Abstract
Adoptive transfer of T cells genetically engineered to express a tumor-targeting chimeric antigen receptor (CAR) or T cell receptor (CAR) can mediate cancer regression in some patients. CARs are synthetic single chain proteins that employ antibody domains to target cell surface antigens. TCRs are natural heterodimeric proteins that can target intracellular antigens through recognition of peptides bound to human leukocyte antigens. CARs have shown promise in B cell malignancies and TCRs in melanoma, but neither approach has achieved clear success in an epithelial cancer. Treatment of epithelial cancers may be particularly challenging because of a paucity of target antigens expressed by carcinomas and not by important healthy tissues. In addition, epithelial cancers may be protected by inhibitory ligands and soluble factors in the tumor microenvironment. One strategy to overcome these negative regulators is to modulate expression of T cell genes to enhance intrinsic T cell function. Programmable nucleases, which can suppress inhibitory genes, and inducible gene expression systems, which can enhance stimulatory genes are entering clinical testing. Other work is delineating whether control of genes for immune checkpoint receptors (e.g. PDCD1, CTLA4), and cytokine and TCR signaling regulators (e.g. CBLB, CISH, IL12, IL15) can increase the anti-tumor activity of therapeutic T cells.
BACKGROUND
Antigen receptor gene therapy, the adoptive transfer of T cells genetically engineered to express a tumor-targeting chimeric antigen receptor (CAR) or T cell receptor (TCR), is an emerging class of cancer treatments that may hold promise for wide-ranging cancers. This type of treatment generally is performed by first harvesting mononuclear cells from the peripheral blood of the patient through a leukapheresis procedure. T cells from the leukapheresis product are then transduced with a viral vector other gene transfer platform encoding a CAR to TCR. The genetically engineered T cells are expanded in the laboratory and administered to the patient intravenously. Lymphocyte-depleting chemotherapy may be given prior to cell infusion to enhance engraftment and function of the engineered T cells. T cell infusion may be followed by systemic administration of cytokines, such as interleukin-2, to support the T cells.
CARs are synthetic molecules composed of antibody single-chain variable fragments (ScFv) that bind to a target tumor antigen, and domains from CD3 signaling chains and T cell costimulatory receptor molecules that provide intracellular signaling (Figure 1). Investigators from several medical centers have reported remarkable results with CARs targeting CD19 in diverse types of B cell malignancies.(1–6) TCRs are natural heterodimeric proteins composed of an α- and β-chain (Figure 1). Antigen recognition is mediated by the complementarity determining regions of the receptor. Signaling is accomplished by endogenous CD3 molecules that associate with the TCR α- and β-chains to form the TCR complex. Certain TCR gene therapies appear to have activity in melanoma, with the most promising approach targeting the cancer germline antigen, cancer/testis antigen 1 (alternative name NY-ESO-1).(7)
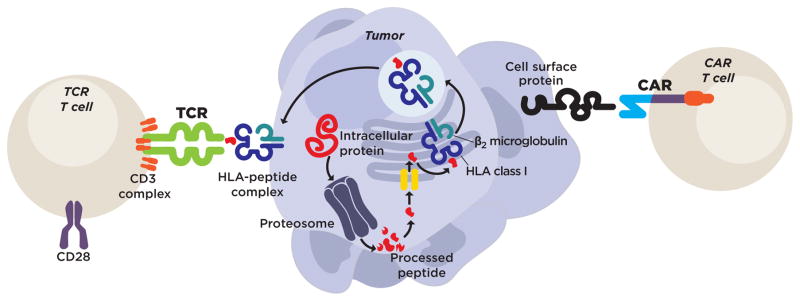
Figure 1. TCRs and CARs recognize tumor cells though distinct mechanisms.
Genetically engineered TCRs complex with endogenous CD3 units to form structures with recognition and signaling capability. They signal with a physiological CD3 signal, and costimulation requires engagement of a separate coreceptor. TCRs recognize a peptide-HLA complex on the cell surface. The peptide in this complex is derived from a protein that is processed by intracellular machinery. Thus, TCRs can recognize intracellular antigens, but they also require antigen processing and presentation by the tumor cell, and their antigen recognition is HLA-restricted. CARs are synthetic proteins that do not exist in nature. They are composed of an antibody-derived recognition unit linked to a signaling domain from the CD3 molecule as well as a costimulatory domain from a costimulatory receptor. CARs recognize cell surface but not intracellular antigens. They do not require antigen processing or presentation by the tumor, and their target recognition is not HLA-restricted.
TCRs but not CARs can target intracellular antigens
The antigen recognition mechanisms of CARs and TCRs differ in crucial ways that affect which antigens they can target and how tumors might evade attack. CARs, through their ScFv domain, recognize membrane-bound cell surface antigens (Figure 1). In contrast, TCRs recognize peptides that are generated by intracellular processing of proteins then presented to T cells by histocompatibility complex (MHC) molecules (Figure 1). CAR recognition of targets is not MHC-restricted; all tumors with the target antigen, regardless of human leukocyte antigen (HLA) haplotype, can be targeted. Because CARs recognize antigens that are not presented by MHC molecules, tumors cannot evade recognition through downregulation or loss of antigen processing and presentation genes such as transporter associated with antigen processing, proteasome subunits, or HLA molecules. However, CARs can bind to soluble antigens, which if present in blood can inhibit the intended recognition of membrane-bound molecules. In addition, most CARs have some degree of inherent ScFv clustering that can trigger tonic signaling, premature T cell exhaustion, and loss of anti-tumor activity.(8) In contrast, TCRs are not inhibited by soluble antigen because their targets are presented by MHC molecules, and they do not exhibit substantial tonic signaling because they are composed of physiological TCR signaling proteins (Figure 1).
Advantages to targeting tumor-specific antigens
Antigen receptor gene therapy is based on the infusion of high numbers of T cells directed against a tumor antigen. The strength of this T cell attack can be further augmented by host conditioning with chemotherapy that decreases negative regulatory cells and increases homeostatic cytokines.(9,10) The potency of the treatment can be additionally increased by the administration of exogenous cytokines.(10) The intent of the treatment is to completely eliminate every cell expressing the target antigen with a single infusion of T cells, and in some cases this goal appears to be achieved. However, the ability of antigen receptor gene therapy to destroy target cells located throughout the body has important consequences for target antigen selection.
A review of clinical experience with various target antigens is informative. CD19 (a B cell antigen) CAR therapy has been reported to eliminate not only malignant B cells but also normal B cells, which have broad tissue distribution.(3) MART1 and gp100 (melanocyte antigens) TCR therapy were shown to mediate not only melanoma regression but also sometimes-severe injury to melanocytes populating skin, eyes, and ears causing rash, and vision and hearing loss.(11) Carcinoembryonic antigen TCR therapy, which targeted an antigen expressed by both healthy colonic epithelial cells and colon cancers, was found to induce severe diarrhea that led to discontinuation of a clinical trial after three patients.(12) Finally, carbonic anhydrase IX (CAIX) CAR therapy, which was directed against an antigen present on the surface of renal cell carcinomas and biliary epithelium was found to cause cholangitis by CAR-mediated recognition of CAIX on the epithelial cells of normal bile ducts.(13,14) From these studies it is evident that antigen receptor gene therapy can induce destruction of diverse target cells regardless of their location. One implication of this finding is that, for treatments that are intended to induce complete regression of metastatic epithelial tumors, it may be important to target antigens that are expressed by tumors but not by vital healthy tissues.
Targeting tumor-specific antigens that are shared between patients
Few antigens are shared by the tumors of different patients and not expressed by healthy tissues.(15) Cancer germline antigens, are expressed in development but silenced in non-germline adult tissues, and reexpressed by some cancers. Successful treatment of some patients with melanoma or synovial sarcoma with TCR gene therapy targeting the germline cancer antigen NY-ESO-1 has been reported.(7) Mutated gene products can also be attractive targets, especially when they are oncogenic drivers. Certain oncogenic Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations occur at relatively high frequency in pancreatic, colorectal, lung, endometrial, ovarian, and prostate cancers. A TCR recognizing an HLA-A11 restricted epitope of the G12D KRAS variant has been described and may permit the first KRAS-targeted TCR gene therapy.(16) Perhaps the most attractive targets for T cell therapy in solid tumors are the viral antigens of tumors such cervical, oropharyngeal, and anal cancer (caused by human papillomavirus (HPV)) or undifferentiated nasopharygeal carcinoma (uNPC) (caused by Epstein-Barr virus (EBV)). In a recent clinical trial, adoptively transferred tumor infiltrating-T cells from cultures selected for their recognition of HPV oncoproteins mediated durable, complete tumor regression in some patients with HPV+ cervical cancer.(17) The magnitude of HPV reactivity of the infused cells correlated with objective tumor response, but “bystander” cells with non-HPV specificities were also infused and may have played a role in the clinical responses. A TCR targeting an HLA-A2-restricted epitope of HPV-16 E6 has been identified, and T cells expressing this receptor demonstrate recognition of cervical and oropharyngeal cancer cell lines.(18) Results of an ongoing clinical trial of TCR gene therapy with this receptor in cervical, oropharyngeal, anal, vaginal, vulvar, and penile cancers will more directly interrogate the HPV-16 E6 oncoprotein as a therapeutic target (NCT02280811). As with HPV, EBV can drive oncogenesis, and adoptive transfer of EBV-specific T cells has been studied and clinical activity reported in hematological malignancies(19,20) and possibly undifferentiated nasopharyngeal carcinoma.(21)
CLINICAL-TRANSLATIONAL ADVANCES
Inhibition of negative regulators of T cell function
Effective treatment of metastatic epithelial cancers by CAR or TCR genetically engineered T cells may require enhancement of T cell function. Enhanced T cell function can be achieved through reduced expression or blockade of inhibitory molecules, or through increased expression of stimulatory molecules. Decreased gene expression can be accomplished by strategies that target either genomic DNA or mRNA. Emerging genome editing technologies can precisely introduce indels to prevent gene expression, or replace nucleotide sequences to modify gene expression (Figure 2).(22) At least four types of programmable nucleases for genome editing have been described: meganucleases, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR-associated nuclease Cas9. Although programmable nuclease technologies hold great promise for manipulating gene expression in therapeutic T cells, their clinical development is in its infancy. A small clinical trial has tested ZFN editing of CCR5 for the treatment of patients with human immunodeficiency virus (HIV) suggesting the clinical feasibility of this approach in humans.(23) Recombinant Cas9 protein complexed with an in vitro transcribed single-guide RNA (RNPs) has been reported to efficiently edit primary human CD4+ T cell CXCR4 and PD-1 genes.(24) A megaTAL nuclease introduced with adeno-associated virus-mediated delivery of a CCR5-targeting template has also been described to efficiently modify CCR5 in human T cells.(25) Genome editing strategies have the advantage that they can completely eliminate expression of a functional gene product in some cells but the disadvantages that the platforms for high-efficiency editing and scaled up clinical application may require further development.
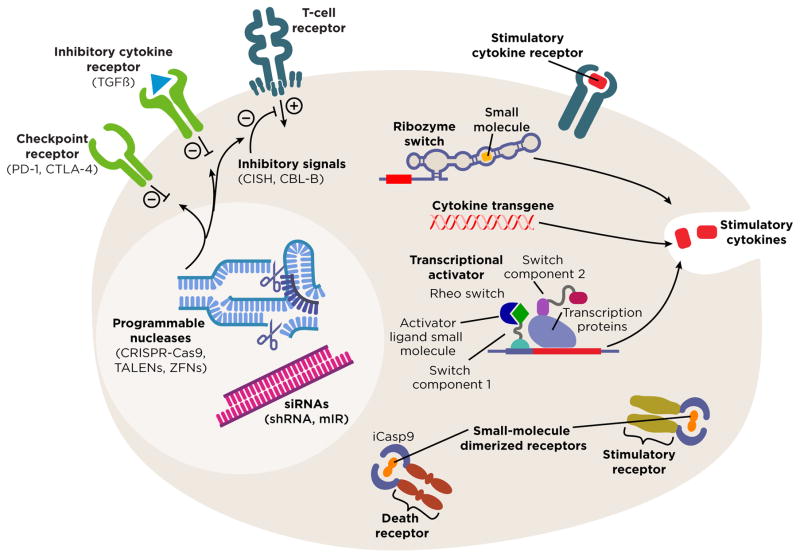
Figure 2. Emerging technologies and potential target genes for modified expression in therapeutic T cells.
Genome editing with programmable nucleases (light blue) such as CRISPR-Cas9, TALENs, ZFNs, and meganucleases may decrease the presence of specific inhibitory genes in T cells. Inhibitory RNAs (magenta) can decrease the expression of negative regulators of T cell anti-tumor function. Target genes for these approaches might include inhibitory cell surface receptors (green), such as PD-1 or CTLA-4, or antagonists of TCR or cytokine signals such as CISH, CBL-B, or SHP-1 (dark blue).
Another approach to inhibiting gene expression is to increase degradation of a target mRNA through RNA interference (RNAi) with short hairpin RNA (shRNA) or artificial microRNA (mIR) (Figure 2). These technologies are easily adapted to clinical application by integrating expression of the targeting RNA into established clinical gene transfer systems. They also have the advantage that tandem hairpin designs may permit simultaneous targeting of multiple genes. A disadvantage is that this approach can decrease but not completely eliminate expression of a gene.
Potential targets for gene silencing or knockdown, or monoclonal antibody blockade
A host of molecules have been reported to inhibit T cell function, some of which have been studied in mouse models or clinical trials of T cell-based cancer therapy. Much of this work has centered on inhibitory receptors expressed by T cells. Monoclonal antibodies that block interactions of the inhibitory receptor programmed death 1 (PD-1), with its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2), have clinical activity in melanoma, non-small cell lung cancer, renal cell carcinoma, urothelial cancer, head and neck squamous cells carcinoma, and other tumors.(26) PD-1 axis blockade with monoclonal antibodies also has been reported to improve adoptive T cell therapy in mouse models of CAR and TCR therapy.(27–29) Hence, PD-1 is an attractive molecule to target in combination with antigen receptor gene therapy. Another T cell inhibitory receptor that has been targeted in cancer therapy is cytotoxic T-lymphocyte antigen 4 (CTLA-4). Inhibition of CTLA-4 binding to its ligands, CD80 and CD86, can induce regression of melanoma and renal cell carcinoma.(30,31) A clinical trial for melanoma that combines CTLA-4 blockade with TIL infusion is ongoing (NCT01701674); tumor response in 5/11 patients has been reported.(32) Checkpoint blockade with the combination of anti-PD-1 and anti-CTLA-4 monoclonal antibodies has greater clinical activity than blockade with either agent alone in melanoma.(33) Dual PD-1 and CTLA-4 blockade combined with adoptive T cell therapy is a potentially interesting area for further exploration.(34) Further study in animal models and in clinical trials will be required to determine the optimal combinations of inhibitory receptors to antagonize. Emerging data also support strategies to inhibit intrinsic regulators of TCR and cytokine signaling, such as Src Homology Region 2 Domain-Containing Phosphatase 1 (SHP-1),(35,36) cytokine inducible SH-2-Containing Protein (CISH)(37), or E3 ubiquitin-protein ligase CBL-B.(38,39)
Controlled overexpression of genes that stimulate T cell function
The function of anti-tumor T cells for adoptive transfer may be improved by transgenic expression of molecules that enhance T cell activation and proliferation. It may be important to have an element of control over the timing and magnitude of expression of these molecules and the survival of the cells that express them. For example, constitutive IL-15 transgene expression enhances the anti-tumor function of T cells in a mouse model of TCR gene therapy, but some mice die from delayed hyper-proliferation of the infused cells,(40) and human T cells transduced to constitutively express IL-15 can display uncontrolled proliferation in vitro.(41) Thus, while transgenic IL-15 expression can improve T cell function, its expression may need to be controlled. In another example of increased treatment risk from transgenic cytokine expression, in a clinical trial of TIL for melanoma in which T cells were transduced to express single-chain IL-12 under a nuclear factor of the activated T cells (NFAT) promoter, tumor regression occurred in some patients, but IL-12 related toxicities prevented further development of the approach.(42)
Technologies to modulate transgene expression or activation, or to terminate T cell survival in vivo might be required. One system for controlling T cell stimulatory signals is to administer T cells that express an engineered costimulatory receptor that is reversibly dimerized by a small molecule (i.e. rimiducid) that is given systemically to the patient (Figure 2).(43) Another strategy is to use the same type of system as a “suicide gene” to induce cell death through dimerization of inducible caspase 9 (iCasp9) (Figure 2). This approach was reported to eliminate donor-derived iCasp9-engineered T cells in patients with graft-versus-host disease in a stem cell transplantation clinical trial.(44) Other technologies in which transgene expression can be controlled by small molecules include the RheoSwitch® inducible promotor system(45) and synthetic ribozyme switches; these technologies require vetting in clinical trials but have the potential to allow titration of transgene expression in vivo (Figure 2).(46,47)
Future directions
Few tumor-specific antigens are present on the surface of epithelial cancer cells and thus accessible to antibody or CAR therapy. TCR therapy can reach the larger pool of intracellular antigens but is limited by HLA restriction and the potential for tumor escape through defects in antigen processing and presentation. The identification and targeting of carefully selected epithelial tumor antigens is crucial for advancement of the field. Successful treatments may also require enhancement of T cell function through genetic manipulation of intrinsic T cell signals. Technologies for control of gene expression in T cells are evolving rapidly and might soon provide the decisive steps forward that are needed.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garfall AL, Maus MV, Hwang W-T, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373:1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Kassim SH, Tran TLN, Crystal JS, Morgan RA, Feldman SA, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–27. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–52. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2010;19:620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 14.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol. 2013;31:999–1008. doi: 10.1038/nbt.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novel Cancer Immunotherapy: HLA-A11 Restricted T Cell Receptor That Recognizes G12D Variant of Mutated KRAS [about 2 screens] [cited 2015 Oct 16]. Available from: https://www.ott.nih.gov/technology/e-028-2015.
- 17.Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543–50. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draper LM, Kwong MLM, Gros A, Stevanović S, Tran E, Kerkar S, et al. Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6. Clin Cancer Res. 2015;21:4431–9. doi: 10.1158/1078-0432.CCR-14-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis CU, Straathof K, Bollard CM, Ennamuri S, Gerken C, Lopez TT, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–90. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox DBT, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21:121–31. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–10. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol. 2015;33:985–9. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sather BD, Ibarra GSR, Sommer K, Curinga G, Hale M, Khan IF, et al. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci Transl Med. 2015;7:307ra156–307ra156. doi: 10.1126/scitranslmed.aac5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John LB, Devaud C, Duong CPM, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–46. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 28.Blake SJP, Ching ALH, Kenna TJ, Galea R, Large J, Yagita H, et al. Blockade of PD-1/PD-L1 promotes adoptive T-cell immunotherapy in a tolerogenic environment. PLoS One. 2015;10:e0119483. doi: 10.1371/journal.pone.0119483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–18. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–8. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhakaran S, Woods D, Royster E, Zager J, Sondak V, Pilon-Thomas S, et al. Pilot trial of ipilimumab and adoptive tumor-infiltrating lymphocyte (TIL) cell therapy. Ann Surg Oncol. 2015;22 [Google Scholar]
- 33.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations. Semin Oncol. 2015;42:363–77. doi: 10.1053/j.seminoncol.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Hebeisen M, Baitsch L, Presotto D, Baumgaertner P, Romero P, Michielin O, et al. SHP-1 phosphatase activity counteracts increased T cell receptor affinity. J Clin Invest. 2013;123:1044–56. doi: 10.1172/JCI65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stromnes IM, Fowler C, Casamina CC, Georgopolos CM, McAfee MS, Schmitt TM, et al. Abrogation of SRC homology region 2 domain-containing phosphatase 1 in tumor-specific T cells improves efficacy of adoptive immunotherapy by enhancing the effector function and accumulation of short-lived effector T cells in vivo. J Immunol. 2012;189:1812–25. doi: 10.4049/jimmunol.1200552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer DC, Guittard GC, Franco Z, Crompton JG, Eil RL, Patel SJ, et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J Exp Med. 2015;212:2095–113. doi: 10.1084/jem.20150304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz-Nicoladoni C, Wallner S, Stoitzner P, Pircher M, Gruber T, Wolf AM, et al. Reinforcement of cancer immunotherapy by adoptive transfer of cblb-deficient CD8+ T cells combined with a DC vaccine. Immunol Cell Biol. 2012;90:130–4. doi: 10.1038/icb.2011.11. [DOI] [PubMed] [Google Scholar]
- 39.Stromnes IM, Blattman JN, Tan X, Jeevanjee S, Gu H, Greenberg PD. Abrogating Cbl-b in effector CD8(+) T cells improves the efficacy of adoptive therapy of leukemia in mice. J Clin Invest. 2010;120:3722–34. doi: 10.1172/JCI41991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–74. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu C, Jones SA, Cohen CJ, Zheng Z, Kerstann K, Zhou J, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109:5168–77. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21:2278–88. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayanan P, Lapteva N, Seethammagari M, Levitt JM, Slawin KM, Spencer DM. A composite MyD88/CD40 switch synergistically activates mouse and human dendritic cells for enhanced antitumor efficacy. J Clin Invest. 2011;121:1524–34. doi: 10.1172/JCI44327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komita H, Zhao X, Katakam AK, Kumar P, Kawabe M, Okada H, et al. Conditional interleukin-12 gene therapy promotes safe and effective antitumor immunity. Cancer Gene Ther. 2009;16:883–91. doi: 10.1038/cgt.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Win MN, Smolke CD. A modular and extensible RNA-based gene-regulatory platform for engineering cellular function. Proc Natl Acad Sci U S A. 2007;104:14283–8. doi: 10.1073/pnas.0703961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YY, Jensen MC, Smolke CD. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc Natl Acad Sci U S A. 2010;107:8531–6. doi: 10.1073/pnas.1001721107. [DOI] [PMC free article] [PubMed] [Google Scholar]