Abstract
Antiphospholipid syndrome (APS) is defined by clinical manifestations that include thrombosis and/or fetal loss or pregnancy morbidity in patients with antiphospholipid antibodies (aPL). Antiphospholipid antibodies are among the most common causes of acquired thrombophilia, but unlike most of the genetic thrombophilias are associated with both venous and arterial thrombosis. Despite an abundance of clinical and basic research on aPL, a unified mechanism that explains their prothrombotic activity has not been defined; this may reflect the heterogeneity of aPL and/or the fact that they may influence multiple pro- and/or antithrombotic pathways. Antiphospholipid antibodies are directed primarily toward phospholipid binding proteins rather than phospholipid per se, with the most common antigenic target being β2-glycoprotein 1 (β2GPI) although antibodies against other targets such as prothrombin are well described. Laboratory diagnosis of aPL depends upon the detection of a lupus anticoagulant (LA), which prolongs phospholipid-dependent anticoagulation tests, and/or anticardiolipin and anti-β2-glycoprotein 1 antibodies. Indefinite anticoagulation remains the mainstay of therapy for thrombotic APS, although new strategies that may improve outcomes are emerging. Preliminary reports suggest caution in the use of direct oral anticoagulants in patients with APS-associated thrombosis. Based on somewhat limited evidence, aspirin and low molecular weight heparin are recommended for obstetrical APS. There remains a pressing need for better understanding of the pathogenesis of APS in humans, for identification of clinical and laboratory parameters that define patients at greatest risk for APS-related events, and for targeted treatment of this common yet enigmatic disorder.
The antiphospholipid syndrome (APS) is characterized by arterial or venous thrombosis and/or pregnancy morbidity accompanied by persistently positive tests for antiphospholipid antibodies (aPL).1 The deep veins of the lower extremities and the cerebral circulation are the most common sites of venous and arterial thrombosis, respectively.2 Obstetrical morbidity includes both recurrent early, or a single late (beyond 10 weeks) pregnancy loss and/or premature birth associated with preeclampsia and placental insufficiency.1 A severe form of APS termed catastrophic antiphospholipid syndrome (CAPS) occurs in <1% patients with aPL,1 and is defined as thrombosis affecting 3 or more organs within a period of 1 week with histologic confirmation of small vessel thrombosis.3 CAPS has a mortality rate of 33%–50%, mostly due to cerebral and cardiac thrombosis, or renal failure.4–6 Patients with APS may also demonstrate thrombocytopenia, livedo reticularis, skin ulcers, valvular heart disease, and transient ischemic attacks.4 Although these are considered “non-criteria” manifestations of APS, their presence along with thrombosis or pregnancy loss, should alert clinicians to this diagnosis.
Pathogenesis of APS
Though originally thought to react with anionic or polar phospholipids, such as cardiolipin, subsequent studies demonstrated that most aPL are directed against phospholipid binding proteins. β2GPI is the primary antigenic target of aPL, although many other antigenic targets, such as prothrombin, have been described.7,8 β2GPI is comprised of 5 “sushi” domains, the fifth domain being atypical and mediating binding to anionic phospholipid, while pathologic anti-β2GPI antibodies have been reported to bind primarily to domain 1.9 β2GPI has been proposed to circulate in a “circular” conformation in which interactions between domains 1 and 5 result in shielding of the domain 1 epitope (Figure 1); this may account for the absence of circulating β2GPI-containing immune complexes in patients with aPL.10 Unfolding of β2GPI with assumption of a “fishhook” conformation is presumed to occur upon binding to phospholipid or cellular receptors, exposing the antigenic region in domain 1.
Figure 1. Proposed structures of the open and closed forms of β2GPI DI-DV represent the 5 domains of β2GPI.
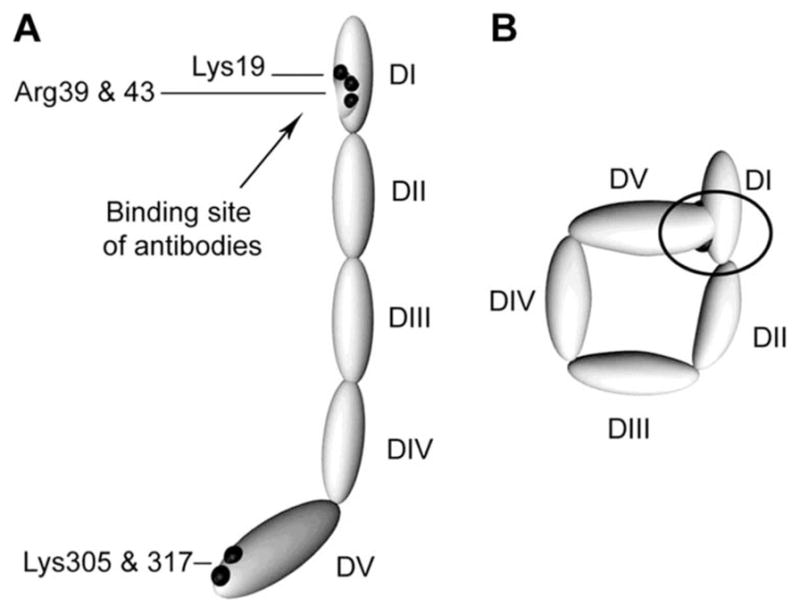
(A) β2GPI structure in the “open” form, as identified by its crystal structure. In this conformation, often referred to the “fish hook” conformation, an epitope containing Lys19, Arg39, and Arg43 that is recognized by anti-β2GPI domain 1 antibodies is exposed. β2GPI incubated at high pH adopts this conformation, and it is proposed that binding of anionic phospholipid results in similar conformational changes. (B) The “circular” form of β2GPI. This conformation is suggested by electron microscopy of circulating plasma β2GPI. In this conformation, the epitopes recognized by anti-β2GPI domain 1 antibodies are not available, which is thought to explain the fact that circulating immune complexes are not present in patients with antiphospholipid antibodies. This conformation is proposed to be maintained by interactions between domain 1 and domain 5. Reprinted with permission from Agar et al.10
Anti-β2GPI antibodies are central to the pathogenesis of APS, and recognize β2GPI bound to the surface of endothelial cells, monocytes, and immobilized platelets, in some cases leading to cellular activation and expression of procoagulant activity.11 Human anti-β2GPI autoantibodies potentiate arterial and venous thrombus formation in a mouse model,11 and lupus anticoagulants whose effects are mediated via interactions with β2GPI, or anti-β2GPI antibodies, are associated with a higher risk of thrombosis than anticardiolipin (aCL) or anti-prothrombin antibodies.7,12 Other mechanisms by which aPL have been proposed to effect a hypercoagulable state include inhibition of the anticoagulant activity of protein C and S, disruption of the annexin A5 shield on cell surfaces,13 inhibition of the ability of β2GPI to inhibit VWF-dependent platelet aggregation,14 and complement activation.15 Anti-β2GPI antibody binding to β2GPI on placental trophoblasts results in inhibition of growth and differentiation, and in animal models causes tissue factor and complement-mediated neutrophil activation, trophoblast injury, and fetal loss.16
Diagnosis of APS
The Sapporo criteria, the first consensus criteria for the diagnosis of “definite APS,” were proposed in 1999, and updated in 2006 after a conference in Sydney, Australia.1 These include both clinical and laboratory criteria, and diagnosis rests on the presence of at least one of each. Clinical criteria include either objectively confirmed venous, arterial, or small vessel thrombosis, or obstetric morbidity including the unexplained death of one or more morphologically normal fetuses at or beyond the 10th week of gestation, the premature birth of 1 or more morphologically normal neonates before the 34th week of gestation, and/or 3 or more unexplained, consecutive spontaneous abortions before the 10th week of gestation (Table 1).1 The laboratory criteria require demonstration of a persistent lupus anticoagulant detected according to guidelines published by the ISTH (Table 2),17 aCL antibody (IgG or IgM) exceeding 40 IgG or IgM antiphospholipid units, or anti-β2GPI antibody (IgG or IgM) at levels exceeding the 99th percentile.1 These three laboratory tests detect antibodies with overlapping, but not always identical specificity. These criteria were proposed to standardize the diagnosis of APS and aPL for clinical trials, however, they have several shortcomings in practice. For example, patients with “non-criteria” manifestations other than thrombosis or pregnancy morbidity, and those with thrombosis but only low-to-moderate titers of anti-β2GPI and ACL are not formally recognized as having APS. The clinical implications of IgA aCL or anti-β2GPI antibodies, and aPL directed against antigens such as phosphatidylserine or phosphatidylethanolamine remain controversial and routine testing for these is not recommended.
Table 1.
Summary of the Sydney Consensus Statement on Classification of APS
| Antiphospholipid antibody syndrome (APS) is present if at least one of the clinical criteria and one of the laboratory criteria are met |
|---|
Clinical criteria
|
Laboratory criteria
|
Table 2.
ISTH criteria for diagnosis of lupus anticoagulants
| Positive screening test (phospholipid-dependent coagulation assay) |
| Two or more screening tests recommended: dilute Russell’s viper venom time (DRVVT) and a sensitive aPTT (low phospholipids with silica as activator) |
| Evidence of inhibition in mixing studies (exclude factor deficiency) |
| 1:1 mixing of patient plasma with normal plasma does not correct the prolonged clotting time |
| LA cannot be conclusively identified if thrombin time is prolonged |
| Evidence that inhibition is phospholipid-dependent |
| Confirmatory test(s) performed by performing tests in which increased phospholipid corrects or reduces the prolonged clotting time |
| Examples: DRVVT confirm, hexagonal phase phospholipid, platelet neutralization test, others |
| Exclusion of coagulation inhibitors |
| Heparin |
| Direct thrombin or factor Xa inhibitors (DOAC) |
| Coagulation factor inhibitors (eg, factor VIII inhibitor) |
Modified from Pengo et al.17
Laboratory investigation is central to the diagnosis of APS. Unfortunately, aPL assays, particularly solid-phase assays for anti-β2GPI and aCL antibodies, are plagued by substantial interlaboratory variability.7,18 Patient factors also affect testing. For example, false-positive LA assays may result from anticoagulation with heparins or direct oral anticoagulants (DOACs).19,20 Martinuzzo et al studied patients on rivaroxaban, dabigatran, or LMWH, who had tested negative for lupus anticoagulant at baseline. Samples were drawn 4 hours after administration of enoxaparin and between 1.5 and 4 hours after taking enoxaparin or dabigatran. Nearly all patients taking dabigatran had prolongation of the activated partial thromboplastin time (APTT), silica clotting time (SCT), and dilute Russell’s viper venom time (DRVVT). There was also a high prevalence of DRVVT prolongation (81%–100%) with LMWH, whereas prolongation of the APTT and SCT was less common (13%–100% depending on dose).21 Though warfarin prolongs clotting times, its effect on clotting time ratios in LA assays is variable. The uncertainty concerning this conundrum is reflected in discrepant recommendations within different guidelines.22 Many authors advocate performance of LA studies using a 1:1 mix of patient and normal plasma, to reduce the effects of warfarin-induced reduction in clotting factor levels on LA testing. Others have found that certain venoms, for example Taipan snake venom, is a more “LA-specific” reagent that can be used for LA diagnosis even in the presence of warfarin or rivaroxaban; however, these tests are not widely available.23,24
A positive LA test may be caused by aPL directed against β2GPI, prothrombin or other less commonly identified antigens. LA whose effects are mediated via interactions with β2GPI confer a higher risk of thrombosis than those due to anti-prothrombin antibodies,7,12,25 although it has been suggested that antibodies to phosphatidylserine–prothrombin complexes may detect a subtype of antibodies more closely associated with thrombosis than those against prothrombin alone.8 In a recent study, the presence of a β2GPI dependent LA was strongly associated with thrombosis [odds ratio (OR) 42.3; 95% confidence interval (CI) 9.9–194.3], whereas there was no increased frequency of thrombosis in a group of 33 patients with non-β2GPI dependent LA (OR 1.6; 95%CI 0.8–3.9).26 Discriminating anti-β2GPI–dependent and independent aCL and LA may have important implications for identifying high-risk patients, although these studies are not commonly available in practice.
A disorder with clinical manifestations of APS but lacking positivity in any of the standard diagnostic laboratory studies has been termed “seronegative APS.” The relationship of this disorder to APS is uncertain.
Assessment of thrombotic risk
Despite advances in understanding the pathologic processes underlying APS, the ability to identify individuals at greatest risk of thrombosis remains challenging. It is particularly difficult to predict the risk of first thrombosis in asymptomatic aPL carriers.
aPL profile and thrombotic risk
LA positivity is a stronger risk factor for both arterial and venous thrombosis than anticardiolipin antibodies.6 However, there is a significant variation in strength of association in different studies that may reflect different methods used to detect LA, or the variable inclusion of LA that were not persistently positive.12 Retrospective and prospective studies have shown no consistent association between thrombosis and aCL.7,27 Because β2GPI is the primary antigen in APS, the anti-β2GPI antibody has been proposed as the more clinically significant and predictive aPL. Several retrospective studies showed that anti-β2GPI antibodies indeed correlate with thrombotic risk,28–30 although these results have not been universally confirmed and recent studies suggest that the thrombotic risk conferred by anti-β2GPI antibodies is modest, with odds ratios between 1.5 and 2.5.28
Based on reports demonstrating that positivity for 2 or more LA, aCL, and/or anti-β2GPI antibodies is more strongly associated with thrombosis and may be useful in assessing thrombotic risk. The updated Sydney criteria advise classification of patients into those with positivity for 1, 2, or all 3 “criteria” aPL (Table 1). For example, data from the Warfarin in Antiphospholipid Syndrome (WAPS) study showed that patients with LA and anti-β2GPI antibodies had a significantly increased risk for thrombosis (OR 4.1, 95% CI 1.3–13.5).7 Pengo et al reported a cumulative incidence of recurrent thrombosis of 12.2%, 26.1%, and 44.2% after 1, 5, and 10 years of follow-up in a retrospective analysis of 160 APS patients positive for LA, aCL, and anti-β2GPI—so-called “triple-positive” patients—123 of whom were on long-term anticoagulation.31 Similarly, in a recent study of 119 female aPL carriers, the annual rate of a first thrombotic event in individuals with double- or triple-positivity (1.27%) was twice as high as that in women with single-positivity (0.65%).32
Recent reports suggest that IgG anti-β2GPI-domain1 antibodies are more strongly associated with a history of thrombosis and obstetrical morbidity compared to antibodies to other regions of the protein.33,34 A prospective study reported that IgG anti-β2GPI domain 1 antibodies were more often persistent at 12 weeks, associated with triple-positivity, and correlated with thrombotic risk.35 Commercial assays for domain 1 antibodies are in development.
How to treat asymptomatic patients with positive aPL is a challenging question for which little evidence-based guidance is available, and is discussed in the “How I treat” section.
Other considerations
Persistence and high levels of aPL are associated with an increased risk of thrombosis.1 The presence of systemic lupus erythematosus (SLE), and other thrombotic risk factors, such as inherited thrombophilia, cancer, immobilization, smoking, pregnancy, and the use of oral contraceptives, and previous thrombosis also increase thrombotic risk.
Obstetrical APS
The literature addressing the association of aPL with obstetrical complications is confounded by different definitions of recurrent fetal loss and aPL positivity, as well as the heterogeneity of aPL assays performed in different labs. Clark et al demonstrated persistently positive lupus anticoagulants in 2.7% of women with recurrent pregnancy loss.36 The presence of LA correlated with thromboembolism during pregnancy, ≥1 stillbirth (beyond 32 weeks), intrauterine growth retardation, and the HELLP syndrome, but not with ≥2 spontaneous abortions (≤12 weeks). The PROM-ISSE study examined the correlation of LA and aCL with poor pregnancy outcomes, observing that positivity for LA was most strongly associated, with aCL >40 IgG phospholipid (GPL) units less strongly, but still significantly associated.37 However, most patients with positive aCL and poor outcomes also had positive LA. The Nimes Obstetrician and Haematologists Cohort Study, a large prospective study analyzing an initial cohort of more than 32 000 primagravidae, confirmed a significant association of LA, aCL or the combination with early pregnancy loss, although only LA were associated with preeclampsia.38
Treatment of thrombosis in APS
Long-term anticoagulation with a vitamin K antagonist (VKA) is the mainstay of therapy for thrombotic APS. However, a significant proportion of patients have recurrent thrombosis despite therapeutic anticoagulation,4,31 and patients with aPL and thrombosis are at higher risk for subsequent cardiovascular mortality than those without. Further complicating treatment is the fact that patients with aPL may be at higher risk of bleeding because of thrombocytopenia or other comorbidities.
Venous thrombotic events
Anticoagulation with unfractionated heparin or low molecular weight heparin transitioned to a VKA, most commonly warfarin, is the standard treatment for a first venous thrombotic event. A target INR of 2.5 (2.0–3.0) is recommended. Given the high rate of recurrent thrombosis in APS, two randomized trials compared standard intensity (INR 2.0–3.0) versus high intensity (INR 3.0–4.0) anticoagulation with warfarin in patients with APS but found no difference in the rates of recurrent thrombosis or major bleeding, supporting the use of standard intensity anticoagulation.39,40 The optimal duration of anticoagulation is unclear, however given that recurrence rates as high as 19%–29% have been reported in patients not receiving long-term anticoagulation, it is recommended that treatment be continued indefinitely. In a recent systematic review of 8 prospective studies, Garcia et al reported that patients with aPL had a higher rate of recurrent thrombosis after stopping anticoagulation with a relative risk of 1.41 (95% CI 0.99–2.00).41 The fact that the confidence interval included “no effect” questions the need for indefinite anticoagulation. However, the studies analyzed were of limited methodologic quality, and most patients did not have confirmed APS. Hence, as in any patient with thrombosis, the duration of anticoagulation must determined based upon the risk-benefit ratio of the individual patients, taking into account not only the risk of recurrent thrombosis, but the likelihood of bleeding, falls and compliance. Short-term anticoagulation may be considered in patients who develop thrombosis in the setting of a reversible risk factor, and those who subsequently test negative for aPL. Also, children with APS have a lower risk of recurrent thrombosis compared with adults and may not need long-term anticoagulation.42
Arterial thrombosis
In the Antiphospholipid Antibodies and Stroke (APASS) study, a subgroup of the Warfarin-Aspirin Recurrent Stroke Study (WARSS) that compared warfarin versus aspirin for secondary stroke prevention, no association was observed between aPL positivity and recurrent stroke, and there was no difference between the rate of recurrent stroke in the warfarin (INR 1.4–2.8) and aspirin groups.43 However, the results from this study are not generalizable to all patients with APS and stroke, because aPL were tested only once at baseline, low-positive anticardiolipin antibodies were included, and several patients had other cardiovascular risk factors. Other than one small randomized trial that reported lower rates of recurrent stroke in patients treated with aspirin plus warfarin versus aspirin alone,44 there are no prospective trials of secondary stroke prevention in APS. Patients with APS and non-stroke arterial events are frequently treated with combination antiplatelet and anticoagulant therapy, continued indefinitely. There is currently no consensus on the use of high-intensity anticoagulation for the secondary prophylaxis of arterial thrombosis. This may be considered, however, in APS patients with a high-risk aPL profile and other cardiovascular risk factors if the potential benefit outweighs the risk of bleeding.
The direct oral anticoagulants (DOACs)
The use of VKAs may be problematic in some patients because of food and drug interactions, bleeding complications, and need for frequent monitoring. Also, aPL differentially affect thromboplastin reagents, potentially affecting the international normalized ratio. DOACs have the potential to overcome some of these issues; they are fixed-dose, do not need routine monitoring, and are effective in the treatment of venous thrombosis in unselected individuals. However, there is limited experience with these agents in patients with APS. Three recent case series, comprising 18 patients in all, reported recurrent thrombosis in 8 of 18 individuals, suggesting caution when considering the use of DOACs in patients with APS.45–47 The Rivaroxaban in Thrombotic AntiPhospolipid Syndrome (TRAPS) trial (www.clinicaltrials.gov; NCT02157272) is an open-label, prospective, non-inferiority randomized trial evaluating the efficacy of rivaroxaban in patients with thrombotic APS, and is currently open. Results of this study should provide definitive insight into the role of DOACS in APS. However, in the absence of prospective data, DOACs should be used cautiously in these patients, and limited to individuals who either fail or are intolerant of a VKA or low molecular weight heparin.
Non-anticoagulant treatment of thrombotic APS
Advances in the understanding of pathogenic mechanisms involved in APS have led to the identification of new therapeutic approaches. These include inhibition of intracellular signaling pathways, novel antiplatelet agents, and immunomodulatory therapies that may be especially useful in patients with recurrent thrombosis and underlying SLE. Plasma exchange, defibrotide, and complement-directed therapies have shown efficacy in case reports of catastrophic APS as well as in animal models.
Hydroxychloroquine
The antimalarial hydroxychloroquine has anti-inflammatory and immunomodulatory effects and is an established first-line treatment for SLE. It has been reported to protect against both arterial and venous thrombosis in patients with SLE, with and without APS.48 Potential mechanisms include a decrease in lupus activity as well as modulation of APS effects. Rand et al have demonstrated that hydroxychloroquine protects the annexin A5 shield on endothelium and placental syncytiotrophoblast from disruption.49
Hydroxychloroquine is recommended for aPL-positive patients with SLE. Hydroxychloroquine decreased aPL titers and lowered the odds of having persistently positive aPL in an observational study. Another small prospective study comparing anticoagulation with a VKA (fluindone), with or without hydroxychloroquine in patients with primary APS reported VTE in 6/20 (30%) patients in the monotherapy group but none in the hydroxychloroquine group over 36 months of follow-up.50 There is currently no strong data to support the use of hydroxychloroquine in patients without autoimmune disorders, however, a multicenter randomized control trial of hydroxychloroquine for primary thrombosis prevention in patients with aPL without systemic autoimmune disease is currently underway (www.clinicaltrials.gov; NCT01784523).
Statins
Statins have anti-inflammatory properties and inhibit the activation of vascular cells by aPL in vitro. Simvastatin and pravastatin also inhibit aPL-induced fetal loss in a murine model.16 A small prospective study demonstrated that fluvastatin treatment for 3 months reduced the levels of inflammatory biomarkers and thrombosis in patients with aPL.51 Further clinical studies are needed to establish the role of statins as adjuvant therapy and primary thromboprophylaxis in patients with APS. Statins may be considered in patients with a history of arterial events.
B-cell directed therapy
A recent open-label, phase II trial of rituximab in primary APS reported some efficacy in controlling noncriteria manifestations, such as thrombocytopenia, hemolytic anemia, and skin ulcers.52 There is equivocal data supporting its use in CAPS. Twenty patients from the CAPS registry were treated with rituximab; eight (40%) received it in combination with first-line treatment because of severe presentations or associated lymphoproliferative disorders and the remainder as second-line therapy due to refractory CAPS.53 Seventy-five percent of these patients recovered from the acute episode.53 Other B cell-directed therapies, such as cytotoxic T lymphocyte antigen 4 (CTLA4) Ig and B-cell activating factor (BAFF), have shown efficacy in preclinical models.54,55
Treatment of obstetrical APS
The treatment of obstetrical APS remains controversial, with numerous studies reporting different outcomes in similar patient populations. A recent Cochrane review concluded that aspirin alone has not been demonstrated to improve pregnancy outcomes.56 Two prospective studies comparing aspirin with aspirin and unfractionated heparin have demonstrated an increased rate of live births in the latter group, although different doses of heparin were used.57,58 However, these results were not replicated in two prospective studies comparing aspirin with aspirin and LMWH due primarily to the better than expected outcomes in the aspirin alone group. Current ACCP guidelines recommend the use of low-dose aspirin with prophylactic or low-dose unfractionated or low molecular weight heparin in patients with aPL and ≥3 pregnancy losses.59
Treatment of catastrophic APS
Early diagnosis and treatment of CAPS is essential in the face of a rapidly progressive, potentially fatal condition. CAPS is characterized by microvascular thrombosis, multiorgan involvement, and a systemic inflammatory response to ischemic tissue necrosis. Anticoagulation with heparin and high-dose steroids (methylprednisolone 1000 mg daily for 3 days or longer) are first-line therapy for CAPS. Plasma exchange has improved mortality in observational studies and in the CAPS registry.5 Intravenous immunoglobulin alone does not appear to be beneficial in patients with CAPS, but may be used with plasma exchange in patients with concomitant immune thrombocytopenia.5
Case reports describe the successful use of eculizumab in patients with refractory CAPS and APS-related post-renal transplant thrombotic microangiopathy.60 The role of eculizumab in preventing recurrent CAPS after renal transplantation in patients with a prior history of CAPS is under investigation (www.clinicaltrials.gov; NCT 01029587). Eculizumab carries a risk of infection with encapsulated organisms and patients should be immunized against meningococcus before starting treatment.
Defibrotide is an adenosine receptor antagonist with antithrombotic, profibrinolytic, and anti-inflammatory effects on vascular endothelial cells that also blocks neutrophil tissue factor expression. It is been successfully used in hepatic veno-occlusive disease and multiorgan failure after stem cell transplant. Defibrotide has also been used for the treatment of refractory CAPS and is thought to act by decreasing endothelial inflammation.61
Conclusions
APS is a prothrombotic, autoimmune disorder with heterogeneous clinical presentations. Despite advances in diagnosis, correctly identifying patients at risk is a challenge. “Triple positivity” for criteria aPL is associated with the highest risk of clinical events, although of the individual assays for aPL, lupus anticoagulants are associated with the highest risk. β2GPI-specific LA and anti-β2GPI-domain 1 antibodies may be more sensitive markers of thrombotic risk, but are not widely available in practice. Long-term anticoagulation remains the mainstay of treatment of thrombotic APS. Innovative therapeutic approaches, such as immune modulation, complement inhibition, and targeting inflammation, are under study. Further mechanistic and clinical studies are needed to develop improved therapies for this potentially devastating illness.
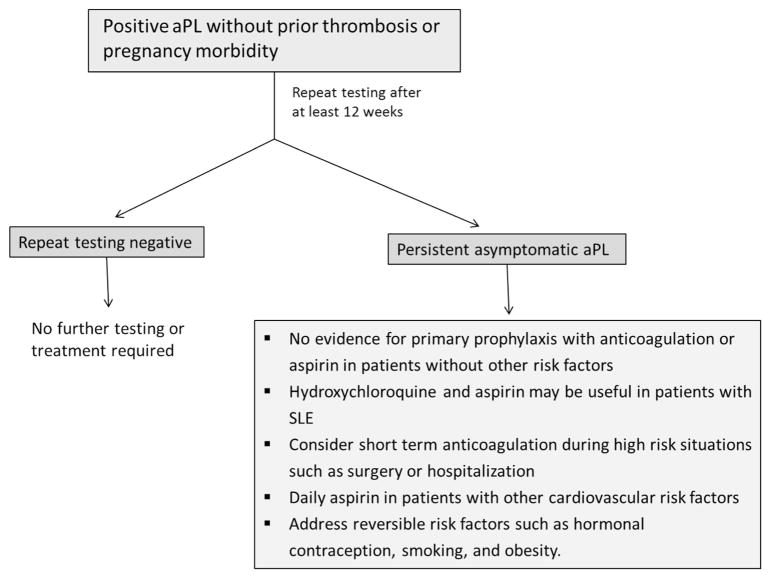
Figure 2. Approach to the asymptomatic patient with antiphospholipid antibodies.
For a description, see the “How I Treat” section.
Learning Objectives.
To review the definition of antiphospholipid antibody syndrome (APS) and its relevance to clinical manifestations in patients with antiphospholipid antibodies
To discuss selected aspects of APS pathogenesis and how they may impact targeted therapies
To review current recommendations for treatment of thrombotic and obstetric APS
How I treat asymptomatic patients with aPL.
The prevalence of asymptomatic aPL in the general, healthy population has been reported to be as high as 1%–5% (although is likely lower when assessed carefully), and may be as high as 11%–86% in patients with SLE.62 There has been no clear benefit demonstrated for primary thrombopropylaxis of such patients. The Antiphospholipid Antibody Acetylsalicylic acid (APLASA) trial, the only randomized study addressing this question, was terminated early due to an unexpectedly low rate of thrombosis. Of the 98 participants, only 3 had a thrombotic event, all of which were in the aspirin group.63 Other retrospective studies have yielded conflicting results. However, prophylaxis with LMWH or aspirin may reduce thrombotic complications in high-risk periods, such as surgery and hospitalization, and should be considered.64 Hydroxychloroquine is recommended in patients with SLE and aPL; it reduces aPL levels and may reduce the rate of thrombosis. A recent meta-analysis concluded that primary prophylaxis with aspirin does not improve obstetric outcomes in otherwise healthy women who are asymptomatic aPL carriers.65 Estrogen containing oral contraceptives (OCs) are clearly a risk factor for thrombosis. Although the risk of thrombosis with OCs in asymptomatic aPL carriers has not been studied, it is reasonable to assume that this combination may significantly increase thrombotic risk, and therefore alternative methods of contraception should be utilized. In a randomized trial comparing combined (estrogen containing) or progresterone-only OCs in women with SLE, only 2 of 54 patients in each group had a thrombotic event. However, all 4 of these patients were positive for aPL. The approach to asymptomatic patients with aPL is summarized in Figure 2.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheum. 2002;46(4):1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 3.Asherson RA, Cervera R, de Groot PG, et al. Catastrophic antiphospho-lipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12(7):530–534. doi: 10.1191/0961203303lu394oa. [DOI] [PubMed] [Google Scholar]
- 4.Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74(6):1011–1018. doi: 10.1136/annrheumdis-2013-204838. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Espinosa G. Update on the catastrophic antiphospholipid syndrome and the “CAPS Registry”. Semin Thromb Hemost. 2012;38(4):333–338. doi: 10.1055/s-0032-1304718. [DOI] [PubMed] [Google Scholar]
- 6.Bucciarelli S, Espinosa G, Cervera R. The CAPS Registry: morbidity and mortality of the catastrophic antiphospholipid syndrome. Lupus. 2009;18(10):905–912. doi: 10.1177/0961203309106833. [DOI] [PubMed] [Google Scholar]
- 7.Galli M, Borrelli G, Jacobsen EM, et al. Clinical significance of different antiphospholipid antibodies in the WAPS (warfarin in the antiphospholipid syndrome) study. Blood. 2007;110(4):1178–1183. doi: 10.1182/blood-2007-01-066043. [DOI] [PubMed] [Google Scholar]
- 8.Bertolaccini ML, Sciascia S, Murru V, Garcia-Fernandez C, Sanna G, Khamashta MA. Prevalence of antibodies to prothrombin in solid phase (aPT) and to phosphatidylserine-prothrombin complex (aPS/PT) in patients with and without lupus anticoagulant. Thromb Haemost. 2013;109(2):207–213. doi: 10.1160/TH12-07-0527. [DOI] [PubMed] [Google Scholar]
- 9.Iverson GM, Victoria EJ, Marquis DM. Anti-beta2 glycoprotein I (beta2GPI) autoantibodies recognize an epitope on the first domain of beta2GPI. Proc Natl Acad Sci U S A. 1998;95(26):15542–15546. doi: 10.1073/pnas.95.26.15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agar C, van Os GM, Morgelin M, et al. Beta2-glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospho-lipid syndrome. Blood. 2010;116(8):1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 11.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci U S A. 1990;87(11):4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devreese K, Peerlinck K, Hoylaerts MF. Thrombotic risk assessment in the antiphospholipid syndrome requires more than the quantification of lupus anticoagulants. Blood. 2010;115(4):870–878. doi: 10.1182/blood-2009-09-244426. [DOI] [PubMed] [Google Scholar]
- 13.Rand JH, Wu XX, Lapinski R, et al. Detection of antibody-mediated reduction of annexin A5 anticoagulant activity in plasmas of patients with the antiphospholipid syndrome. Blood. 2004;104(9):2783–2790. doi: 10.1182/blood-2004-01-0203. [DOI] [PubMed] [Google Scholar]
- 14.Hulstein JJ, Lenting PJ, de Laat B, Derksen RH, Fijnheer R, de Groot PG. beta2-Glycoprotein I inhibits von Willebrand factor dependent platelet adhesion and aggregation. Blood. 2007;110(5):1483–1491. doi: 10.1182/blood-2006-10-053199. [DOI] [PubMed] [Google Scholar]
- 15.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 16.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118(10):3453–3461. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection: Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7(10):1737–1740. doi: 10.1111/j.1538-7836.2009.03555.x. [DOI] [PubMed] [Google Scholar]
- 18.Willis R, Lakos G, Harris EN. Standardization of antiphospholipid antibody testing–historical perspectives and ongoing initiatives. Semin Thromb Hemost. 2014;40(2):172–177. doi: 10.1055/s-0033-1364207. [DOI] [PubMed] [Google Scholar]
- 19.Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost. 2011;105(2):385–386. doi: 10.1160/TH10-08-0511. [DOI] [PubMed] [Google Scholar]
- 20.Tripodi A, Chantarangkul V, Clerici M, Mannucci PM. Laboratory diagnosis of lupus anticoagulants for patients on oral anticoagulant treatment.: performance of dilute Russell viper venom test and silica clotting time in comparison with Staclot LA. Thromb Haemost. 2002;88(4):583–586. [PubMed] [Google Scholar]
- 21.Martinuzzo ME, Barrera LH, Da MA, Otaso JC, Gimenez MI, Oyhamburu J. Frequent false-positive results of lupus anticoagulant tests in plasmas of patients receiving the new oral anticoagulants and enoxaparin. Int J Lab Hematol. 2014;36(2):144–150. doi: 10.1111/ijlh.12138. [DOI] [PubMed] [Google Scholar]
- 22.Moore GW. Recent guidelines and recommendations for laboratory detection of lupus anticoagulants. Semin Thromb Hemost. 2014;40(2):163–171. doi: 10.1055/s-0033-1364185. [DOI] [PubMed] [Google Scholar]
- 23.Parmar K, Lefkou E, Doughty H, Connor P, Hunt BJ. The utility of the Taipan snake venom assay in assessing lupus anticoagulant status in individuals receiving or not receiving an oral vitamin K antagonist. Blood Coagul Fibrinolysis. 2009;20(4):271–275. doi: 10.1097/mbc.0b013e3283256037. [DOI] [PubMed] [Google Scholar]
- 24.van Os GM, de Laat B, Kamphuisen PW, Meijers JC, de Groot PG. Detection of lupus anticoagulant in the presence of rivaroxaban using Taipan snake venom time. J Thromb Haemost. 2011;9(8):1657–1659. doi: 10.1111/j.1538-7836.2011.04395.x. [DOI] [PubMed] [Google Scholar]
- 25.De Laat B, Derksen RH, Reber G, et al. An international multicentre-laboratory evaluation of a new assay to detect specifically lupus anticoagulants dependent on the presence of anti-beta2-glycoprotein autoantibodies. J Thromb Haemost. 2011;9(1):149–153. doi: 10.1111/j.1538-7836.2010.04068.x. [DOI] [PubMed] [Google Scholar]
- 26.de Laat HB, Derksen RH, Urbanus RT, Roest M, de Groot PG. beta2-glycoprotein I-dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood. 2004;104(12):3598–3602. doi: 10.1182/blood-2004-03-1107. [DOI] [PubMed] [Google Scholar]
- 27.Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol. 2009;8(11):998–1005. doi: 10.1016/S1474-4422(09)70239-X. [DOI] [PubMed] [Google Scholar]
- 28.Urbanus RT, de Groot PG. Antiphospholipid antibodies: we are not quite there yet. Blood Rev. 2011;25(2):97–106. doi: 10.1016/j.blre.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Devreese K, Hoylaerts MF. Challenges in the diagnosis of the antiphospholipid syndrome. Clin Chem. 2010;56(6):930–940. doi: 10.1373/clinchem.2009.133678. [DOI] [PubMed] [Google Scholar]
- 30.de Groot PG, Lutters B, Derksen RH, Lisman T, Meijers JC, Rosendaal FR. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost. 2005;3(9):1993–1997. doi: 10.1111/j.1538-7836.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 31.Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost. 2010;8(2):237–242. doi: 10.1111/j.1538-7836.2009.03674.x. [DOI] [PubMed] [Google Scholar]
- 32.Mustonen P, Lehtonen KV, Javela K, Puurunen M. Persistent antiphospholipid antibody (aPL) in asymptomatic carriers as a risk factor for future thrombotic events: a nationwide prospective study. Lupus. 2014;23(14):1468–1476. doi: 10.1177/0961203314545410. [DOI] [PubMed] [Google Scholar]
- 33.de Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of beta 2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105(4):1540–1545. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]
- 34.de Laat B, Pengo V, Pabinger I, et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: an international multicenter study. J Thromb Haemost. 2009;7(11):1767–1773. doi: 10.1111/j.1538-7836.2009.03588.x. [DOI] [PubMed] [Google Scholar]
- 35.Pengo V, Ruffatti A, Tonello M, et al. Antiphospholipid syndrome: antibodies to Domain 1 of beta2-glycoprotein 1 correctly classify patients at risk. J Thromb Haemost. 2015;13(5):782–787. doi: 10.1111/jth.12865. [DOI] [PubMed] [Google Scholar]
- 36.Clark CA, Davidovits J, Spitzer KA, Laskin CA. The lupus anticoagulant: results from 2257 patients attending a high-risk pregnancy clinic. Blood. 2013;122(3):341–347. doi: 10.1182/blood-2013-02-485839. quiz 466. [DOI] [PubMed] [Google Scholar]
- 37.Lockshin MD, Kim M, Laskin CA, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum. 2012;64(7):2311–2318. doi: 10.1002/art.34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chauleur C, Galanaud JP, Alonso S, et al. Observational study of pregnant women with a previous spontaneous abortion before the 10th gestation week with and without antiphospholipid antibodies. J Thromb Haemost. 2010;8(4):699–706. doi: 10.1111/j.1538-7836.2010.03747.x. [DOI] [PubMed] [Google Scholar]
- 39.Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349(12):1133–1138. doi: 10.1056/NEJMoa035241. [DOI] [PubMed] [Google Scholar]
- 40.Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS) J Thromb Haemost. 2005;3(5):848–853. doi: 10.1111/j.1538-7836.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 41.Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood. 2013;122(5):817–824. doi: 10.1182/blood-2013-04-496257. [DOI] [PubMed] [Google Scholar]
- 42.Hunt BJ. Pediatric antiphospholipid antibodies and antiphospholipid syndrome. Semin Thromb Hemost. 2008;34(3):274–281. doi: 10.1055/s-0028-1082271. [DOI] [PubMed] [Google Scholar]
- 43.Levine SR, Brey RL, Tilley BC, et al. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. JAMA. 2004;291(5):576–584. doi: 10.1001/jama.291.5.576. [DOI] [PubMed] [Google Scholar]
- 44.Okuma H, Kitagawa Y, Yasuda T, Tokuoka K, Takagi S. Comparison between single antiplatelet therapy and combination of antiplate-let and anticoagulation therapy for secondary prevention in ischemic stroke patients with antiphospholipid syndrome. Int J Med Sci. 2009;7(1):15–18. doi: 10.7150/ijms.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Son M, Wypasek E, Celinska-Lowenhoff M, Undas A. The use of rivaroxaban in patients with antiphospholipid syndrome: a series of 12 cases. Thromb Res. 2015;135(5):1035–1036. doi: 10.1016/j.thromres.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer JK, McBane RD, Black DF, Williams LN, Moder KG, Wysokinski WE. Failure of dabigatran and rivaroxaban to prevent thromboembolism in antiphospholipid syndrome: a case series of three patients. Thromb Haemost. 2014;112(5):947–950. doi: 10.1160/TH14-03-0272. [DOI] [PubMed] [Google Scholar]
- 47.Win K, Rodgers GM. New oral anticoagulants may not be effective to prevent venous thromboembolism in patients with antiphospholipid syndrome. Am J Hematol. 2014;89(10):1017. doi: 10.1002/ajh.23797. [DOI] [PubMed] [Google Scholar]
- 48.Petri M. Use of hydroxychloroquine to prevent thrombosis in systemic lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep. 2011;13(1):77–80. doi: 10.1007/s11926-010-0141-y. [DOI] [PubMed] [Google Scholar]
- 49.Wu XX, Guller S, Rand JH. Hydroxychloroquine reduces binding of antiphospholipid antibodies to syncytiotrophoblasts and restores annexin A5 expression. Am J Obstet Gynecol. 2011;205(6):576.e577–514. doi: 10.1016/j.ajog.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11(10):1927–1929. doi: 10.1111/jth.12363. [DOI] [PubMed] [Google Scholar]
- 51.Erkan D, Willis R, Murthy VL, et al. A prospective open-label pilot study of fluvastatin on proinflammatory and prothrombotic biomarkers in antiphospholipid antibody positive patients. Ann Rheum Dis. 2014;73(6):1176–1180. doi: 10.1136/annrheumdis-2013-203622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erkan D, Vega J, Ramon G, Kozora E, Lockshin MD. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis Rheum. 2013;65(2):464–471. doi: 10.1002/art.37759. [DOI] [PubMed] [Google Scholar]
- 53.Berman H, Rodriguez-Pinto I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun Rev. 2013;12(11):1085–1090. doi: 10.1016/j.autrev.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Kim KS, Denton MD, Chandraker A, et al. CD28-B7-mediated T cell costimulation in chronic cardiac allograft rejection: differential role of B7-1 in initiation versus progression of graft arteriosclerosis. Am J Pathol. 2001;158(3):977–986. doi: 10.1016/S0002-9440(10)64044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahn P, Ramanujam M, Bethunaickan R, et al. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58(9):2824–2834. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;(2):Cd002859. doi: 10.1002/14651858.CD002859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Backos M, Rai R, Baxter N, Chilcott IT, Cohen H, Regan L. Pregnancy complications in women with recurrent miscarriage associated with antiphospholipid antibodies treated with low dose aspirin and heparin. Br J Obstet Gynaecol. 1999;106(2):102–107. doi: 10.1111/j.1471-0528.1999.tb08208.x. [DOI] [PubMed] [Google Scholar]
- 58.Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies) BMJ. 1997;314(7076):253–257. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e691S–736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14(2):459–465. doi: 10.1111/ajt.12540. [DOI] [PubMed] [Google Scholar]
- 61.Burcoglu-O’Ral A, Erkan D, Asherson R. Treatment of catastrophic antiphospholipid syndrome with defibrotide, a proposed vascular endothelial cell modulator. J Rheumatol. 2002;29(9):2006–2011. [PubMed] [Google Scholar]
- 62.Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15(2):145–151. doi: 10.1006/jaut.2000.0409. [DOI] [PubMed] [Google Scholar]
- 63.Erkan D, Harrison MJ, Levy R, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum. 2007;56(7):2382–2391. doi: 10.1002/art.22663. [DOI] [PubMed] [Google Scholar]
- 64.Giron-Gonzalez JA, Garcia del Rio E, Rodriguez C, Rodriguez-Martorell J, Serrano A. Antiphospholipid syndrome and asymptomatic carriers of antiphospholipid antibody: prospective analysis of 404 individuals. J Rheumatol. 2004;31(8):1560–1567. [PubMed] [Google Scholar]
- 65.Amengual O, Fujita D, Ota E, et al. Primary prophylaxis to prevent obstetric complications in asymptomatic women with antiphospholipid antibodies: a systematic review. Lupus. 2015;24(11):1135–1142. doi: 10.1177/0961203315578765. [DOI] [PubMed] [Google Scholar]