | 4099a |
AGIOS, average genomic identity of orthologous gene sequences; BS, Bacillus subtilis; PD, Planococcus donghaensis; PG, Planomicrobium glaciei; PH, Planococcus halocryophilus; PM, Planococcus massiliensis.
Number of proteins per genome.
TABLE 5.
Pairwise comparisons of Planomicrobium species using GGDC, formula 2 (DDH estimates based on identities/HSP length)a
| PM | PD | PG | PH | BS | |
|---|---|---|---|---|---|
| PM | 100.00% | 19.1% ± 2.76 | 20.9% ± 2.91 | 18.9% ± 2.76 | 27.4% ± 2.54 |
| PD | 100.00% | 19.2% ± 2.73 | 39.2% ± 3.34 | 27.7% ± 2.54 | |
| PG | 100.00% | 19.2% ± 2.73 | 28.6% ± 2.54 | ||
| PH | 100.00% | 29.7% ± 2.54 | |||
| BS | 100.00% |
BS, Bacillus subtilis; DDH, DNA-DNA hybridization; GGDC, Genome-to-Genome Distance Calculator; PD, Planococcus donghaensis; PG, Planomicrobium glaciei; PH, Planococcus halocryophilus; PM, Planococcus massiliensis.
Confidence intervals indicate inherent uncertainty in estimating DDH values from intergenomic distances based on models derived from empirical test data sets (which are always limited in size) [27]. Distance formulas are explained in Auch et al. [26]. Formula 2 is recommended, particularly for draft genomes.
Conclusion
In the context of culturomics studies, several new bacterial species are isolated and then characterized. It is in this context that we studied the phenotypic and phylogenetic characteristics and conducted genomic analyses on strain ES2T. Results allowed us to formally propose the creation of Planococcus massiliensis sp. nov., represented by the strain ES2T. P. massiliensis represents the eighth halophilic bacterium isolated from human stool. Because the colon is not a high-salinity environment, it would be interesting to examine the role of salt or salty products as a potential source of any unusual taxon, such as halophilic.
Taxonomic and nomenclatural proposals
Description of Planococcus massiliensis sp. nov.
Planococcus massiliensis (mas.si.li.en'sis, L., masc. adj., massiliensis for Massilia, the old Roman name for Marseille, where the strain was isolated).
Strain ES2T grows at an optimum temperature of 37°C, at pH 7.0–8.0 and at NaCl concentration of 75 g/L. Cells are Gram-positive, strictly aerobic, straight or curved rods (0.6–0.9 μm), motile, and nonendospore forming. Colonies are orange, circular, entire, smooth and convex, 1.0–2.2 mm in diameter.
P. massiliensis shows positive reactions for esterase, lipase, trypsin, naphthol-AS-BI-phosphohydrolase, α-glucosidase, d-glucose, d-fructose, d-mannose, d-ribose and d-arabinose. The strain is also oxidase positive but catalase negative.
Strain ES2T is susceptible to doxycycline, rifampicin, vancomycin, nitrofurantoin, amoxicillin, erythromycin, ampicillin, ceftriaxone, ciprofloxacin, gentamicin, penicillin, trimethoprim/sulfamethoxazole and imipenem.
P. massiliensis ES2T (= CSUR P1103, = DSM 28915) was isolated from a stool sample of a healthy Senegalese man. It exhibited a genome size of 3 357 017 bp with a 46.0% G+C content. The 16S rRNA sequence was deposited in GenBank under accession number LK021122, and the whole genome shotgun sequence has been deposited in GenBank under accession number CCXS00000000.
Acknowledgements
The authors thank the Xegen Company (http://www.xegen.fr/) for automating the genomic annotation process and C. Andrieu for administrative assistance. This study was funded by the Fondation Méditerranée Infection.
Conflict of Interest
None declared.
References
- 1.Lagier J.C., Armougom F., Million M., Hugon P., Pagnier I., Robert C. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 2.Lagier J.C., Hugon P., Khelaifia S., Fournier P.E., La Scola B., Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;1:237–264. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wayne L.G., Brenner D.J., Colwell R.R., Grimont P.A.D., Kandler O., Krichevsky M.I. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int J Syst Bacteriol. 1987;37:463–464. [Google Scholar]
- 4.Welker M., Moore E.R.B. Applications of whole-cell matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry in systematic microbiology. Syst Appl Microbiol. 2011;34:2–11. doi: 10.1016/j.syapm.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Ramasamy D., Mishra A.K., Lagier J.C., Padhmanabhan R., Rossi M., Sentausa E. A polyphasic strategy incorporating genomic data for the taxonomic description of novel bacterial species. Int J Syst Evol Microbiol. 2011;64:384–391. doi: 10.1099/ijs.0.057091-0. [DOI] [PubMed] [Google Scholar]
- 6.Stackebrandt E. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152–155. [Google Scholar]
- 7.Tindall B.J., Rosselló-Móra R., Busse H.J., Ludwig W., Kämpfer P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol. 2000;60:249–266. doi: 10.1099/ijs.0.016949-0. [DOI] [PubMed] [Google Scholar]
- 8.Vandamme P., Pot B., Gillis M., de Vos P., Kersters K., Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadia C., Mykytczuk S., Wilhelm R.C., Whyte L.G. Planococcus halocryophilus sp. nov., an extreme sub-zero species from high Arctic permafrost. Int J Syst Evol Microbiol. 2012;62:1937–1944. doi: 10.1099/ijs.0.035782-0. [DOI] [PubMed] [Google Scholar]
- 10.Yoon J.H., Kang S.J., Lee S.Y., Oh K.H., Oh T.K. Planococcus salinarum sp. nov., isolated from a marine solar saltern, and emended description of the genus Planococcus. Int J Syst Evol Microbiol. 2010;60:754–758. doi: 10.1099/ijs.0.013136-0. [DOI] [PubMed] [Google Scholar]
- 11.Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steven B., Briggs G., McKay C.P., Pollard W.H., Greer C.W., Whyte L.G. Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture dependent and culture-independent methods. FEMS Microbiol Ecol. 2007;59:513–523. doi: 10.1111/j.1574-6941.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. c: Molecular Evolutionary Genetics Analysis, version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 16.Hyatt D., Chen G.L., Locascio P.F., Land M.L., Larimer F.W., Hauser L.J. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson D.A., Karsch-Mizrachi I., Clark K., Lipman D.J., Ostell J., Sayers E.W. GenBank. Nucleic Acids Res. 2012;40:D48–D53. doi: 10.1093/nar/gkr1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe T.M., Eddy S.R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagesen K., Hallin P., Rodland E.A., Staerfeldt H.H., Rognes T., Ussery D.W. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendtsen J.D., Nielsen H., von Heijne G., Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Krogh A., Larsson B., von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford K., Parkhill J., Crook J., Horsnell T., Rice P., Rajandream M.A. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 23.Carver T., Thomson N., Bleasby A., Berriman M., Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics. 2009;25:119–120. doi: 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darling A.C., Mau B., Blattner F.R., Perna N.T. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechner M., Findeib S., Steiner L., Marz M., Stadler P.F., Prohaska S.J. Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics. 2011;12:124. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auch A.F., von Jan M., Klenk H.P., Göker M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci. 2010;2:117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier-Kolthoff J.P., Auch A.F., Klenk H.P., Göker M. Genome sequence–based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]