Abstract
Background: Temporality between socioeconomic status (SES), depressive symptoms (DS), dietary quality (DQ), and central adiposity (CA) is underexplored.
Objectives: Alternative pathways linking SES to DQ, DS, and CA were tested and models compared, stratified by race and sex.
Methods: With the use of data from the Healthy Aging in Neighborhoods of Diversity across the Life Span (baseline age: 30–64 y; 2 visits; mean follow-up: 4.9 y), 12 structural equation models (SM) were conducted and compared. Time-dependent factors included the Center for Epidemiologic Studies–Depression [CES-D total score, baseline or visit 1 (v1), follow-up or visit 2 (v2), mean across visits (m), and annual rate of change (Δ)], 2010 Healthy Eating Index (HEI) (same notation), and central adiposity principal components' analysis score of waist circumference and trunk fat (kg) (Adipcent) (same notation). Sample sizes were white women (WW, n = 236), white men (WM, n = 159), African American women (AAW, n = 395), and African American men (AAM, n = 274), and a multigroup analysis within the SM framework was also conducted.
Results: In the best-fitting model, overall, ∼31% of the total effect of SES→Adipcent(v2) (α ± SE: −0.10 ± 0.03, P < 0.05) was mediated through a combination of CES-D(v1) and ΔHEI. Two dominant pathways contributed to the indirect effect: SES→(−)CES-D(v1)→(+)Adipcent(v2) (−0.015) and SES→(+) ΔHEI→(−)Adipcent(v2) (−0.017), with a total indirect effect of −0.031 (P < 0.05). In a second best-fitting model, SES independently predicted Adipcent(v1, −0.069), ΔHEI(+0.037) and CES-D(v2, −2.70) (P < 0.05), with Adipcent(v1) marginally predicting ΔHEI(−0.014) and CES-D(v2, +0.67) (P < 0.10). These findings were indicative of DS's and CA's marginally significant bidirectional association (P < 0.10). Although best-fit–selected models were consistent across race × sex categories, path coefficients differed significantly between groups. Specifically, SES→Adipcent[v1(+0.11), v2(+0.14)] was positive among AAM (P < 0.05), and the overall positive association of Adipcent(v1)→CES-D(v2) was specific to AAW (+0.97, P < 0.10).
Conclusions: Despite consistent model fit, pathways linking SES to DQ, DS, and CA differed markedly among the race × sex groups. Our findings can inform the potential effectiveness of various mental health and dietary interventions.
Keywords: depression, dietary quality, central adiposity, socioeconomic status, urban adults
Introduction
Depression and obesity are 2 global public health problems, with major depressive disorder ranking among the top 10 disability causes worldwide (1, 2). Obesity is often comorbid with depression (3). Obesity is also an independent risk factor for cardiovascular disease, with visceral fat or central adiposity (CA)9 suggested as the primary mechanism, particularly among women (4).
There are inconsistent findings on the association between depression and obesity. Cross-sectional studies suggest that obesity causes depression (5–9), although others support an opposite temporal direction (10–14) A U-shaped relation between adiposity and depression was also uncovered (9), a few studies found an inverse relation (9, 15, 16), but several detected no association (17–21). Cohort studies positively associated depression with adiposity in one or both temporal directions (6–8, 10, 22–28).
Sociodemographic factors such as sex (5, 6, 8–15, 27–29), age, race, and socioeconomic status (SES) play a role in the association between depression and obesity (5, 7, 11–13, 28, 30). CA is influenced by lifestyle factors such as diet quality (DQ) and physical activity (11, 12, 14, 31, 32), which also have been linked to depressive symptoms (DS) (5, 11, 12, 14, 33–38). Because parental SES and cultural influences are stable factors predetermined early in life, it is likely the most antecedent variable in the causal pathway, potentially affecting DS, DQ, and CA, perhaps differentially by sex and race. Many of the studies examining similar research questions were unidirectional and cross-sectional in nature. Only a few recent studies looked at the relation between depressive symptoms and obesity in a bidirectional manner (6, 22, 24). Our study goes a step beyond this to include DQ and examine SES as an antecedent factor, while uncovering the most likely temporal relations between diet, DS, and CA in a sample of urban adults.
The Healthy Aging in Neighborhood of Diversity across the Life Span (HANDLS) provides an opportunity to study the association between SES, DS, DQ, and CA over a mean period of ∼5 y and test temporal associations between those key variables. As an extension to a previous study that used only baseline cross-sectional data in HANDLS (11), the present study uses longitudinal data to examine 1) SES disparities in CA, DS, and DQ, as well as moderation by sex and race, and 2) alternative structural equations models (SMs) linking SES, DS, DQ, and CA, stratified by sex and race, and with SES considered the most antecedent variable in all models.
Specifically, this article focuses on the following subaims: the associations between SES and the key measures of interest both at visit 1 (v1), visit 2 (v2), mean across visits (m), and annual rate of change (Δ); testing heterogeneity of those associations by sex and race; testing alternative SMs and finding best fit; changing the temporal relation between the key variables, with the exception of SES; testing heterogeneity by sex and race; examining more closely the mediating effects of intermediate variables in the best-fitting models; and finally, examining those mediating effects within each sex × race group.
Methods
Database
HANDLS is a prospective cohort study of a representative sample of African American men (AAM), African American women (AAW), white men (WM), and white women (WW) aged 30–64 y at v1. Study participants were recruited by household screenings as a fixed cohort, with the use of an area probability sampling design covering 13 census segments in Baltimore. Data were collected in 2 separate phases at v1 [2004–2009; also known as wave 1 (39)]. Phase 1 assessed sociodemographic information, as well as physiologic and psychologic chronic exposure, and included the first 24-h dietary recall. Phase 2 consisted of in-depth examinations in mobile medical research vehicles and included a second 24-h dietary recall and psychometric, anthropometric, and body composition measurements (40). V2 of HANDLS was initiated in 2009 and completed in 2013 (also known as wave 3). The protocol for v2 was a medical research vehicle examination followed by a telephone interview.
The study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences, NIH. All participants provided written informed consent.
Study population
Follow-up time between v1 and v2 ranged from <1 to ∼8 y, with a mean ± SE of 4.88 ± 0.03 y. HANDLS initially recruited 3720 participants (sample 1), of whom 669 had complete data on CA measures [waist circumference (WC) and trunk fat (TF) in kilograms] only at v1 (sample 2a); 202 had those CA measures available only at follow-up (sample 2b) and 1821 at both visits (sample 2c). Among those, DQ (with the use of two 24-h recalls) was available at both visits simultaneously in 1332 (sample 3). Moreover, participants with missing data on DS and education (y) were excluded, yielding 1064 (sample 4) (Supplemental Figure 1). Participants in sample 4 compared with others in sample 1 had a higher proportion African Americans (69% compared with 60%; P = 0.001), a marginally lower percentage of men (42.5% compared with 47.9, P < 0.10), and a marginally higher proportion of self-rated health as very good/excellent (41% compared with 37%, P < 0.10).
Central adiposity outcomes.
Two CA measurements were used. First, WC was assessed with a tape measure applied to the hip bone, wrapping around the waist at the navel, keeping the tape parallel to the floor, and ensuring that the person was not holding his or her breath and that the tape was not wrapped too tight or too loose. WC was estimated to the nearest 1/10th of a centimeter. DXA was performed with a Lunar DPX-IQ (Lunar Corp.) at v1 and Hologic Discovery QDR (Bedford, Massachusetts) at v2, with scans measuring total tissue, fat and lean mass, and regional fat mass, among others. A comparability substudy indicated that findings from Lunar and Hologic scans were valid and comparable at v1. From DXA scans, TF provided a second measure of CA. Principal components analysis combined WC and TF into a single measure at each visit. The central adiposity factor score derived from a principal components analysis of waist circumference and trunk fat (kg) (Adipcent) (a standardized z score), was entered into a mixed-effects linear regression model with time. An empirical Bayes estimator of the slope for each individual was obtained, reflecting individual-level Δ in CA (ΔAdipcent). Moreover, m of Adipcent [Adipcent(m)] was also computed for each individual. Those variables were included in the SMs whenever they were the outcomes of a baseline variable aside from SES and the predictors of the final follow-up outcome.
Depressive symptoms assessment
Cognitive and neuropsychologic tests were administered at both visits by trained psychometricians (41). Tests included the Center for Epidemiologic Studies–Depression (CES-D) scale, a 20-item self-report symptom rating scale that emphasizes the affective, depressed mood component (42). Factorial invariance in the CES-D structure was demonstrated when contrasting results from NHANES I with HANDLS (43). We used only the CES-D total continuous score. Similar to Adipcent, CES-D total score was measured at v1 and v2, CES-D(m), and as ΔCES-D with a mixed-effect regression model.
Dietary assessment and dietary quality measurement: 2010 Healthy Eating Index
All 24-h dietary recalls were obtained with the USDA Automated Multiple Pass Method, a computerized structured interview (44). Measuring cups, spoons, ruler, and an illustrated Food Model Booklet were used as measurement aids. Trained nutrition professionals coded recall data, matching foods consumed with 8-digit codes from the Food and Nutrient Database for Dietary Studies (45).
DQ was assessed with the 2010 Healthy Eating Index (HEI). Steps for calculating the HEI are available from the National Cancer Institute (46) and the National Institute on Aging (47). The HEI was calculated for each day of the two 24-h recalls (days 1 and 2) and then averaged to obtain the mean 2010 total and component scores. Only the total score of HEI for v1 and v2 was used in the present study. With the use of a mixed-effect regression model, the Δ for HEI total score (ΔHEI) was measured. Similarly, the mean between the 2 visits was another intermediate variable of interest [HEI(m)].
SES
SES was measured by completed years of education and poverty status (poverty income ratio <125%: below poverty; poverty income ratio ≥125%: above poverty). A principal components analysis of education (y) and poverty status was conducted, yielding a standardized SES factor score.
Covariates
Most analyses were stratified simultaneously by race (white compared with African American) and sex. Other covariates included age (y), marital status (married compared with unmarried), smoking status (0 = never, 1 = former smoker, and 2 = current smoker), and drug use (marijuana, cocaine, and/or opiates; coded as 0 = never, 1 = former user, and 2 = current user).
Statistical methods
STATA release 13.0 (StataCorp LP) was used in all analyses. The descriptive part took into account design complexity and unequal probability of sampling by including sampling weights and obtaining representative estimates of means and proportions with standard errors with the use of Taylor series linearization. The Wald test from regression models (linear regression for continuous variables and logistic regression for categorical variables), taking into account sampling weights (Stata survery command:linear regression), was used to compare means across race × sex groups, considering WW as the referent category.
Beyond the descriptive parts of the analysis, several multiple regression models and path analyses were run for 2 specific purposes: 1) testing SES differences in HEI, CES-D, and Adipcent measures (v1 and v2, m, and Δ), both overall and stratifying by race and sex, while testing the significance of interaction terms in a separate model, specifically SES × race × sex, at a type I error of 0.05. Sampling design complexity was also adjusted for in those models by adding the sampling weights. In those models, v1 age, marital status, smoking, and drug use were adjusted for, in addition to the inverse Mills ratio. The latter was predicted from a probit model with a binary outcome (1 = selected compared with 0 = not selected into final sample), with key predictors being v1 age, sex, race, poverty income ratio, and education. This method to adjust for sample selectivity (2-stage Heckman selection) is described elsewhere in more detail (11, 48). 2) Testing relations between SES, CES-D, HEI, and Adipcent by switching temporal relations between those key variables while controlling for exogenous variables of v1 age, marital status, smoking, and drug use behaviors, as well as the inverse Mills ratio, and keeping SES as the most antecedent endogenous variable. It is assumed in this model that a baseline variable other than SES precedes change between the visits (or mean between visits) in a second distinctive variable, which in turn precedes the follow-up value of a third variable. Thus, permutation of the temporal relations between CES-D, HEI, and Adipcent gave 6 possible models, and additional permutations of the 2 different ways of measuring the intermediate variable yielded a total 12 possible models. Consequently, a set of 12 SMs was estimated, first in the total sample and, second, stratifying by sex and race. In all those models, SES was an endogenous variable, which was allowed to predict all other outcome variables (i.e., CES-D, HEI, and Adipcent measures). The set of equations and hypothesized models is presented in Supplemental Figure 2. This part of the analysis was not adjusted for sampling design complexity to obtain appropriate model fit indexes (49).
Global model fit often evaluated in SMs include the comparative fit index (close fit when close to 1), the Akaike information criterion (AIC), the Bayesian information criterion (BIC) (smaller numbers indicate better fit), the root mean error of approximation with its 90% CI [close fit when root mean error of approximation close to zero (<0.05), reasonable fit when between 0.05 and 0.08], and the standardized root mean squared residual (<0.08 for close fit) (49). However, given that our models were saturated (just-identified, with zero degrees of freedom) and our goal was to compare nonnested models, the only fit indexes that were available were the AIC and BIC. Thus, best fit was determined with the lowest AIC/BIC criteria with a relative margin of difference of 5%. Two or more models were chosen if they were within this 5% margin of difference from the model with the lowest AIC/BIC (49). For the selected model(s), a multigroup analysis was conducted to determine whether the hypothesized model was equal across groups, with the use of a standard Wald test (49).
Furthermore, for all models, the mediation proportion (MP, %) was estimated to quantify the proportion of the total effect of a variable explained by a particular pathway or indirect effect (50, 51). MP is presented only when the total effect’s associated P is <0.10. A cutoff of 10% or higher for MP is used to indicate substantial mediation. Moreover, the significance of the indirect, direct, and total effects is also reported and described.
Results
Characteristics of study population: Sex × race differences.
Taking WW as referent, educational attainment was lower and poverty status was more prevalent among AAM and AAW than among WW (Table 1). Proportion married was higher among WM than among WW (44.7% compared with 35.7, P < 0.05), and a higher prevalence of current smoking was found among AAM and AAW than among WW (62.1% and 40.7% compared with 23.7%, P < 0.05). This was also the case for current illicit drug use, when AAM was compared with WW (28.1% compared with 9.0%, P < 0.05). CES-D(v1, m) were more elevated among WW than among WM. However, no race × sex differentials were noted for ΔCES-D, which indicated an increase in depressive symptoms over time.
TABLE 1.
Study characteristics of selected HANDLS participants (baseline age: 30–64 y, n = 1064)1
| Whites (n = 395) |
African Americans (n = 669) |
|||
| Women (n = 236) | Men (n = 159) | Women (n = 395) | Men (n = 274) | |
| Age at v1, y | 46.3 ± 0.8 | 48.5 ± 1.0 | 47.3 ± 0.7 | 47.5 ± 0.8 |
| Age at v2, y | 51.0 ± 0.8 | 53.4 ± 1.0 | 52.3 ± 0.7 | 52.3 ± 0.8 |
| Follow-up time, y | 4.78 ± 0.04 | 4.89 ± 0.07 | 4.92 ± 0.05* | 4.87 ± 0.05 |
| Marital status, % | ||||
| Married | 35.7 | 44.7* | 21.6 | 32.5 |
| Missing | 3.5 | 3.1 | 3.2 | 3.5 |
| Education, y | 15.2 ± 0.4 | 14.3 ± 0.4 | 12.6 ± 0.2* | 12.6 ± 0.3* |
| Poverty income ratio, % | ||||
| <125%: Poor | 13.4 | 12.0 | 26.9* | 21.1* |
| ≥125%: Not poor | 86.5 | 87.9 | 73.1 | 78.9 |
| SES factor score | 1.1 ± 0.1 | 0.9 ± 0.1 | 0.3 ± 0.1* | 0.4 ± 0.1* |
| Smoking status, % | ||||
| Never | 41.5 | 36.4 | 34.6 | 20.3 |
| Former smoker | 21.9 | 22.1 | 18.9 | 13.3 |
| Current smoker | 23.7 | 28.4 | 40.7* | 62.1* |
| Missing | 12.9 | 13.1 | 5.8* | 4.3* |
| Illicit drug use, % | ||||
| Never | 47.9 | 36.4 | 49.0 | 24.6 |
| Former | 30.2 | 39.7 | 30.6 | 43.0 |
| Current | 9.0 | 10.8 | 14.6 | 28.1* |
| Missing | 12.9 | 13.1 | 5.8 | 4.3* |
| CES-D score | ||||
| CES-D(v1) | 10.7 ± 0.8 | 7.7 ± 0.6* | 10.6 ± 0.6 | 9.8 ± 0.6 |
| CES-D(v2) | 13.6 ± 1.2 | 11.8 ± 1.0 | 14.5 ± 0.9 | 13.0 ± 0.9 |
| CES-D(m) | 12.1 ± 0.9 | 9.7 ± 0.7* | 12.6 ± 0.7 | 11.4 ± 0.6 |
| ΔCES-D | 0.68 ± 0.16 | 0.73 ± 0.10 | 0.79 ± 0.10 | 0.68 ± 0.11 |
| HEI score | ||||
| HEI(v1) | 49.7 ± 1.4 | 45.0 ± 1.1* | 44.3 ± 0.8* | 42.7 ± 0.9* |
| HEI(v2) | 55.5 ± 1.2 | 48.7 ± 1.2* | 46.5 ± 0.9* | 45.6 ± 1.1* |
| HEI(m) | 52.6 ± 1.2 | 46.9 ± 1.0* | 45.4 ± 0.7* | 44.2 ± 0.8* |
| ΔHEI | 0.86 ± 0.02 | 0.75 ± 0.02* | 0.71 ± 0.02* | 0.70 ± 0.02* |
| CA, Adipcent score | ||||
| Adipcent(v1) | −0.26 ± 0.10 | −0.11 ± 0.08 | 0.18 ± 0.08* | −0.65 ± 0.08* |
| Adipcent(v2) | −0.40 ± 0.11 | −0.21 ± 0.12 | 0.12 ± 0.09* | −0.66 ± 0.10 |
| Adipcent(m) | −0.33 ± 0.10 | −0.16 ± 0.09 | 0.15 ± 0.08* | −0.66 ± 0.09* |
| Δ Adipcent | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| WC, cm | ||||
| WC(v1) | 91.2 ± 1.5 | 100.4 ± 1.3* | 97.9 ± 1.3* | 92.7 ± 1.2 |
| WC(v2) | 95.8 ± 1.5 | 104.3 ± 1.6* | 102.4 ± 1.2* | 98.4 ± 1.2 |
| WC(m) | 93.5 ± 1.5 | 102.4 ± 1.4* | 100.1 ± 1.2* | 95.5 ± 1.2 |
| TF, kg | ||||
| TF(v1) | 14.3 ± 0.6 | 13.0 ± 0.4 | 16.8 ± 0.5* | 9.9 ± 0.5* |
| TF(v2) | 16.2 ± 0.6 | 14.2 ± 0.7* | 19.4 ± 0.6* | 11.6 ± 0.6* |
| TF(m) | 15.2 ± 0.6 | 13.6 ± 0.5* | 18.1 ± 0.5* | 10.7 ± 0.5* |
Values are means ± SEMs or percentages. CA is measured with Adipcent, DQ is measured with HEI, and DS is measured with CES-D. *Different from white women, P < 0.05, based on Wald tests from ordinary least squares linear regression models for continuous variables with race × sex as the only predictor. Logistic regression models are used for binary variables. All analyses accounted for sampling design complexity by including population weights. Adipcent, central adiposity factor score derived from a principal components analysis of waist circumference and trunk fat (kg); CA, central adiposity; CES-D, Center for Epidemiologic Studies–Depression; DQ, diet quality; DS, depressive symptoms; HANDLS, Healthy Aging in Neighborhoods of Diversity across the Life Span; HEI, Healthy Eating Index; m, mean across visits; SES, socioeconomic status (z score derived from a principal components analysis of education in years and poverty status); TF, trunk fat; v1, visit 1; v2, visit 2; WC, waist circumference; Δ, annual rate of change.
ΔHEI indicated an improvement in overall dietary quality in all groups, with the fastest rate of increase observed in WW (mean: 0.86 compared with 0.70–0.75/y in other race × sex groups, P < 0.05). WW had a higher mean total score on the HEI(v1, v2) than did the other groups. No race × sex group difference in ΔCA was noted when using WW as the referent, although Adipcent was increasing over time at 0.05–0.07 SDs/y. The mean WC was significantly higher among WM and AAW than among WW, whereas the mean TF was higher in AAW than in WW and significantly lower among WM and AAM than among WW.
Socioeconomic differences in DS, DQ, and CA within each race × sex group.
Adjusted SES differences in CES-D, HEI, and Adipcent are presented in Table 2. SES factor score was consistently inversely linked to CES-D(v1, v2, m) and to ΔCES-D among WW and AAW. SES was also positively related to HEI(v1, v2, m) and ΔHEI with a significantly and consistently stronger association observed in WW than in AAW. Among WW, SES was inversely related to Adipcent across waves but not to ΔAdipcent. SES’s inverse association with Adipcent was stronger among WW compared with both AAW and AAM (P-interaction < 0.05 for the SES × sex × race term).
TABLE 2.
Multiple ordinary least squares linear models: SES factor score predicting CES-D, HEI, and Adipcent across waves and moderation by sex and race among selected HANDLS participants (baseline age: 30–64 y; n = 1064)1
| n | v1 | v2 | Mean between visits | Annual rate of change, Δ | |
| CES-D | |||||
| White women | 236 | −2.04 ± 0.60* | −3.39 ± 0.71* | −2.72 ± 0.55* | −0.26 ± 0.10* |
| White men | 159 | −2.39 ± 0.65* | −2.95 ± 1.02* | −2.67 ± 0.80* | −0.15 ± 0.10 |
| African American women | 395 | −1.57 ± 0.56* | −2.64 ± 0.77* | −2.11 ± 0.60* | −0.19 ± 0.09* |
| African American men | 274 | −1.54 ± 0.45* | −2.21 ± 0.82* | −1.88 ± 0.59* | −0.12 ± 0.10 |
| HEI | |||||
| White women | 236 | 3.93 ± 0.97* | 5.40 ± 0.70* | 4.66 ± 0.63 | 0.08 ± 0.02* |
| White men | 159 | 2.43 ± 0.89* | 4.49 ± 0.97* | 3.46 ± 0.78* | 0.08 ± 0.02* |
| African American women | 395 | 0.41 ± 0.67* | 1.16 ± 0.782 | 0.79 ± 0.612 | 0.02 ± 0.022 |
| African American men | 274 | 1.66 ± 0.91* | 2.02 ± 0.82*,2 | 1.84 ± 0.69*,2 | 0.03 ± 0.02 |
| Adipcent | |||||
| White women | 236 | −0.30 ± 0.06* | −0.30 ± 0.07* | −0.30 ± 0.06* | 0.00 ± 0.00 |
| White men | 159 | −0.13 ± 0.072 | −0.16 ± 0.10 | −0.14 ± 0.082 | −0.00 ± 0.01 |
| African American women | 395 | 0.14 ± 0.072 | 0.07 ± 0.09 2 | 0.10 ± 0.082 | −0.01 ± 0.00 |
| African American men | 274 | −0.00 ± 0.062 | 0.04 ± 0.08 2 | 0.02 ± 0.072 | 0.01 ± 0.00 |
Values are linear regression coefficients β ± SE. CA is measured with Adipcent, DQ is measured with HEI, and DS is measured with CES-D. *P < 0.05 for null hypothesis that β = 0 in the multiple linear regression model. All analyses accounted for sampling design complexity by including population weights. Each model is adjusted for v1 age, marital status (unmarried = 0 compared with married = 1), smoking (never = 0 compared with former = 1 or current = 2), and illicit drug use status (never = 0 compared with former = 1 or current = 2) and the inverse Mills ratio. Adipcent, central adiposity factor score derived from a principal components analysis of waist circumference and trunk fat (kg); CA, central adiposity; CES-D, Center for Epidemiologic Studies–Depression; DQ, dietary quality; DS, depressive symptoms; HANDLS, Healthy Aging in Neighborhoods of Diversity across the Life Span; HEI, Healthy Eating Index; m, mean across visits; SES, socioeconomic status (z score derived from a principal components analysis of education in years and poverty status); v1, visit 1 or baseline; v2, visit 2 or follow-up.
P-interaction < 0.05 for null hypothesis of no difference by sex and race in the effect of SES on the outcome variable, based on a model with race × sex and SES × race × sex entered in addition to the main effects and the potential confounders. “White women” is the referent category being compared with all other sex and race groups.
Findings from SM: overall study population pathways.
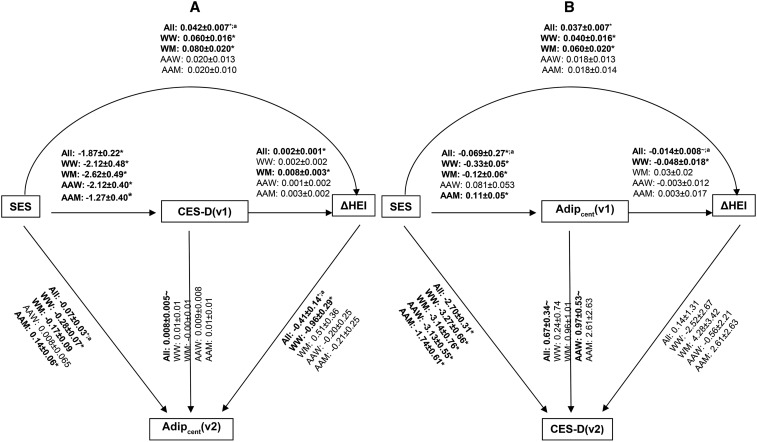
Supplemental Table 1 presents findings for all 12 SMs for the total study sample. With the use of the lowest AIC/BIC criteria, best fit was found for models 3 and 11. Key findings from those models are highlighted in Figure 1. Based on model 3, ∼31% of the total effect of SES on Adipcent(v2) was mediated through a combination of CES-D(v1) and ΔHEI. The overall indirect relation between SES and Adipcent(v2) was composed of the following pathways: SES→(−)CES-D(v1)→(+)ΔHEI→(−)Adipcent(v2), SES→(−)CES-D(v1)→(+)Adipcent(v2), and SES→(+)ΔHEI→(−)Adipcent(v2), with the latter being the most dominant indirect effect [+0.042 × (−0.41) = −0.017 compared with total indirect effect = −0.031], followed by SES→CES-D(v1)→Adipcent(v2) (−1.87 × 0.008 = −0.015).
FIGURE 1.
Best-fit model [(A) model 3] and second best-fit model [(B) model 11] out of the 12 SMs and compared within race × sex with the use of the lowest AIC/BIC criteria. (A) Model 3. All: AIC/BIC = 38,751/38,999; n = 1064; total effects: SES→ΔHEI: +0.037* SES→Adipcent(v2): −0.10* CES-D(v1)→Adipcent(v2): 0.07; indirect effects: SES→ΔHEI: +0.004* SES→Adipcent(v2): −0.031* CES-D(v1)→Adipcent(v2): −0.001* mediation proportions: SES→ΔHEI: −10.8%; SES→Adipcent(v2): +31.0%; CES-D(v1)→Adipcent(v2): NA. WW: AIC/BIC = 8030/8176; n = 236. WM: AIC/BIC = 5108/5237; n = 159. AAW: AIC/BIC = 12,463/12,630; n = 395. AAM: AIC/BIC = 9313/9465; n = 274. (B) Model 11. All: AIC/BIC = 39,044/39,293; n = 1064; total effects: SES→ΔHEI: +0.038* SES→CES-D(v2): −2.73* Adipcent(v1)→CES-D(v2): +0.66~; indirect effects: SES→ΔHEI: +0.0010* SES→CES-D(v2): −0.030~; Adipcent(v1)→CES-D(v2): −0.006~; mediation proportions: SES→ΔHEI: +2.6; SES→CES-D(v2): 1.1; Adipcent(v1)→CES-D(v2): −0.9. WW: AIC/BIC = 8037/8182; n = 236. WM: AIC/BIC = 5148/5277; n = 159. AAW: AIC/BIC = 12,563/12,729; n = 395. AAM: AIC/BIC = 9464/9616; n = 274. ~P < 0.10, *P < 0.05 for null hypothesis that path coefficient = 0 or that total/indirect effect = 0. Detailed findings are presented in Supplemental Tables 1–4. aP < 0.05 based on Wald test for path equality constraint after multigroup analysis. AAM, African American men; AAW, African American women; Adipcent, central adiposity; AIC, Akaike information criterion; BIC, Bayesian information criterion; CES-D, Center for Epidemiologic Studies–Depression; HEI, Healthy Eating Index; NA, not applicable; SES, socioeconomic status; SM, structural equations model; WM, white men; WW, white women; Δ, annual rate of change.
Conversely, model 11 suggested that most total effects of interest were accounted for by direct associations. Specifically, the SES→CES-D(v2) total effect was not explained by the Adipcent(v1)→ΔHEI pathway based on the values of MP, although the indirect association of SES→ΔHEI through Adipcent(v1) was statistically significant (+0.0010, P < 0.05). Thus, in model 11, SES was an independent predictor of Adipcent(v1) (−0.069), ΔHEI (+0.037), and CES-D(v2) (−2.70), with Adipcent(v1) also marginally predicting ΔHEI (−0.014) and CES-D(v2) (+0.67) (P < 0.10).
Findings from SM: across race and sex pathways.
Supplemental Tables 2–4 present findings for all 12 SMs, stratified by race and sex. The selected models (models 3 and 11) are highlighted in Figure 1. Moreover, statistically significant differences across groups are also indicated for each path coefficient based on multigroup analysis. Among WW in model 3 (Figure 1A, Supplemental Table 2), ∼22% of the total effect of SES on Adipcent(v2) (−0.35) was mediated through several pathways involving CES-D(v1) and ΔHEI, particularly the pathway going from SES→(+0.008)ΔHEI→(−0.96)Adipcent(v2), thus bypassing CES-D(v1).
In model 11 (Figure 1B, Supplemental Table 4), among WW, the total effect of SES→ΔHEI(+0.056) was partially mediated through Adipcent(v1) (MP = 28.6%). In fact, SES was inversely related to Adipcent(v1), which was in turn inversely associated with the rate of change in HEI [SES→Adipcent(v1): −0.033; Adipcent(v1)→ΔHEI: −0.048], yielding a significant positive indirect effect of SES→ΔHEI through Adipcent(v1) (+0.016).
Among WM and in model 3 (Figure 1A, Supplemental Table 2), the direct association between SES and ΔHEI was positive (+0.080), whereas the indirect association through CES-D(v1) was an inverse one (−0.021). Similarly, the indirect relation between SES and Adipcent(v2) in WM was a positive one overall (+0.04), with an inverse direct relation found between SES and Adipcent(v2) (−0.28). This uncovers that the direct and indirect pathways from SES to Adipcent(v2) had opposing effects among WM.
Model 11 findings in WM (Figure 1B, Supplemental Table 4) were comparable to the overall population, with only direct unmediated associations found between SES→(+0.060)ΔHEI and SES→(−3.14)CES-D(v2) and a direct inverse relation (−0.12) between SES and Adipcent(v2).
Among AAW (models 3 and 11), the only associations in those models that were significant were an inverse direct relation (−2.12) between SES and CES-D(v1) in model 3 and a similar relation between SES and CES-D(v2) in model 11 (−3.13). Based on model 11, Adipcent(v1) was only marginally positively associated with CES-D(v2) in this group (+0.97, P < 0.10).
In addition to an inverse SES→CES-D(v1, v2) among AAM [models 3 (−1.27) and 11(−1.74)], a direct positive relation between SES and Adipcent [v2(+0.14), v1(+0.11)] was also detected in both models as well. Thus, unlike the pattern found in the total population, a higher SES among AAM was linked to higher amounts of Adipcent that was not mediated by CES-D(v1) or ΔHEI.
Discussion
To our knowledge, our present study is the first to compare models depicting longitudinal relations between SES, DS, DQ, and CA with the use of extensive data on white and African American urban adults that included DXA TF measurements. Several key findings emerged. In the best-fitting model, overall, ∼31% of SES→(−)Adipcent(v2) total effect was mediated through a combination of CES-D(v1) and ΔHEI. Two dominant pathways contributed to the indirect effect: SES→(−)CES-D(v1)→(+)Adipcent(v2) and SES→(+)ΔHEI→(−)Adipcent(v2). In a second best-fitting model, SES independently predicted Adipcent(v1, −), ΔHEI(+), and CES-D(v2, −) (P < 0.05), with Adipcent(v1) marginally predicting ΔHEI(−) and CES-D(v2, +) (P < 0.10). These findings indicated, among others, that depressive symptoms and central adiposity had a marginally significant bidirectional association. Although best fit was consistent across race × sex categories, path coefficients differed significantly between groups. Specifically, SES→Adipcent(v1, v2) was a positive association among AAM (P < 0.05), and the positive direct relation between Adipcent(v1) and CES-D(v2) found in the total population was specific to AAW (P < 0.10).
Only a few cohort studies (6–8, 10, 22–28) have reported a direct association between obesity and depression, of which 3 found bidirectional associations (6, 22, 24). With the use of a large Finnish birth cohort (n = 8451, aged 14 y at v1, 31 y at v2), Herva et al. (6) observed that abdominal obesity among males was closely linked to concomitant depression, whereas being overweight/obese in both adolescence and adulthood may be a risk of depression among females. Moreover, Pan et al. (22) (Nurse’s Health Study; n = 65,955; follow-up time: 10 y; women/age range = 54–79 y/white and other) found that baseline depression was associated with an increased risk of obesity at follow-up, whereas baseline obesity was linked to an increased risk of depression at follow-up. Singh et al. (24) demonstrated a similar bidirectional relation among women in a slightly younger age group (45–50 y; follow-up: 12 y), whereby weight gain was associated with an increased prevalence and incidence of depression, and women with prevalent and incident depression had an increased risk of weight gain.
In contrast, the remaining cohort studies found an association in 1 of 2 temporal directions. For instance, with the use of data on 2251 adults residing in Baltimore, with a mean baseline age of 57.9 y, Sutin and Zonderman (26) found that women who experienced depressed affect had greater increases in BMI and waist and hip circumference across the adult life span. In contrast, baseline adiposity was unrelated to DS trajectory for both sexes. Another study based in Baltimore, covering several ethnicities (men and women, aged 30–89 y at baseline, n = 1071), found that baseline depression predicted weight gain during the 11-y follow up (23). Conversely, 3 other cohort studies found an association within specific sociodemographic groups, between baseline obesity or adiposity and follow-up depression (7, 8, 27). Our study suggested that the association between v1 DS and v2 CA, although a marginally significant one, was among 2 mechanisms mediating the SES disparity in v2 CA. Moreover, v1 CA was also directly linked to v2 DS, specifically among AAW (P < 0.10). Despite best fit being ascribed to model 3, the closeness of fit to model 11 makes both pathways biologically plausible, although each one entails a very different mechanism by which SES is linked to DQ, DS, and CA and different bidirectional relations between those endogenous variables.
A causal pathway for the direct link between depression and leptin resistance, altering appetite and reducing DQ, which in turn increases adiposity, is supported in the literature (52, 53). However, our findings indicated that higher baseline DS can potentially improve DQ over time, particularly among WM. Thus, the direct DS-CA relation is better explained by hypercortisolemia, previously shown to be associated with depression, greater abdominal fat deposits, and the metabolic syndrome, independently of food intake (54–56).
The second main pathway explaining the SES disparity in CA, particularly among WW, bypasses DS and is mediated through a faster improvement in HEI with higher SES. The latter phenomenon [i.e., SES→(+)ΔHEI] is potentially mediated by better food security (57–60), better access to a healthy food environment in wealthier neighborhoods (61–64), lower concerns about food prices (51, 65, 66), and knowledge of healthy dietary habits (51, 66, 67).
In the second best-fitting model, WW experienced a positive relation between SES and change in DQ, which was partially mediated by v1 CA’s inverse relation with both SES and change in DQ. This meant that WW with greater abdominal fat accumulation were less likely to improve their diets over time compared with women with less fat accumulation. This is a novel finding that, to our knowledge, has not been previously reported in longitudinal studies.
Moreover, higher v1 CA was directly but marginally associated with higher v2 DS, particularly among AAW, a finding reported by others (7, 8, 22, 24, 27). This temporal relation can be explained by a lower level of physical activity, body image dissatisfaction, and poor self-esteem, all of which can increase DS severity (14, 68).
Furthermore, our finding of a positive total effect of SES on both v1 and v2 CA among AAM may be attributed to differential ideal body image and body dissatisfaction in this race × sex group, particularly compared with whites (69), with a gap in values becoming more apparent with increased wealth. This finding may also be indicative of reduced physical activity with increased wealth among this race × sex group.
Despite its much strength, including measuring TF with DXA and using SM, our study had a few limitations. First, the residual or direct effect of SES on DS and CA may partly be due to SES disparities in physical activity. HANDLS lacked a reliable baseline measure for physical activity, precluding a test this pathway. Second, DQ was based on 2 self-reported 24-h recalls carrying both random and systematic errors. Although random errors in relation to outcomes (e.g., DS and CA measures) may bias the effect toward the null value, systematic errors could cause bias in either direction. Third, the unequal sample sizes between the race × sex groups may yield more statistical power for AAW compared with the remaining 3 groups.
In conclusion, despite consistent model fit, longitudinal pathways linking SES, DQ, DS, and CA differed markedly between race × sex groups. Specifically, although in AAW, unhealthy eating may not underlie the DS-CA association, and SES is directly and positively associated with CA among AAM, overall and among WW, DS and unhealthy DQ may both contribute to an inverse relation between SES and CA. Therefore, the potential effects of depressive symptoms on dietary behavior or CA or vice versa should be examined more closely within each of those 4 groups to assess the potential effectiveness of various interventions, particularly those targeting mental health, healthy eating behavior, and CA.
Acknowledgments
We thank Salman Tajuddin and Megan Williams for their internal review of the manuscript. MAB, MTF-K, DS, GAD, HAB, OSR, MKE, and ABZ wrote and revised the manuscript; MAB planned the analysis, performed the data management and statistical analysis, and had primary responsibility for the final content; MTF-K, MKE, and ABZ participated in the data acquisition; MTF-K, DS, GAD, and HAB participated in the literature review; and OSR and ABZ participated in the plan of the analysis. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: AAM, African American men; AAW, African American women; Adipcent, central adiposity factor score derived from a principal components analysis of waist circumference and trunk fat (kg); AIC, Akaike information criterion; BIC, Bayesian information criterion; CA, central adiposity; CES-D, Center for Epidemiologic Studies–Depression; DQ, diet quality; DS, depressive symptoms; HANDLS, Healthy Aging in Neighborhoods of Diversity across the Life Span; HEI, Healthy Eating Index; m, mean across visits; MP, mediation proportion; SES, socioeconomic status; SM, structural equations model; TF, trunk fat; v1, visit 1; v2, visit 2; WC, waist circumference; WM, white men; WW, white women; Δ, annual rate of change.
References
- 1.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007;29:6–28. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res 2010;178:230–5. [DOI] [PubMed] [Google Scholar]
- 4.Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med 2009;71:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes (Lond) 2006;30:513–9. [DOI] [PubMed] [Google Scholar]
- 6.Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Laksy K, Joukamaa M. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes (Lond) 2006;30:520–7. [DOI] [PubMed] [Google Scholar]
- 7.Sachs-Ericsson N, Burns AB, Gordon KH, Eckel LA, Wonderlich SA, Crosby RD, Blazer DG. Body mass index and depressive symptoms in older adults: the moderating roles of race, sex, and socioeconomic status. Am J Geriatr Psychiatry 2007;15:815–25. [DOI] [PubMed] [Google Scholar]
- 8.Wild B, Herzog W, Lechner S, Niehoff D, Brenner H, Muller H, Rothenbacher D, Stegmaier C, Raum E. Gender specific temporal and cross-sectional associations between BMI-class and symptoms of depression in the elderly. J Psychosom Res 2012;72:376–82. [DOI] [PubMed] [Google Scholar]
- 9.Zhao G, Ford ES, Dhingra S, Li C, Strine TW, Mokdad AH. Depression and anxiety among US adults: associations with body mass index. Int J Obes (Lond) 2009;33:257–66. [DOI] [PubMed] [Google Scholar]
- 10.Hasler G, Pine DS, Kleinbaum DG, Gamma A, Luckenbaugh D, Ajdacic V, Eich D, Rossler W, Angst J. Depressive symptoms during childhood and adult obesity: the Zurich Cohort Study. Mol Psychiatry 2005;10:842–50. [DOI] [PubMed] [Google Scholar]
- 11.Beydoun MA, Kuczmarski MT, Mason MA, Ling SM, Evans MK, Zonderman AB. Role of depressive symptoms in explaining socioeconomic status disparities in dietary quality and central adiposity among US adults: a structural equation modeling approach. Am J Clin Nutr 2009;90:1084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beydoun MA, Wang Y. Pathways linking socioeconomic status to obesity through depression and lifestyle factors among young US adults. J Affect Disord 2010;123:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper DC, Trivedi RB, Nelson KM, Reiber GE, Zonderman AB, Evans MK, Waldstein SR. Sex differences in associations of depressive symptoms with cardiovascular risk factors and metabolic syndrome among African Americans. Cardiovasc Psychiatry Neurol 2013;2013:979185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, Penninx BW. Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depress Anxiety 2010;27:1057–65. [DOI] [PubMed] [Google Scholar]
- 15.Gil K, Radzillowicz P, Zdrojewski T, Pakalska-Korcala A, Chwojnicki K, Piwonski J, Ignaszewska-Wyrzykowska A, Zaluga L, Mielczarek M, Landowski J, et al. Relationship between the prevalence of depressive symptoms and metabolic syndrome: results of the SOPKARD Project. Kardiol Pol 2006;64:464–9. [PubMed] [Google Scholar]
- 16.Ho RC, Niti M, Kua EH, Ng TP. Body mass index, waist circumference, waist-hip ratio and depressive symptoms in Chinese elderly: a population-based study. Int J Geriatr Psychiatry 2008;23:401–8. [DOI] [PubMed] [Google Scholar]
- 17.Hach I, Ruhl UE, Klotsche J, Klose M, Jacobi F. Associations between waist circumference and depressive disorders. J Affect Disord 2006;92:305–8. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins MA, Miller DK, Stewart JC. A 9-year, bidirectional prospective analysis of depressive symptoms and adiposity: the African American Health Study. Obesity (Silver Spring) 2015;23:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dogan Y, Onat A, Kaya H, Ayhan E, Can G. Depressive symptoms in a general population: associations with obesity, inflammation, and blood pressure. Cardiol Res Pract 2011;2011:740957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy JM, Horton NJ, Burke JD Jr, Monson RR, Laird NM, Lesage A, Sobol AM. Obesity and weight gain in relation to depression: findings from the Stirling County Study. Int J Obes (Lond) 2009;33:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patten SB, Williams JV, Lavorato DH, Brown L, McLaren L, Eliasziw M. Major depression, antidepressant medication and the risk of obesity. Psychother Psychosom 2009;78:182–6. [DOI] [PubMed] [Google Scholar]
- 22.Pan A, Sun Q, Czernichow S, Kivimaki M, Okereke OI, Lucas M, Manson JE, Ascherio A, Hu FB. Bidirectional association between depression and obesity in middle-aged and older women. Int J Obes (Lond) 2012;36:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriksen CA, Mather AA, Mackenzie CS, Bienvenu OJ, Sareen J. Longitudinal associations of obesity with affective disorders and suicidality in the Baltimore epidemiologic catchment area follow-up study. J Nerv Ment Dis 2014;202:379–85. [DOI] [PubMed] [Google Scholar]
- 24.Singh G, Jackson CA, Dobson A, Mishra GD. Bidirectional association between weight change and depression in mid-aged women: a population-based longitudinal study. Int J Obes (Lond) 2014;38:591–6. [DOI] [PubMed] [Google Scholar]
- 25.Kivimäki M, Jokela M, Hamer M, Geddes J, Ebmeier K, Kumari M, Singh-Manoux A, Hingorani A, Batty GD. Examining overweight and obesity as risk factors for common mental disorders using fat mass and obesity-associated (FTO) genotype-instrumented analysis: the Whitehall II Study, 1985–2004. Am J Epidemiol 2011;173:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutin AR, Zonderman AB. Depressive symptoms are associated with weight gain among women. Psychol Med 2012;42:2351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogelzangs N, Kritchevsky SB, Beekman AT, Brenes GA, Newman AB, Satterfield S, Yaffe K, Harris TB, Penninx BW, Health ABCS. Obesity and onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J Clin Psychiatry 2010;71:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong SY, Leung JC, Leung PC, Woo J. Depressive symptoms and change in abdominal obesity in the elderly: positive or negative association? Am J Geriatr Psychiatry 2011;19:730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milaneschi Y, Simonsick EM, Vogelzangs N, Strotmeyer ES, Yaffe K, Harris TB, Tolea MI, Ferrucci L, Penninx BW, Health A, et al. Leptin, abdominal obesity, and onset of depression in older men and women. J Clin Psychiatry 2012;73:1205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicken MT, Lee H, Mezuk B, Kershaw KN, Rafferty J, Jackson JS. Racial and ethnic differences in the association between obesity and depression in women. J Womens Health (Larchmt) 2013;22:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panagiotakos DB, Chrysohoou C, Pitsavos C, Stefanadis C. Association between the prevalence of obesity and adherence to the Mediterranean diet: the ATTICA study. Nutrition 2006;22:449–56. [DOI] [PubMed] [Google Scholar]
- 32.Sichieri R. Dietary patterns and their associations with obesity in the Brazilian city of Rio de Janeiro. Obes Res 2002;10:42–8. [DOI] [PubMed] [Google Scholar]
- 33.Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr 2014;99:181–97. [DOI] [PubMed] [Google Scholar]
- 34.Quirk SE, Williams LJ, O’Neil A, Pasco JA, Jacka FN, Housden S, Berk M, Brennan SL. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry 2013;13:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julien D, Gauvin L, Richard L, Kestens Y, Payette H. Longitudinal associations between walking frequency and depressive symptoms in older adults: results from the VoisiNuAge study. J Am Geriatr Soc 2013;61:2072–8. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Hirvensalo M, Hintsanen M, Hintsa T, Pulkki-Raback L, Jokela M, Telama R, Tammelin T, Hutri-Kahonen N, Viikari JS, et al. Longitudinal associations between changes in physical activity and depressive symptoms in adulthood: the young Finns study. Int J Behav Med 2014;21:908–17. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y, Iwasa H, Kumagai S, Suzuki T, Awata S, Yoshida H. Longitudinal association between habitual physical activity and depressive symptoms in older people. Psychiatry Clin Neurosci 2015;69:686–92. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Yen ST. Physical activity, gender difference, and depressive symptoms. Health Serv Res 2015;50:1550–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Healthy aging in neighborhoods of diversity across the lifespan. Bethesda (MD): National Institute on Aging, Intramural Research Program, NIH; 2004. [Internet]. [cited 2016 Feb 18]. Available from: http://handls.nih.gov/.
- 40.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis 2010;20:267–75. [PMC free article] [PubMed] [Google Scholar]
- 41.Lezak M, Lezak M, editors. Neuropsychological assessment. 4th ed New York: Oxford University Press; 2004. [Google Scholar]
- 42.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 43.Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res 2004;126:177–87. [DOI] [PubMed] [Google Scholar]
- 44.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 45.USDA ARS, Food Surveys Research Group. USDA Food and Nutrient Database for Dietary Studies, 3.0 Beltsville (MD): USDA Agricultural Research Service; 2014 [Internet]. [cited 2016 Mar 29]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=12089.
- 46.National Cancer Institute, Division of Cancer Control and Population Sciences. Rockville (MD); 2013 [Internet]. [cited 2016 Feb 18]. Available from: http://epi.grants.cancer.gov/hei/tools.html.
- 47.Healthy Eating Index 2010 calculation. Baltimore (MD): National Institute on Aging, Intramural Research Program, NIH; 2014. [Internet] [cited 2016 Feb 18]. Available from: http://handls.nih.gov/06Coll-dataDoc.htm.
- 48.Heckman JJ. Sample selection bias as a specification error. Econometrica 1979;47:153–61. [Google Scholar]
- 49.Acock AC. Discovering structural equation modeling using Stata. College Station (TX): StataCorp LP; 2013. [Google Scholar]
- 50.Ditlevsen S, Christensen U, Lynch J, Damsgaard MT, Keiding N. The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology 2005;16:114–20. [DOI] [PubMed] [Google Scholar]
- 51.Beydoun MA, Wang Y. How do socio-economic status, perceived economic barriers and nutritional benefits affect quality of dietary intake among US adults? Eur J Clin Nutr 2008;62:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munzberg H, Myers MG Jr. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 2005;8:566–70. [DOI] [PubMed] [Google Scholar]
- 53.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol 2007;7:648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogelzangs N, Suthers K, Ferrucci L, Simonsick EM, Ble A, Schrager M, Bandinelli S, Lauretani F, Giannelli SV, Penninx BW. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology 2007;32:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber-Hamann B, Hentschel F, Kniest A, Deuschle M, Colla M, Lederbogen F, Heuser I. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med 2002;64:274–7. [DOI] [PubMed] [Google Scholar]
- 56.Young AH. Cortisol in mood disorders. Stress 2004;7:205–8. [DOI] [PubMed] [Google Scholar]
- 57.Hadley C, Zodhiates A, Sellen DW. Acculturation, economics and food insecurity among refugees resettled in the USA: a case study of West African refugees. Public Health Nutr 2007;10:405–12. [DOI] [PubMed] [Google Scholar]
- 58.Ruel MT. Is dietary diversity an indicator of food security or dietary quality? A review of measurement issues and research needs. Food Nutr Bull 2003;24:231–2. [DOI] [PubMed] [Google Scholar]
- 59.Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004;79:6–16. [DOI] [PubMed] [Google Scholar]
- 60.Monsivais P, Drewnowski A. The rising cost of low-energy-density foods. J Am Diet Assoc . 2007;107:2071–6. [DOI] [PubMed] [Google Scholar]
- 61.Cheadle A, Psaty BM, Curry S, Wagner E, Diehr P, Koepsell T, Kristal A. Community-level comparisons between the grocery store environment and individual dietary practices. Prev Med 1991;20:250–61. [DOI] [PubMed] [Google Scholar]
- 62.Murakami K, Sasaki S, Takahashi Y, Uenishi K. Neighborhood food store availability in relation to food intake in young Japanese women. Nutrition 2009;25:640–6. [DOI] [PubMed] [Google Scholar]
- 63.Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. Fruit and vegetable access differs by community racial composition and socioeconomic position in Detroit, Michigan. Ethn Dis 2006;16:275–80. [PubMed] [Google Scholar]
- 64.Black JL, Macinko J. Neighborhoods and obesity. Nutr Rev 2008;66:2–20. [DOI] [PubMed] [Google Scholar]
- 65.Beydoun MA, Powell LM, Wang Y. The association of fast food, fruit and vegetable prices with dietary intakes among US adults: is there modification by family income? Soc Sci Med 2008;66:2218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turrell G, Kavanagh AM. Socio-economic pathways to diet: modelling the association between socio-economic position and food purchasing behaviour. Public Health Nutr 2006;9:375–83. [DOI] [PubMed] [Google Scholar]
- 67.Beydoun MA, Wang Y. Do nutrition knowledge and beliefs modify the association of socio-economic factors and diet quality among US adults? Prev Med 2008;46:145–53. [DOI] [PubMed] [Google Scholar]
- 68.Chaiton M, Sabiston C, O’Loughlin J, McGrath JJ, Maximova K, Lambert M. A structural equation model relating adiposity, psychosocial indicators of body image and depressive symptoms among adolescents. Int J Obes (Lond) 2009;33:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wildes JE, Emery RE, Simons AD. The roles of ethnicity and culture in the development of eating disturbance and body dissatisfaction: a meta-analytic review. Clin Psychol Rev 2001;21:521–51. [DOI] [PubMed] [Google Scholar]