Abstract
Background
Myeloproliferative neoplasms are Philadelphia chromosome-negative diseases characterized by hyperproliferation of mature myeloid cells, associated or not with the Janus kinase 2 tyrosine kinase mutation, JAK2V617F. As there is no curative therapy, researchers have been investigating new drugs to treat myeloproliferative neoplasms, including l-amino acid oxidase from Calloselasma rhodostoma snake venom (CR-LAAO), which is a toxin capable of eliciting apoptosis in several tumor cell lines.
Objective
To evaluate the effects of l-amino acid oxidase from C. rhodostoma snake venom in the apoptotic machinery of JAK2-mutated cell lines.
Methods
The HEL 92.1.7 and SET-2 cell lines were cultured with l-amino acid oxidase and catalase for 12 h at 37 °C in 5% carbon dioxide. The cell viability was assessed by the multi-table tournament method, the level of apoptosis was measured by flow cytometry, and the expression of cysteine-dependent aspartate-specific proteases and cleaved Poly(ADP-ribose) polymerase were analyzed by Western blotting.
Results
l-Amino acid oxidase from C. rhodostoma snake venom was cytotoxic to HEL 92.1.7 and SET-2 cells (50% inhibitory concentration = 0.15 μg/mL and 1.5 μg/mL, respectively) and induced apoptosis in a concentration-dependent manner. Cell treatment with catalase mitigated the l-amino acid oxidase toxicity, indicating that hydrogen peroxide is a key component of its cytotoxic effect.The activated caspases 3 and 8 expression and cleaved PARP in HEL 92.1.7 and SET-2 cells confirmed the apoptosis activation by CR-LAAO.
Conclusions
l-Amino acid oxidase from C. rhodostoma snake venom is a potential antineoplastic agent against HEL 92.1.7 and SET-2 JAK2V617F-positive cells as it activates the extrinsic apoptosis pathway.
Keywords: Myeloproliferative neoplasms, Apoptosis, l-Amino acid oxidase, Calloselasma rhodostoma, Janus kinase 2 mutation
Introduction
Myeloproliferative neoplasms (MPN) are hematological neoplasms with similar phenotypic features, preserved cell maturation, and hyperproliferation of one or more blood cell types. MPN comprise chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), chronic neutrophilic leukemia, chronic eosinophilic leukemia, mastocytosis, and non-classifiable MPN.1 Patients with PV, ET, and PMF are negative for the Breakpoint Cluster Region-Abelson Leukemia (Bcr-Abl) oncogene, and their hematopoietic progenitors are independent and hypersensitive to numerous cytokines.2, 3 Mutation in the Janus kinase 2 (JAK2) tyrosine kinase is the predominant molecular alteration in MPN; it is characterized by a guanine-to-thymine transversion at nucleotide 1849 of exon 14 of the gene (chromosome 9), resulting in the substitution of valine for phenylalanine at position 617 (JAK2V617F).4
The amino acid exchange occurs in the JH2 pseudokinase domain and leads to the loss of the autoinhibitory control of the JH2 domain over the JH1 domain, and the consequent constitutive activation of the protein.5 Jekarl et al.6 reported that the JAK2V617F mutation is found in 95% of PV patients and in 50% of patients with ET and MF. In addition to the JAK2V617F mutation, other gene mutations such as JAK2 exon 12, MPL and calreticulin may help in the differential diagnosis, pathogenesis, and prognosis of Philadelphia chromosome-negative (Ph−) MPN.7 It is well known that the pathogenesis of MPN is also linked to genetic and epigenetic alterations and myeloid cell resistance to apoptosis.5, 8, 9
Apoptosis is the physiological process of programmed cell death that plays a role in the maintenance of cell number and integrity, and tissue development in a variety of body systems. Activation of apoptosis occurs mainly via the intrinsic and extrinsic pathways,10 both of which require the participation of cysteine-dependent aspartate-specific proteases (caspases). Caspases are proteases bearing a catalytic cysteine residue that cleaves other proteins at their aspartic acid residue. Caspases are synthesized as inactive precursors (zymogens) that require cleavage by proteases to form active enzymes, which in turn trigger a reaction cascade that culminates in cell apoptosis.10
Many types of stimuli can elicit the intrinsic or mitochondrial apoptosis pathway, including hypoxia, intracellular stress, lack of growth factors, irradiation, chemotherapeutic agents, bacteria, and viruses. These stimuli induce the release of cytochrome c, apoptosis inducing factor (AIF), second mitochondria derived activator of caspases/direct inhibitor of apoptosis-binding protein with low pI (SMAC/DIABLO), which culminates in the activation of apoptotic protease activating factor-1 (APAF-1). The binding of APAF-1 to deoxyadenosine triphosphate (ATP)/2 deoxy-ATP (dATP) induces the formation of the apoptosome, a multimeric complex that activates the initiator caspase-9 and subsequently activates the executioner caspases -3, -6, and -7.11
The extrinsic apoptosis pathway is triggered by the binding of ligands to death receptors. These include FAS (FAS/CD95), tumor necrosis factor (TNF) death receptors 1 and 2 (TNF-R1 and R2, respectively), TNF-related apoptosis-inducing ligand receptors (TRAIL) R1 and R2 (DR4 and DR5, respectively); a family of transmembrane proteins bearing a cysteine-rich extracellular domain and an intracellular domain named “death domain” (DD), which is responsible for transducing the apoptotic signal. The receptor-ligand binding recruits adaptor molecules such as FADD (FAS associated with DD) and TRADD (TNFR1 associated with DD); it also elicits intracellular signaling pathways that activate the initiator caspases -8, -9, and -10 and the executioner caspases -3, -6, and -7, and promotes the formation of apoptotic bodies (cell death). Finally, macrophages phagocytose the apoptotic bodies.12
Despite extensive knowledge on the pathogenesis of MPN, scientists have neither stratified the disease nor discovered effective treatments to cure it yet. The current treatments for PV, ET, and PMF rely on palliative therapies (bleeding, hydroxycarbamide, interferon-α, busulfan, corticoids, and androgens), supportive therapies (blood transfusion, growth factors, erythropoietin, and antibiotics), allogeneic bone marrow transplant, and JAK2 inhibitors.13 Bone marrow transplant is the only treatment that can change the course of the disease; however, the success of this therapy is restricted to a small group of young patients who receive bone marrow from a human leukocyte antigen (HLA)-compatible donor. JAK2 inhibitors have exerted promising effects in patients with PMF, including those who are negative for the JAK2V617F mutation. Recent studies have evidenced the beneficial effects of JAK2 inhibitors on the treatment of patients with PMF, such as decreased spleen volume, body weight gain, delayed bone marrow fibrosis, and improved overall survival.14
In this sense, researchers have sought for new drugs and therapies to treat and cure patients with MPN. Snake venoms are one of the antitumor agents under investigation; these proteins exert a variety of pharmacological activities that influence the clinical manifestations of the disease, such as apoptosis induction.15 Snake venoms have complex compositions with different contents of proteins, peptides, carbohydrates, lipids, biogenic amines, nucleotides, and amino acids. The major component of the venom of the Calloselasma rhodostoma, a snake from Vietnam, Cambodia, Thailand and Malaysia is l-amino acid oxidase (LAAO), which is a flavoenzyme that catalyzes the oxidative deamination of l-amino acids to α-ketoacid and produces ammonia and H2O2 as byproducts.16 The secondary effects of H2O2 seem to account for most of the biological effects of C. rhodostoma LAAO (CR-LAAO), including apoptosis induction.17
To improve the understanding on the biological activity and pharmacological potential of LAAOs, the present study aims to examine the effects of CR-LAAO on the apoptosis machinery of JAK2V617F-positive cell lines obtained from MPN patients.
Method
Cell lines and culture
JAK2V617F-positive cell lines HEL 92.1.7 and SET-2 were obtained from patients with erythroleukemia and post-ET megakaryoblastic leukemia, respectively. The cell lines were kindly provided by Dr. Thomas Radimerski (Novartis, Switzerland). The HEL 92.1.7 and SET-2 cells were cultured for 12 h with CR-LAAO, in complete RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 25 mM Hepes, under a humidified 5% CO2 atmosphere at 37 °C.
Determination of the enzymatic activity of the l-amino acid oxidase
Dr. Sandro Ghisla (University of Konstanz, Germany) kindly provided the CR-LAAO, which was obtained according to the method described by Macheroux et al.18 The enzymatic activity of CR-LAAO was determined in 0.1 M Tris–HCl buffer pH 7.2, at 25 °C. The qualitative test was performed to verify whether the protein was active, which is a requisite for biological assays.
l-Amino acid oxidase cytotoxicity assay
The ability of living cells to reduce 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to insoluble violet formazan crystals was used as the parameter to determine the cytotoxicity of CR-LAAO to HEL 92.1.7 and SET-2 cells. Both cell lines (1 × 105 cells/well) were cultured in 96-well plates, in the presence of CR-LAAO (0.03–3.0 μg/mL), 25 μM etoposide (VP-16; positive control), or buffer (negative control), for 12 h under a humidified 5% CO2 atmosphere at 37 °C. To assess the contribution of H2O2 to the overall effect of CR-LAAO, both cell lines were treated with CR-LAAO in combination with 150 U/mL catalase.
The plates were centrifuged at 240 × g for 10 min at 4 °C and 200 μL of MTT (5 mg/mL) were added to each well. After 4 h of incubation at 37 °C under 5% CO2, the formazan crystals were dissolved by adding 100 μL of a lysing solution (10% sodium dodecyl sulfate plus 0.01 M HCl) to the wells. The final absorbance was recorded at 540 nm. Controls in the absence of CR-LAAO had 100% of cell viability. The percentage of cell viability was calculated and used to determine the 50% inhibitory concentration (IC50) values.
Measurement of apoptosis
HEL 92.1.7 and SET-2 cells (2 × 105 cells/well) were cultured in 24-well plates in the presence of CR-LAAO (0.05–2.5 μg/mL), for 12 h under a humidified 5% CO2 atmosphere at 37 °C. The cells were washed with phosphate buffered saline at pH 7.4, centrifuged, and suspended in 400 μL of hypotonic fluorescent solution (50 μg/mL propidium iodide; Sigma–Aldrich, St. Louis, MO, USA). After 45 min of incubation at 4 °C in the dark, the samples were analyzed in a FACSCanto flow cytometer (Becton-Dickinson, San Jose, CA, USA). Ten thousand events were collected and analyzed by the Diva software and the results are expressed as percentage of cells with hypodiploid nuclei, which represent apoptotic cells.
Western blotting analysis of caspases -3, -8, and -9 and cleaved Poly(ADP-ribose) polymerase
The HEL 92.1.7 and SET-2 cells (1 × 106) treated with CR-LAAO (0.03–0.15 μg/mL and 1.5–3.0 μg/mL, respectively) were suspended in 200 μL of the Western blotting sample buffer supplemented with 5% mercaptoethanol, 4% sodium dodecyl sulfate (SDS), 20% glycerol, and 100 mM Tris–HCl at pH 6.0. The samples were analyzed by electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes.
The membrane was sequentially labeled overnight with anti-caspase-3 polyclonal rabbit antibody (code 9662, Cell Signaling Technology), anti-tubulin (code T3320, Sigma–Aldrich), anti-caspase-9 polyclonal rabbit antibody (code 9502, Cell Signaling Technology), anti-caspase-8 mouse monoclonal antibody (code 9746, Cell Signaling Technology), and anti-cleaved Poly(ADP-ribose) polymerase (PARP – code 9541, Cell Signaling Technology). The antibodies had been diluted in a blocking solution containing 5% skim milk and 0.01% sodium azide, according to the manufacturers’ instructions.
After labeling with each primary antibody, the membrane was incubated with the peroxidase-labeled anti-mouse or anti-rabbit secondary antibody for 1 h at room temperature. The labeled proteins were detected by chemiluminescence (Amersham ECL Plus, GE Healthcare Life Science, Pittsburgh, BREAK). Then, the membrane was washed with the stripping buffer before being relabeled with the next primary antibody.
Statistical analysis
The percentages of cell viability and apoptosis of CR-LAAO-treated HEL 92.1.7 and SET-2 cells were statistically compared with their respective untreated controls, using one-way analysis of variance (ANOVA) and Dunnett's post-test. To analyze the effect of H2O2 on cell viability, the cell groups treated or not with catalase were statistically compared using Student's t-test. Data were analyzed using the GraphPad Prism software (v.5.0; Graph Pad Software, La Jolla, CA, USA). A p-value <0.05 was considered significant.
Results
C. rhodostoma l-amino acid oxidase decreases the viability of HEL 92.1.7 and SET-2 cells by releasing H2O2
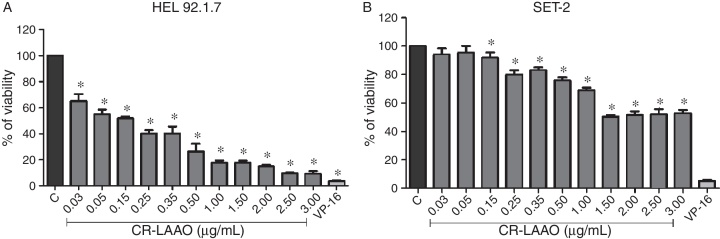
Compared with the control, CR-LAAO diminished the viability of HEL 92.1.7 and SET-2 cells in a concentration-dependent manner, yielding the IC50 values of 0.15 and 1.5 μg/mL, respectively (Figure 1). The standard compound VP-16 decreased the viability of both cell lines by nearly 96.7%.
Figure 1.
Cytotoxicity of l-amino acid oxidase from Calloselasma rhodostoma snake venom (CR-LAAO) to (A) HEL 92.1.7 and (B) SET-2 JAK2V617F positive cells. C: negative control (culture medium). VP-16: 25 μM etoposide (positive control).
Results are expressed as means ± standard deviation/standard error of the mean of triplicate assays. *p-value <0.05 vs. C (one-way ANOVA).
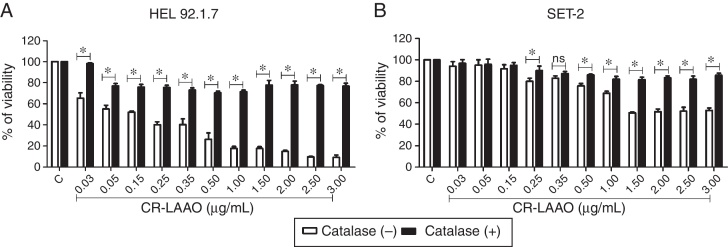
To assess whether H2O2 mediates the cytotoxic effect of CR-LAAO, the cells were treated with the toxin in combination with catalase. Catalase markedly changed the cytotoxic profile of CR-LAAO by increasing the viability of both cell lines to 80–100% (Figure 2). Together, these results demonstrate that (i) CR-LAAO is more cytotoxic to HEL 92.1.7 cells than to SET-2 cells, and (ii) the H2O2 released during the enzymatic reaction catalyzed by this oxidase mediates its cytotoxic action.
Figure 2.
l-Amino acid oxidase from Calloselasma rhodostoma snake venom (CR-LAAO) cytotoxicity, alone or in combination with catalase to (A) HEL 92.1.7 and (B) SET-2 JAK2V617F-positive cells.
C: negative control (culture medium), ns: non-significant. Results are expressed as means ± standard deviation/standard error of the mean of triplicate assays. *p-value <0.05 vs. catalase + (Student's t-test).
C. rhodostoma l-amino acid oxidase induces apoptosis in HEL 92.1.7 and SET-2 cells by activating caspases
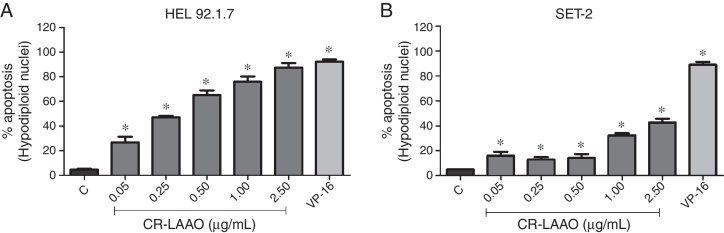
To examine the apoptosis-inducing effect of CR-LAAO, the formation of cells with hypodiploid nuclei was investigated using flow cytometry. CR-LAAO induced the formation of cells with hypodiploid nuclei in HEL 92.1.7 cells more strongly than in the SET-2 cell line, in a concentration-dependent manner (Figure 3).
Figure 3.
Quantification of apoptosis by determining the percentage of cells with hypodiploid nuclei induced by l-amino acid oxidase from Calloselasma rhodostoma snake venom (CR-LAAO) of (A) HEL 92.1.7 and (B) SET-2 JAK2V617F-positive cells. C: negative control (culture medium). VP-16: 25 μM etoposide (positive control).
Results are expressed as means ± standard deviation/standard error of the mean of duplicate assays. *p-value <0.05 vs. C (one-way ANOVA and Dunnett's post-test).
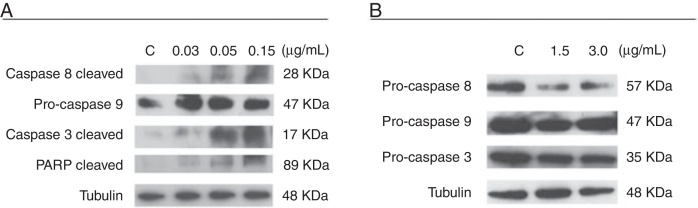
Next, to assess whether CR-LAAO-induced apoptosis occurred via the intrinsic or extrinsic pathway, the activation of caspases -3, -8, -9 and cleaved PARP were analyzed in the HEL 92.1.7 and SET-2 cell lines treated with CR-LAAO for 12 h. The reduced intensity of pro-caspases -3 and -8 bands associated with the increased intensity of caspase-3 and cleaved PARP bands indicated the activation of caspases (Figure 4).
Figure 4.
Caspase activation in HEL 92.1.7 and SET-2 cells treated with the l-amino acid oxidase from Calloselasma rhodostoma snake venom (CR-LAAO), detected by western-blotting. (A) Activation of caspases -3, -8 and cleaved PARP in HEL 92.1.7 cells. (B) Activation of pro-caspases 3 and 8 in SET-2 cells. C: negative control (culture medium).
Therefore, the activation of caspases mediates the apoptosis-inducing effect of CR-LAAO by triggering the extrinsic pathway, which is more pronounced in HEL.92.1.7 cells than in SET-2 cells.
Discussion
Patients with Ph− MPN such as PV, ET, and PMF can display extramedullary hematopoiesis, hyperplasia of megakaryocytes in the bone marrow, and varying degrees of dysplasia. Progression of these MPN types is marked by the appearance of medullary fibrosis (in patients with PV and ET) or transformation into either myelodysplastic syndrome or acute leukemia: 10% of the cases progress to acute myeloid leukemia.19
Description of the JAK2V617F mutation in 2005 prompted the molecular study of the physiopathological mechanisms of Ph− MPN.4 Expression of JAK2V617F induces the proliferation of cell lines independently of their stimulation by cytokines and growth factors. In murine experimental models, the retroviral expression of JAK2V617F in hematopoietic cells elicits the development of a hematopoietic disease whose clinical signs and symptoms resembles those of PV.4 The presence of the JAK2V617F mutation correlates with the most severe clinical and laboratory parameters in patients with PV, ET, and PMF, which is characterized by increased hematocrit, hemoglobin levels, leukocyte count, and neutrophil count; diminished erythropoietin levels; hepatomegaly; splenomegaly; and elevated incidence of thromboembolic events, when compared with JAK2V617F-negative patients.20 Although the pathogenesis of MPN are well known, scientists have still not discovered an effective drug to treat and cure most of the patients with PV, ET, and PMF.
Several researchers have described the resistance of mononuclear cells to apoptosis and deregulation of the expression of apoptosis-regulating genes in patients with PV, ET, and PMF.9, 21
Ruxolitinib, a JAK2 inhibitor, activates caspases -3, -8, and -9 in specific cell lines,22 while diosgenin cleaves caspases -3, -8, and -9 in HEL 92.1.7 cells.23 These reports underscore the relevance of apoptosis activation for the antitumor effect of new drugs against JAK2-mutated cells.
In the present study, the pro-apoptotic and cytotoxic potential of CR-LAAO was examined in HEL 92.1.7 and SET-2 cells with the JAK2V617F mutation from patients with PV and ET who progressed to acute leukemia.
Recent studies have demonstrated that H2O2 accounts for the biological effects of CR-LAAO, including apoptosis induction, which is an interesting therapeutic strategy to treat neoplasms.24 The increased production of H2O2 and other reactive oxygen species elicits apoptosis by damaging the mitochondrial membrane and promoting the release of cytochrome c into the cytosol.25 In the present study, a 12-h treatment with CR-LAAO significantly reduced the viability of HEL 92.1.7 and SET-2 cells; it indicates that CR-LAAO is cytotoxic to JAK2V617F-positive cells from patients with PV, ET, and PMF. Catalase mitigated the cytotoxicity of CR-LAAO, demonstrating that H2O2 plays a central role in the cell death process in JAK2-mutated cell lines. The fact that the HEL.92.1.7 cells were more sensitive to the cytotoxic action of CR-LAAO than the SET-2 cells is extremely relevant because the former is homozygous for the JAK2V617F mutation, which makes it more leukemogenic than the SET-2 cell line. These data also stress that analysis of the overall effect of CR-LAAO in different cellular contexts can help to predict the effect of this toxin in other mutated cell lines and in cells obtained from JAK2V617F-positive patients with PV, ET, and PMF.
The mononuclear cells of healthy individuals are more resistant to the action of CR-LAAO than the HEL.92.1.7 and SET-2 cells, which suggests that CR-LAAO selectively induces cytotoxicity in tumor cell lines.26 Although the antitumor potential of snake venom LAAO (SV-LAAO) has already been reported,15 this is the first report on the action of CR-LAAO in cell lines obtained from patients with Ph− MPN.
CR-LAAO also elicited apoptosis in HEL.92.1.7 and SET-2 cells by activating caspases -3, -8 and cleaved PARP. Cleavage of pro-caspase-8 can either activate caspase-3 or elicit apoptosis via the mitochondrial pathway or via BID cleavage. The activation of pro-caspases -3 and -8 suggests activation of the extrinsic pathway and BID cleavage. The extrinsic apoptosis pathway is triggered by binding death receptors to their ligands FAS (FAS/CD95), TNF-R1 and -R2, TRAIL R1 (DR4) and R2 (DR5), a family of transmembrane proteins bearing a cysteine-rich extracellular domain and an intracellular DD. The signal transduction via DD activates the working caspases -3, -6, and -7 and elicits the subsequent formation of apoptotic bodies (cell death).12
SV-LAAOs isolated from other species such as Ophiophagus Hannah, Bothrops atrox, and Bungarus fasciatus are cytotoxic against gastric cancer, lung adenocarcinoma, murine melanoma, promyelocytic leukemia, and breast cancer cell lines; the toxins elicited the intrinsic and extrinsic apoptosis pathways.27 It has been reported previously that LAAO isolated from Bothrops pirajai snake venom elicits apoptosis and potentiates the effect of imatinib mesylate – an inhibitor of Bcr-Abl tyrosine-kinase activity used to treat patients with CML – in Bcr-Abl-positive CML cell lines.28 In line with these data, several studies reported that SV-LAAOs exert their cytotoxic action by releasing H2O2, which causes oxidative stress and consequent cell death in tumor cell lines.26, 29 However, catalase only partially inhibited the cytotoxicity of LAAO from Bothrops leucurus.30 Therefore, not only the release of H2O2 but also other mechanisms may mediate the cytotoxic action of SV-LAAO.
In agreement with the aforementioned literature, the results of the present study show that SV-LAAOs act in H2O2-dependent and -independent manners, depending on the cellular context. Although the mechanism of action of CR-LAAO is poorly understood, the results of the current study indicate that this toxin is a potential antineoplastic agent against JAK2V617F-positive cell lines, as demonstrated by its ability to induce cytotoxicity and apoptosis of HEL 92.1.7 and SET-2 cells. These findings may help to develop novel drugs to treat Ph− MPN as well as to improve current knowledge on the snake envenomation mechanism. In addition, LAAOs can be useful tools to investigate the biochemical pathways of the apoptosis process in tumor cells.
It is also important to point out that the present investigation showed results of a CR-LAAO cytotoxicity potential just against JAK2V617F-positive cell lines. Thus, in order to confirm the CR-LAAO therapeutic potential against Ph− MPN, future studies using MPN mice models and in primary samples from myeloproliferative patients must be conducted to evaluate the toxicity of the drug and the effect of the compound on primary cells.
Conclusions
The results of this study suggest that CR-LAAO has an anti-neoplastic potential against HEL 92.1.7 and SET 2-JAK2 positive cell lines demonstrated by the activation of extrinsic apoptotic pathway, which may contribute to the description of new drugs for the treatment the MPN.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank to São Paulo Research Foundation (FAPESP, grants #2011/23236-4 and 2014/19127-3) and Research Support Center on Animal Toxins of USP (NAP-TOXAN-USP, grant #12.1.17615.1.5).
References
- 1.Campo E., Swerdlow S.H., Harres N.L., Pileri S.A., Stein H., Jaffe E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spivak J.L. Narrative review: thrombocytosis, polycythemia vera, and JAK2 mutations – the phenotypic mimicry of chronic myeloproliferation. Ann Int Med. 2010;152(5):300–306. doi: 10.7326/0003-4819-152-5-201003020-00008. [DOI] [PubMed] [Google Scholar]
- 3.Milosevic J.D., Kralovics R. Genetic and epigenetic alterations of myeloproliferative disorders. Int J Hematol. 2013;97(2):183–197. doi: 10.1007/s12185-012-1235-2. [DOI] [PubMed] [Google Scholar]
- 4.James C., Ugo V., Le Couédic J.P., Staerk J., Delhommeau F., Lacout C. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 5.Vainchenker W., Delhommeau F., Constantinescu S.N., Bernard O.A. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118(7):1723–1735. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- 6.Jekarl D.W., Han S.B., Kim M., Lim J., Oh E.J., Kim Y. JAK2 V617F mutation in myelodysplastic syndrome, myelodysplastic syndrome/myeloproliferative neoplasm, unclassifiable, refractory anemia with ring sideroblasts with thrombocytosis and acute myeloid leukemia. Korean J Hematol. 2010;45(1):46–50. doi: 10.5045/kjh.2010.45.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tefferi A., Pardanani A. Genetics CALR mutations and a new diagnostic algorithm for MPN. Nat Rev Clin Oncol. 2014;11(3):125–126. doi: 10.1038/nrclinonc.2014.16. [DOI] [PubMed] [Google Scholar]
- 8.Tognon R., Gasparotto E.P., Leroy J.M., Oliveira G.L., Neves R.P., Carrara R.C. Differential expression of apoptosis-related genes from death receptor pathway in chronic myeloproliferative diseases. J Clin Pathol. 2011;64(1):75–82. doi: 10.1136/jcp.2010.080895. [DOI] [PubMed] [Google Scholar]
- 9.Gasparotto E.P., Tognon R., Ferrerira A.F., Oliveira G.L., Palma P.V., Zanichelli M.A. Deregulated expression of A1, BCL-2, BCL-XL and MCL-1 antiapoptotic proteins and BID, BAD and BAX proapoptotic genes in Polycythemia vera patients. Braz J Pharm Sci. 2011;47(4):873–886. [Google Scholar]
- 10.Dejean L.M., Ryu S.Y., Martinez-Caballero S., Teijido O., Peixoto P.M., Kinnally K.W. MAC and BCL-2 family proteins conspire in a deadly plot. Biochim Biophys Acta. 2010;1797(6–7):1231–1238. doi: 10.1016/j.bbabio.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hail-Junior N., Kim H.J., Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11(10):1677–1694. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 12.Jin Z., El-Deiry W.S. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour E., Cortes J., Giles F., Kantarjian H. Current perspectives on the treatment of patients with chronic myeloid leukemia: an individualized approach to treatment. Cancer J. 2007;13(6):357–365. doi: 10.1097/PPO.0b013e31815b0df7. [DOI] [PubMed] [Google Scholar]
- 14.Choi C.W., Bang S.M., Jang S., Jung C.W., Kim H.J., Kim H.Y. Guidelines for the management of myeloproliferative neoplasms. Korean J Intern Med. 2015;30(6):771–788. doi: 10.3904/kjim.2015.30.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa T.R., Menaldo D.L., Silva C.P., Sorrechia R., Albuquerque S., Pietro R.C. Evaluating the microbicidal, antiparasitic and antitumor effects of CR-LAAO from Calloselasma rhodostoma venom. Int J Biol Macro. 2015;80:489–497. doi: 10.1016/j.ijbiomac.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Guo C1, Liu S., Yao Y., Zhang Q., Sun M.Z. Past decade study of snake venom l-amino acid oxidase. Toxicon. 2012;60(3):302–311. doi: 10.1016/j.toxicon.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues R.S., Silva J.F., França B., Fonseca F.P., Otaviano A.R., Silva F.H. Structural and functional properties of Bp-LAAO, a new l-amino acid oxidase isolated from Bothrops pauloensis snake venom. Biochimie. 2009;91(4):490–501. doi: 10.1016/j.biochi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Macheroux P., Seth O., Bollschweiler C., Schwarz M., Kurfürst M., Au L.C. l-Amino-acid oxidase from the Malayan pit viper Calloselasma rhodostoma – comparative sequence analysis and characterization of active and inactive forms of the enzyme. Eur J Biochem. 2001;268(6):1679–1686. [PubMed] [Google Scholar]
- 19.Thepot S., Itzykson R., Seegers V., Raffoux E., Quesnel B., Chait Y. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM) Blood. 2010;116(19):3735–3742. doi: 10.1182/blood-2010-03-274811. [DOI] [PubMed] [Google Scholar]
- 20.Speletas M., Katodritou E., Daiou C., Mandala E., Papadakis E., Kioumi A. Correlations of JAK2-V617F mutation with clinical and laboratory findings in patients with myeloproliferative disorders. Leuk Res. 2007;31(8):1053–1062. doi: 10.1016/j.leukres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Tognon R., Gasparotto E.P., Neves R.P., Nunes N.S., Ferreira A.F., Palma P.V. Deregulation of apoptosis-related genes is associated with PRV1 overexpression and JAK2 V617F allele burden in Essential Thrombocythemia and Myelofibrosis. J Hematol Oncol. 2012;5:2. doi: 10.1186/1756-8722-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szymańska J., Smolewski P., Majchrzak A., Cebula-Obrzut B., Chojnowski K., Treliński J. Pro-apoptotic activity of ruxolitinib alone and in combination with hydroxyurea, busulphan, and PI3K/mTOR inhibitors in JAK2-positive human cell lines. Adv Clin Exp Med. 2015;24(2):195–202. doi: 10.17219/acem/32934. [DOI] [PubMed] [Google Scholar]
- 23.Cailleteau C., Liagre B., Beneytout J.L. A proteomic approach to the identification of molecular targets in subsequent apoptosis of HEL cells after diosgenin-induced megakaryocytic differentiation. J Cell Biochem. 2009;107(4):785–796. doi: 10.1002/jcb.22176. [DOI] [PubMed] [Google Scholar]
- 24.Costa T.R., Burin S.M., Menaldo D.L., Castro F.A., Sampaio S.V. Snake venom l-amino acid oxidases: an overview on their antitumor effects. J Venom Anim Toxins Incl Trop Dis. 2014;20:23. doi: 10.1186/1678-9199-20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakshit S., Mandal L., Pal B.C. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem Pharmacol. 2010;80(11):1662–1670. doi: 10.1016/j.bcp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Ande S.R., Kommoju P.R., Draxl S., Murkovic M., Ghisla S., Fröhlich K.U. Induction of apoptosis in yeast by l-amino acid oxidase from the Malayan pit viper Calloselasma rhodostoma. Yeast. 2008;25(5):349–357. doi: 10.1002/yea.1592. [DOI] [PubMed] [Google Scholar]
- 27.Fung S.Y., Lee M.L., Tan N.H. Molecular mechanism of cell death induced by king cobra (Ophiophagus hannah) venom l-amino acid oxidase. Toxicon. 2015;96:38–45. doi: 10.1016/j.toxicon.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Burin S.M., Ayres L.R., Neves R.P., Ambrosio L., Morais F.R., Dias-Baruffi M. l-Amino acid oxidase isolated from Bothrops pirajai induces apoptosis in BCR-ABL-positive cells and potentiates imatinib mesylate effect. Basic Clin Pharmacol Toxicol. 2013;113(2):103–112. doi: 10.1111/bcpt.12073. [DOI] [PubMed] [Google Scholar]
- 29.Bregge-Silva C., Nonato M.C., de Albuquerque S., Ho P.L., Azevedo I.L.M.J., Diniz M.R.V. Isolation and biochemical, functional and structural characterization of a novel l-amino acid oxidase from Lachesis muta snake venom. Toxicon. 2012;60(7):1263–1276. doi: 10.1016/j.toxicon.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Naumann G.B., Silva L.F., Silva L., Faria G., Richardson M., Evangelista K. Cytotoxicity and inhibition of platelet aggregation caused by an l-amino acid oxidase from Bothrops leucurus venom. Biochim Biophys Acta. 2011;1810(7):683–694. doi: 10.1016/j.bbagen.2011.04.003. [DOI] [PubMed] [Google Scholar]