Abstract
Objective
Hypophagia and increased energy expenditure under inflammatory conditions, such as that observed after bacterial lipopolysaccharide (LPS) administration, are associated with leptin secretion. The hypophagic effect of leptin depends in part on the activation of PI3K signaling pathway. However, the role of PI3K in the endotoxemia-induced hypophagia has not been determined.
Methods
In an attempt to examine the functional contribution of the PI3K pathway in hypophagia and weight loss induced by LPS (100 ug/Kg, ip), we performed a central pharmacological PI3K inhibition (LY294002). Additionally, to gain mechanistic insights on the role of the catalytic PI3K p110α subunit in leptin responsive cells, mice expressing Cre-recombinase driven by the Lepr promoter (LepR-Cre) were crossed with mice carrying a loxP-modified p110α allele (Pi3kca gene) (LepRΔp110α). As studies have suggested that the PI3K p110β subunit has a dominant role over p110α in energy homeostasis, we further crossed LepR-Cre mice with loxP-modified p110α and p110β (Pi3kcb gene) alleles (LepRΔp110α+β). In order to verify the requirement of leptin in PI3K effects on food intake, we also used leptin-deficient ob/ob mice.
Results
We found that LPS stimulates PI3K and STAT3 signaling pathways in cells expressing the leptin receptor. Central PI3K inhibition prevented LPS-induced hypophagia and weight loss. Genetic deletion of p110α subunit selectively in LepR cells had no effect on LPS-induced hypophagia and weight loss. However, p110α and p110β double deletion in LepR cells prevented LPS-induced hypophagia and partially reversed the weight loss. Leptin deficiency blunted LPS-induced acute pAKT and pSTAT3 phosphorylation and the acute suppression of food intake.
Conclusions
Our studies show that the PI3K p110β subunit in LepR cells is required for acute endotoxemic hypophagia. The data provide promising approaches for PI3K inhibition in preventing low energy balance and cachectic states during inflammatory challenges.
Keywords: LPS, Metabolism, Leptin, Hypothalamus, Inflammation
Highlights
-
•
Bacterial lipopolyssacharide (LPS) stimulates PI3K pathway in hypothalamic LepR expressing cells.
-
•
LPS-induced hypophagia is prevented by central PI3K inhibition.
-
•
PI3K p110α subunit in LepR cells is not required for LPS-induced hypophagia.
-
•
PI3K p110α and p110β double deletion in LepR cells prevents LPS-induced hypophagia.
1. Introduction
Systemic inflammation triggered by bacterial endotoxin is characterized by increased cytokines, altered energy balance via suppression of food consumption and enhanced thermogenesis, body weight loss and behavioral changes [1], [2], [3], [4]. These responses and the resulting undernutrition compromise the recovery of the organism. Experimentally, the innate immune system can be activated by administration of lipopolysaccharide (LPS), a cell wall component derived from Gram-negative bacteria. LPS or cytokine injection increases the gene expression and the circulating levels of the proinflammatory adipokine leptin in rodents [5], [6], [7]. Leptin is primarily secreted by the white adipose tissue and acts in the brain to control energy homeostasis. Leptin administration decreases food intake and increases energy expenditure [8], [9]. The ability of leptin to reduce food intake requires the signal transducer and activation of transcription 3 (STAT3) signaling, which in turn stimulates the transcription of the proopiomelanocortin (Pomc) gene, a well-known anorexigenic factor [10], [11].
Evidence from several studies indicates that phosphoinositide 3- kinase (PI3K) signaling is an important molecular pathway in metabolic regulation also activated by leptin [12], [13], [14], [15]. Leptin triggers PI3K activity via phosphorylation of the insulin receptor substrate-2 (IRS-2) [12], [16]. The regulatory subunit p85 then binds to IRS and localizes the catalytic activity to the cell membrane. The PI3K p110 catalytic subunit in turn catalyzes the phosphorylation of PIP2 (phosphatidylinositol 4,5-bisphosphate) to PIP3 (phosphatidylinositol 3,4,5-trisphosphate) that finally recruits and activates downstream molecules [17]. Reports using pharmacological approaches have demonstrated that the reduction of food intake by central leptin administration is prevented by pretreatment with PI3K inhibitors [12], [13]. The p110α and p110β subunits are critical for the PI3K action on metabolic regulation and are important candidates to mediate leptin's effects [18], [19], [20]. Genetic suppression of PI3K activity in hypothalamic neurons blocks the acute effects of leptin on cells activity [21], [22].
Luyendyk and coworkers [23] reported that PI3K pathway negatively regulates LPS-induced responses in monocytes and macrophages. However, the role of PI3K in the hypophagia triggered by LPS remains an open question. We hypothesized that leptin-mediated PI3K signaling plays a role in LPS-induced hypophagia. To test this hypothesis, we initially used pharmacologic inhibition of PI3K in mice exposed to endotoxin. In addition, we generated mice lacking either p110α or p110α and p110β isoforms selectively in LepR cells, using the Cre-loxP system. We further assessed the metabolic changes (body weight and food intake) in response to acute LPS treatment and the activation of STAT3 and PI3K pathways. The requirement of leptin in LPS-induced hypophagia, pSTAT3 and pAKT expression was evaluated using leptin-deficient ob/ob mice.
2. Materials and methods
2.1. Ethics statement
All animal procedures were carried out with prior approval from the University of Michigan Committee on Use and Care of Animals (IACUC, Animal Protocol: PRO00004380), in accordance with the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals, as well as an approval of the Ethics Committee for Animal Use of the School of Medicine of Ribeirao Preto, University of Sao Paulo.
2.2. Animals
All animals were kept in a light- (12 h on/off) and temperature- (21–23 °C) controlled environment with free access to water and food. The wild type C57BL/6 (JAX® mice, stock # 000664), the ob/ob (JAX® mice, stock # 000632), the LepR-Cre (JAX® mice, stock # 008320), the R26-tdTomato (JAX® mice, stock # 007914), the Pik3ca loxP/loxP (JAX® mice, stock # 017704) [24] and the Pik3cb loxP/loxP (JAX® mice, stock # 017705) [25] mice were kept in the University of Michigan animal facility. Wild type C57BL/6 mice used for the central injection of the PI3K inhibitor were kept in the Medical School Central Animal Facility of the University of Sao Paulo - Campus of Ribeirao Preto.
In order to visualize the LepRb expressing neurons, we crossed the LepR-Cre, a knock-in strain that coexpresses Cre-recombinase with the Lepr gene, previously described and validated [26], [27], with the R26-tdTomato mouse, which have a loxP-flanked transcription-blocking cassette preventing the expression of CAG promoter-driven tdTomato, a red fluorescent protein. Under Cre-mediated excision of the loxP-flanked site, the endogenous red fluorescence is detected only in LepR cells.
2.3. LepR-specific deletion of p110α or p110α+β and genotyping
To inactivate the catalytic subunit p110α or both subunits p110α and p110β in LepRb neurons, LepR-Cre mice were crossed with mice carrying the loxP-modified p110α (Pi3kca gene) and p110β (Pi3kcb gene) alleles [24], [25]. Preliminary observations indicated that complete Cre-mediated excision is only obtained in LepR-Cre homozygous animals. Therefore, our experimental mice were those homozygous for LepR-Cre allele and homozygous for p110α allele (LepRΔp110α) or homozygous for pl10α and p110β alleles (LepRΔp110α+β), compared with their respective homozygous littermate controls, p110αloxP and p110α + βloxP. Deletion of the p110α and p110β subunits was validated by RT-PCR in arcuate nucleus (ARC) punches from LepRΔp110α and LepRΔp110α+β and their respective control mice. Brains were sliced (thickness: 1.0 mm) according to coordinates from the Franklin and Paxinos mouse brain atlas [28] (−1.3 mm to −2.3 mm from Bregma) and punches of the ARC were microdissected using a stainless-steel punch needle of 1.0 mm in diameter. These mouse lines were also previously used and validated [22].
PCR amplification of the floxed (flanked by loxP sites) genomic region, combined with the PCR detection of the Cre transgene in tail-derived DNA, was performed (Sigma RED Extract-N-Amp Tissue PCR Kit -cat# XNAT). Mice were genotyped at weaning and after experiments, using the pairs of primers described in Table 1.
Table 1.
List of primers used for genotyping of mouse models.
| Mice | Forward | Reverse |
|---|---|---|
| LepR cre | 5′ TGCTTCTGTCCGTTTGCCGGT 3′ | 5′ GTGAAACAGCATTGCTGTCAC 3′ |
| R26-tdTomato | 5′ CTGTTCCTGTACGGCATGG 3′ | 5′ GGCATTAAAGCAGCGTATCC 3′ |
| p110α floxed | 5′CTGTGTAGCCTAGTTTAGAGCAACCATCTA 3′ | 5′CCTCTCTGAACAGTTCATGTTTGATGGTGA 3′ |
| p110β floxed | 5′ CTCAAACTAGTGACTAGAAGCTGTGA 3′ | 5′ CTGATCGAGGCCATTAGAGAAGACCG 3′ |
2.4. Drugs and animal treatment protocol
Intraperitoneal (ip) injection of saline (0.15M NaCl, in 5 μl/g), LPS (100 μg/kg, in 5 μl/g) from Escherichia coli (Sigma, Serotype 026:B6) or leptin (Sigma, 2.5 μg/g, in 5 μl/g) was performed between 4:00–4:30 PM, 2 h before lights off. The PI3K inhibitor LY294002 (Calbiochem, 1 μg/mouse, in 3 μl) [13] or its vehicle (2% DMSO in 0.15M NaCl, 3 μl) was intracerebroventricularly (icv) injected 30 min before saline or LPS injections. All procedures were performed in 8–10 weeks old male mice.
2.5. Experimental procedures
2.5.1. Food intake and body weight phenotyping
Intact mice (n = 6–8/group) were single housed and allowed to adapt to the cages and to handling five days prior to the experiment. On the day of the experiment, food was withdrawn at 04:00 PM and mice received the ip injection of saline or LPS. At 06:00 PM (lights off), the animals were re-fed and food consumption was measured 2, 14 and 24 h afterward. Body weight was determined immediately before the injections and 24 h later. A group of naive wild type mice treated with LPS or saline as described above was subsequently treated with intraperitoneal injection of leptin (2.5 μg/g, ip) or saline for food intake and body weight measurements.
Food intake and body weight were also assessed in wild type mice treated with central injections of the PI3K inhibitor LY294002. For this purpose, eight days before the experiment, anesthetized mice were implanted with a cannula in the lateral ventricle. On the day of the experiment, mice were ascribed to four different groups (n = 8/group): 1) Vehicle + Saline, 2) Vehicle + LPS, 3) LY294002 + Saline, 4) LY294002 + LPS. At 3:30 PM food was withdrawn and mice received an icv injection of vehicle or LY294002. At 04:00 PM, mice received the ip injection of saline or LPS, and 2 h later, the animals were re-fed and food consumption and body weight were measured as described.
2.5.2. Implantation of the cannula into the lateral ventricle
Mice were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (7.5 mg/kg) at a volume of 0.1 mL/100 g and placed in a stereotaxic instrument (Kopf, model 900). A stainless-steel guide cannula (10 mm) was implanted into the right lateral ventricle (stereotaxic coordinates: AP = −0.3 mm, LL = −1.0 mm and depth = −2.5 mm from the Bregma). The cannula was held in place using two stainless-steel screws and dental acrylic resin in the skull. To prevent occlusion of the guide cannula, a 30 gage metal wire filled the cannula. After surgery, the mice received a prophylactic injection of penicillin (50,000 U, i.p.). Eight days after mice received an icv injection of vehicle or LY294002, followed by ip injection of saline or LPS, for food consumption and body weight measurement as described.
2.5.3. pSTAT3 and pAKT immunostaining after LPS administration
To assess the co-expression of the LepRb with pSTAT3 and pAKT in response to endotoxin, homozygous LepR-Cre – tdTomato mice were injected ip with saline or LPS (n = 3/group). Two hours later, the mice were anesthetized using isoflurane (Fluriso, Vet One) and transcardially perfused with saline, followed by 4% formaldehyde in 0.1 M phosphate buffer (PBS). Brains were dissected, post-fixed in the same fixative for 1 h, placed in PBS containing 20% sucrose and sectioned on a cryostat (30-μm sections, 5 series) in the frontal plane. Series of brain sections were later processed for pSTAT3 or pAKT immunostaining. The tdTomato red fluorescence expressed specifically in LepR cells does not require additional staining. We also used p110αloxP and LepRΔp110α mice (n = 5/group) to evaluate pAKT immunoreactivity in response to LPS. Finally, to investigate whether LPS induces pSTAT3 and pAKT expression in leptin-deficient mice, ob/ob mice were injected with saline or LPS (n = 3/group). Two or 4 h after treatment the mice were submitted to the above described procedures for perfusion and immunostaining.
Brain coronal sections were rinsed with PBS and nonspecific binding was prevented by immersing the sections in blocking buffer (PBS, normal donkey serum and Triton X-100) for 1 h at room temperature. The sections were incubated for 48 h at 4 °C with primary antibodies: rabbit anti-phospho STAT3 Y705 (1:2000, Cell Signaling # 9145) or rabbit anti-phospho AKT T308 (1:1000, Cell Signaling # 2965). After rinses, sections were incubated for 1 h with the biotinylated goat anti-rabbit secondary antibody (1:1000, Vector Labs, BA1000) and then processed using the Vectastain Elite avidin-biotin immunoperoxidase method (Vector Labs). Solutions of diaminobenzidine, nickel sulfate, and H2O2 were used to generate blue-black immunolabeling. Finally, the sections were mounted on gelatin-coated slides and coverslipped with DPX. Photomicrographs were acquired using an Axio Imager M2 microscope (Carl Zeiss). The number of pSTAT3 immunoreactive cells was obtained by counting the black (nuclear) staining from a constant area of the ARC using ImageJ® software (Version 1.38, NIH, USA). Only one side of one representative section per mouse was counted.
For immunofluorescence, after incubation in primary antibody, sections were incubated for 2 h with donkey anti-rabbit conjugated with AlexaFluor 488 (1:400, Life Technologies # A21206) secondary antibody. Sections were coverslipped with Fluoromont-G™ mounting medium (Southern Biotechnology Associates) and analyzed using a Leica confocal laser scanning microscope. The immunoreactive structures were excited using argon or helium-neon green lasers with the excitation and barrier filters set for the fluorochrome used (green), and we collected epifluorescence using a DS red filter to visualize the tdTomato protein (red). Images showing the fluorescence were obtained from sequentially acquired images of slices excited by the laser. Fluorescence images of pSTAT3 or pAKT and LepR were superposed to identify the presence of dual-labeled neurons (yellow).
2.5.4. Dissection of the mediobasal hypothalamus for gene or protein expression analyses
Mice were injected with saline or LPS (n = 5–7/group) and 2 h later were deeply anesthetized with isoflurane and euthanized by decapitation. Mediobasal hypothalamic (MBH) fragments were dissected out (thickness: 2.0 mm) from an area 1.0 mm lateral to the midline at the anterior border of the optic chiasm and the anterior border of the mammillary bodies. Tissue was processed for RT-PCR or Western blotting analyses.
2.5.5. Immunoblot analysis
Total protein from mediobasal hypothalamus (MBH) was extracted using 1% Triton-X 100, 25 mM Tris (pH 8.0), 1.5 mM EGTA, 0.5 mM EDTA and protease inhibitor cocktail (PhosphoStop, Roche) at 4 °C and 15,000 g for 30 min. Aliquots of the lysates containing 10 mg of protein were denatured in Laemmli buffer and β-mercaptoethanol (Bio-Rad) at 95 °C for 5 min. Samples were blotted onto a nitrocellulose membrane. Nonspecific binding was prevented by immersing the membranes in blocking buffer (3% BSA in Tris-buffered saline-Tween 20, TBS-T) for 60 min at room temperature. The membranes were then exposed overnight to the primary antibodies: rabbit anti-GAPDH (1:4000, Cell signaling # 5174), rabbit anti-mTOR (1:1000, Cell Signaling # 2972), rabbit anti-phospho mTOR S2448 (1:1000, Cell Signaling # 2971), rabbit anti-phospho FoxO1 S256 (1:1000, Cell Signaling # 9461), rabbit anti-AKT (1:3000, Cell Signaling # 4691), rabbit anti-phospho AKT S473 (1:3000, Cell Signaling # 4058) or rabbit anti-phospho AKT T308 (1:3000, Cell Signaling # 2965). The blots were rinsed in TBS-T and then incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:4000, Cell Signaling # 7074) for 1 h at room temperature. Antibody-antigen complexes were visualized by detecting enhanced chemiluminescence using an ECL detection system (Thermo Scientific) and digital images with Chemi Doc™ XRS+ Image Lab™ software (Bio-Rad). Expression of all proteins/phospho-proteins was normalized to the expression of GAPDH. Data were analyzed as relative expression (%) respective to each control mouse line.
2.5.6. Gene expression by real time RT-PCR
Total RNA was isolated from MBH or ARC punches using RNA extraction kit, Qiazol Reagent (miRNeasy, Qiagen) and DNase treatment (RNase-Free; Qiagen). The cDNA was synthesized using Superscript II and random primers (Invitrogen) according to the manufacturer's protocol. Quantitative real-time PCR was performed on a CFX-384 Bio-Rad RT-PCR detection system using SYBR® Green Gene Expression Assays and pairs of primers designed by Sigma, as described in Table 2. All samples and standard curves were run in triplicate. Water instead of cDNA was used as a negative control. One housekeeping gene (Gapdh) was used for each cDNA sample. Determination of gene transcript in each sample was obtained by the ΔΔCT method [29]. For each sample, the threshold cycle (Ct) of mRNA was measured and normalized to the average of the housekeeping genes (ΔCt = CtUnknown − CtHousekeeping gene). The fold change of mRNA in the unknown sample relative to control group was determined by 2−ΔΔCt, where ΔΔCt = ΔCtUnknown − ΔCtControl. Data are shown as a percentage (%) of the relative mRNA expression to the control group.
Table 2.
List of primers used for quantitative RT-PCR.
| Gene | Forward | Reverse |
|---|---|---|
| Pomc | 5′ TGAAAACCCCCGGAAGTACG 3′ | 5′ ACGTTGGGGTACACCTTCAC 3′ |
| Npy | 5′ CAGAAAACGCCCCCAGAACAAGC 3′ | 5′ GGCAGACTGGTTTCAGGGGATGGAT 3′ |
| Agrp | 5′ AAGCTCAGGGCACAAGAGAC 3′ | 5′ CAGTGCCAACAGCAGAACAC 3′ |
| Gapdh | 5′ GCTCATGACCACAGTCCATGC 3′ | 5′ GTTGGGGGGGGATAGGGCCTCTCTTG 3′ |
| Pik3r1 (p85α) | 5′ ACATCTCAAGGGAAGAAGTG 3′ | 5′ GGATCAGAGAAGCCATATTTTC 3′ |
| Pik3r2 (p85β) | 5′ TTGGAGGATCTTCTGAGTC 3′ | 5′ CTTACTGTAGCATTCACTGTGTC 3′ |
| Pik3ca (p110α) | 5′ GAACCAGTAGGCAACCGTGA 3′ | 5′ GCTCTGCTATGAGGCGAGTT 3′ |
| Pik3cb (p110β) | 5′ TTCTGCCCACCGGGATTTAT 3′ | 5′ AGTCTTCGTGTTTCGTCTTCCA 3′ |
| Pik3cd (p110δ) | 5′ GAACAAGGCAGACATCTAAG 3′ | 5′ CATCCTGTTGTGTTACTTCTC 3′ |
2.6. Statistical analysis
The results are expressed as the means ± SEM and were analyzed using the GraphPad Prism 6 software. Comparison between 2 groups (saline and LPS) was carried out using the unpaired 2-tailed Student's t test. Two-way ANOVA followed by Tukey's post hoc test was used to compare the effects of LPS treatment between mouse lines. Differences were accepted as significant at p < 0.05.
3. Results
3.1. Acute decrease in food intake and body weight after LPS or leptin treatment
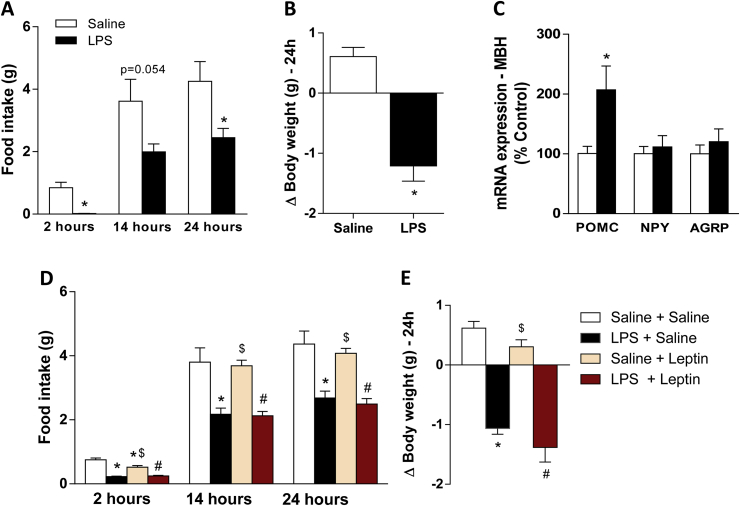
Wild type mice injected with LPS showed a substantial reduction in food intake and body weight from 2 to 24 h after treatment (Figure 1A–B). These responses occurred in parallel to an increase in Pomc gene transcription. No changes in neuropeptide Y (NPY) and agouti related protein (AgRP) mRNA expression were observed (Figure 1C). Exogenous leptin treatment promoted a reduction in food intake only 2 h after treatment, with no effect on body weight (Figure 1D–E). LPS-induced reduction of food intake was significantly higher than leptin-induced reduction of food intake over the course of 24 h. However, we did not observe an exacerbated reduction of food intake in animals treated with both LPS and exogenous leptin, suggesting a ceiling effect of LPS in the inhibition of food consumption.
Figure 1.
LPS- or leptin-induced hypophagia and weight loss. A–B: Graphs showing 2, 14 and 24 h food intake and 24 h change in body weight in wild type mice treated with saline or LPS (100 μg/kg, ip). C: mRNA expression in the mediobasal hypothalamus (MBH), 2 h after treatment. D–E: Graphs showing 2, 14 and 24 h food intake and 24 h change in body weight in wild type mice treated with saline or LPS (100 μg/kg, ip), followed by treatment with saline or leptin (2.5 μg/g, ip). 2-tailed Student's t test was performed. Data are expressed as mean ± SEM. (n = 6–7). *p < 0.05: Saline vs LPS or saline vs leptin; #p < 0.05: Saline + leptin vs LPS + leptin; $ p < 0.05: LPS + Saline vs Saline + leptin.
3.2. Acute LPS induces pSTAT3 and pAKT in ARC LepR neurons
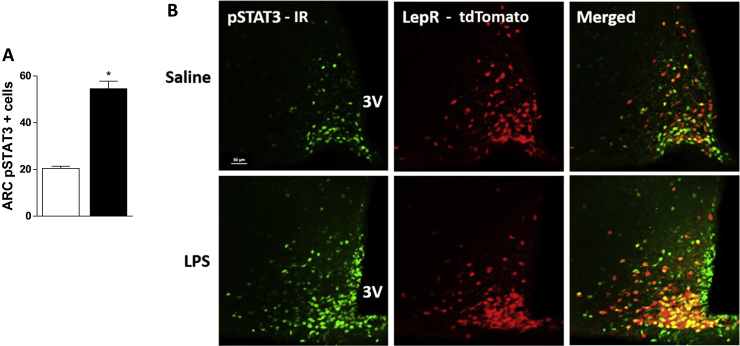
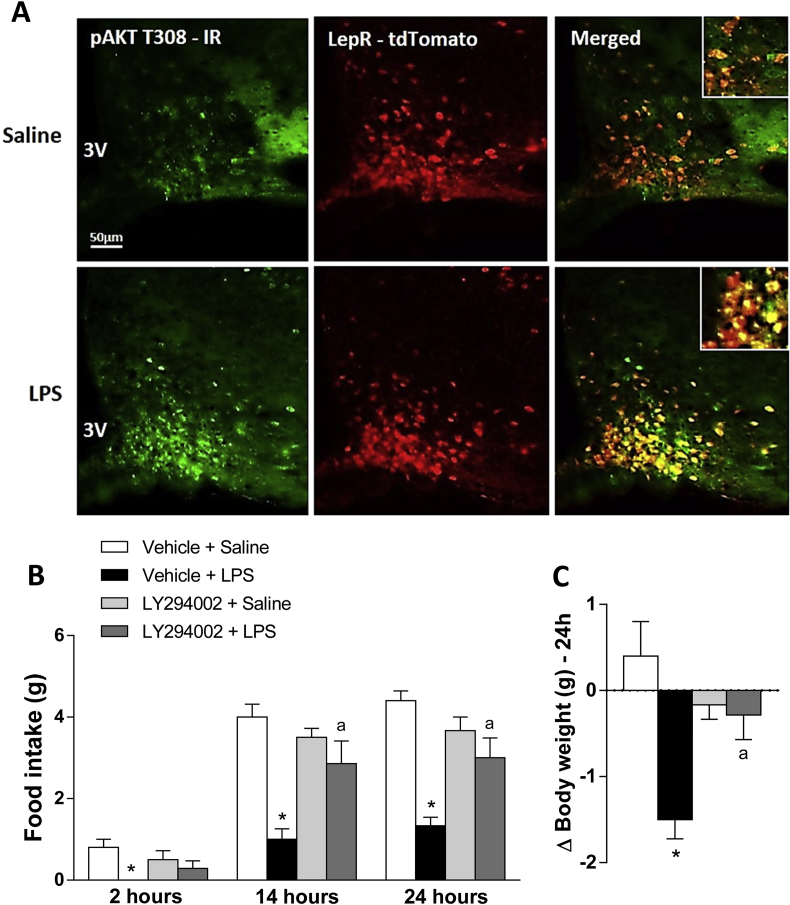
To determine the ARC neuronal population engaged in LPS response, we used LepR-Cre – tdTomato mice. We found that LPS significantly increased pSTAT3 expression in the ARC (Figure 2A). Around 50% of the LPS-induced STAT3 phosphorylation occurs in LepR neurons (Figure 2B). Additionally, LPS increased AKT phosphorylation in the ARC. About 78% of LPS-induced pAKT neurons in the ARC are LepR neurons (Figure 3A), indicating that endotoxin recruits both STAT3 and PI3K pathway in LepR neurons in this nucleus.
Figure 2.
LPS-induced STAT3 phosphorylation. A: number of pSTAT3 positive cells in hypothalamic arcuate nucleus (ARC) and B: representative photomicrographs of ARC 2 h after saline or LPS (100 μg/kg, ip) injection in LepR reporter mice (red), showing pSTAT3 expression (green). 3V = third ventricle. Scale bar: 50 μm 2-tailed Student's t test was performed. Data are expressed as mean ± SEM. (n = 6). *p < 0.05: Saline vs LPS.
Figure 3.
PI3K pathway is required for LPS induced hypophagia. Top panel (A) showing representative photomicrographs of pAKT T308 expression (green) in the hypothalamic arcuate (ARC) nucleus 2 h after saline or LPS (100 μg/kg, ip) injection, in LepR reporter mice (red). 3V = third ventricle. Scale bar: 50 μm. Inset images show examples of LepR neurons co-expressing or not pAKT, 40× magnificent. Graphs showing 2, 14 and 24 h food intake (B) and 24 h changes in body weight (C) in wild type mice treated with icv injection of vehicle (2% DMSO in saline, 3 μl) or PI3K inhibitor LY294002 (1 μg/mouse, 3 μl), followed by saline or LPS (100 μg/kg, ip) injection. Two-way ANOVA followed by Tukey's post hoc test was performed. Data are expressed as mean ± SEM. (n = 8). *p < 0.05: Vehicle + Saline vs Vehicle + LPS; a p < 0.05: Vehicle + LPS vs LY294002 + LPS.
3.3. PI3K pathway is required for LPS-induced hypophagia and weight loss
Considering the acute effect of LPS on food intake and the high localization of LPS-induced pAKT in ARC LepR neurons, we investigated the LPS effect on food intake and body weight in mice devoid of PI3K signaling by means of central injection of the PI3K inhibitor LY294002. We found that LPS treatment no longer had an effect on food intake and weight gain in wild type mice previously injected with PI3K inhibitor (Figure 3B–C), indicating that central PI3K pathway is required for the hypophagia and body weight loss induced by LPS.
3.4. PI3K p110α activity in LepR cells is not required for LPS suppression of food intake and weight loss
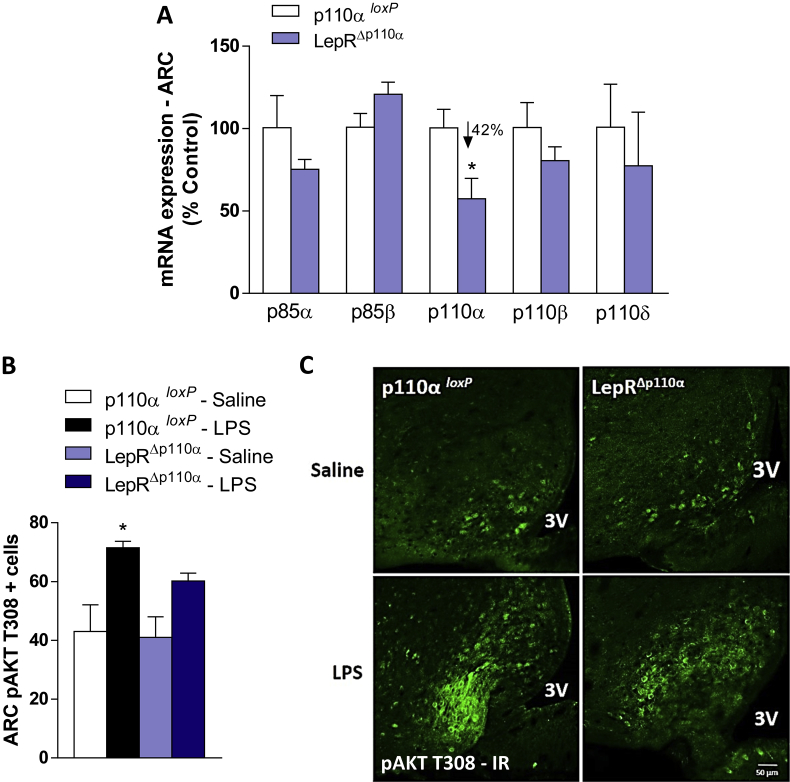
We analyzed if the PI3K p110α catalytic subunit in LepR cells is required for the endotoxemic hypophagia using a genetic mouse model of conditional gene deletion. Mice lacking p110α in LepR cells (LepRΔp110α) presented a 42% reduction of the p110α mRNA expression in the ARC, compared with controls (p110αloxP) (Figure 3A). As PI3K is widely expressed, we do not expect to completely delete p110α from the entire ARC, but selectively from LepR cells. Thus, the remaining ARC p110α expression is likely to originate from neurons other than LepR. The selective p110α deletion did not affect the expression of other, non targeted, PI3K isoforms such as the p85α, p85β, p110β and p110δ (Figure 4A).
Figure 4.
Validation of deletion of PI3K p110α catalytic subunit in LepR cells. The percentage of relative mRNA expression in punches from the hypothalamic arcuate nucleus (ARC) of intact p110α loxP controls in comparison with LepRΔp110α mice (A) (n = 6–7/group). The number of ARC pAKT positive cells in p110αloxP and LepRΔp110α mice 2 h after saline or LPS (100 μg/kg, ip) treatment (B) (n = 5/group). Representative photomicrographs of pAKT T308 expression (green) in the ARC of p110αloxP and LepRΔp110α mice 2 h after treatment (C). 3V = third ventricle. Scale bar: 50 μm 2-tailed Student's t test (A) and Two-way ANOVA followed by Tukey's post hoc test (B) were performed. Data are expressed as mean ± SEM. *p < 0.05: p110α loxPvs LepRΔp110α and p110α loxP Saline vs p110α loxP LPS.
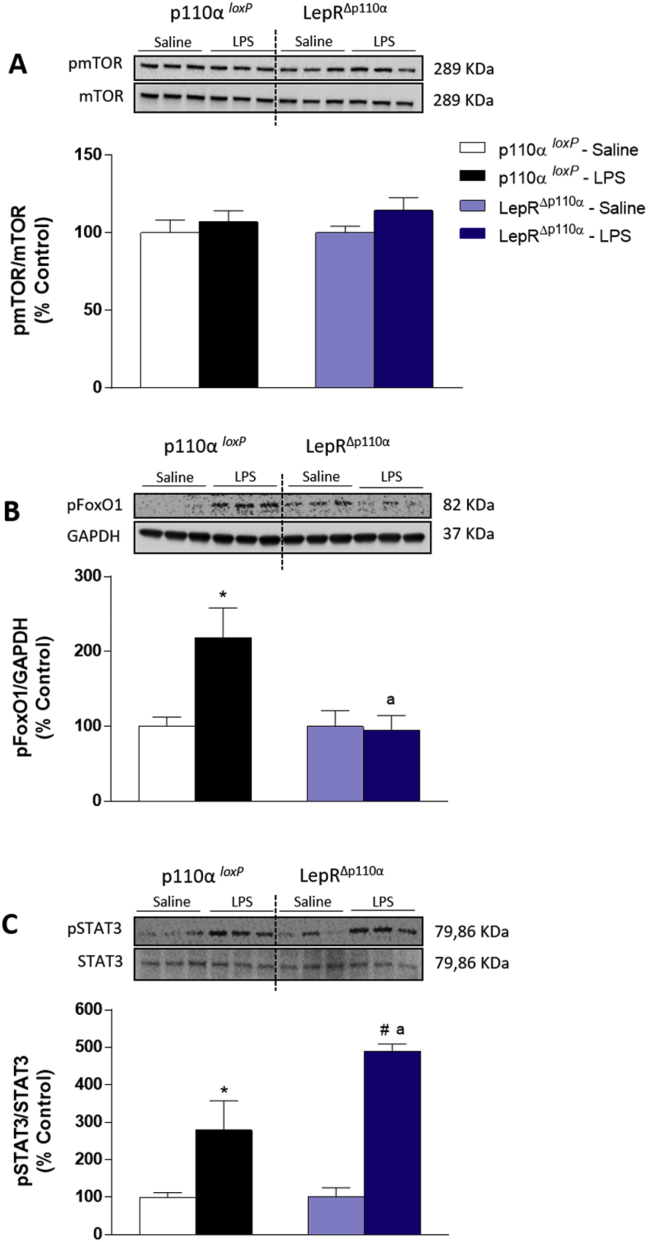
We next assessed PI3K activity in the MBH using pAKT as a read-out. We found that LPS increased AKT phosphorylation in control mice. However, the number of pAKT positive neurons in LepRΔp110α mice did not reach statistical significance, compared with saline treatment, suggesting that the p110α subunit is required for full LPS-induced pAKT expression (Figure 4B–C). LPS did not affect the mTOR phosphorylation in both animal groups (Figure 5A), but it did increase the phosphorylation of the Forkhead box protein O1 (FoxO1) in p110αloxP controls. On the other hand, LPS-induced FoxO1 phosphorylation was blunted in LepRΔp110α mice (Figure 5B), suggesting that p110α in LepR cells is required for full endotoxemia-induced pFoxO1. LPS-induced pSTAT3 expression was potentiated in LepRΔp110α mice, compared with p110αloxP controls (Figure 5C).
Figure 5.
LPS-induced protein phosphorylation in the mediobasal hypothalamus of mice with deletion of p110α catalytic subunit in LepR cells. Percentage of pmTOR (A), pFoxO1 (B) and pSTAT3 expression in the mediobasal hypothalamus of p110αloxP and LepRΔp110α mice 2 h after saline or LPS (100 μg/kg, ip) injection (n = 5–6/group). Two-way ANOVA followed by Tukey's post hoc test was performed. Data are expressed as mean ± SEM. (n = 5/group). *p < 0.05: p110αloxP Saline vs p110αloxP LPS; a p < 0.05: p110αloxP LPS vs LepRΔp110α LPS.
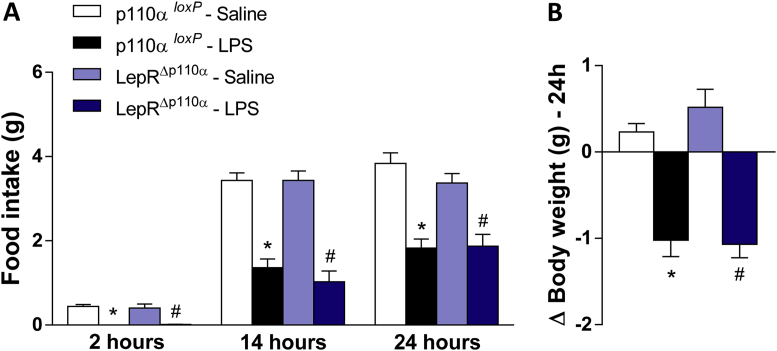
Although LPS-induced phosphorylation of AKT and FoxO1 has been attenuated or even absent in mice with deletion of p110α in LepR neurons, the effects of LPS on food intake and body weight were similar to that found in controls treated with LPS, suggesting that p110α in LepR neurons is not required for the LPS-induced hypophagia and weight loss (Figure 6A–B).
Figure 6.
PI3K p110α catalytic subunit is not required for LPS-induced hypophagia and weight loss. Cumulative food intake 2, 14 and 24 h (A) and 24 h changes in body weight (B) in p110α loxP and LepRΔp110α mice treated with saline or LPS (100 μg/kg, ip). Two-way ANOVA followed by Tukey's post hoc test was performed. Data are expressed as mean ± SEM. (n = 8/group). *p < 0.05: p110α loxP Saline vs p110α loxP LPS; #p < 0.05: LepRΔp110α Saline vs LepRΔp110α LPS.
3.5. PI3K p110β activity in LepR cells is necessary for the LPS suppression of food intake and weight loss
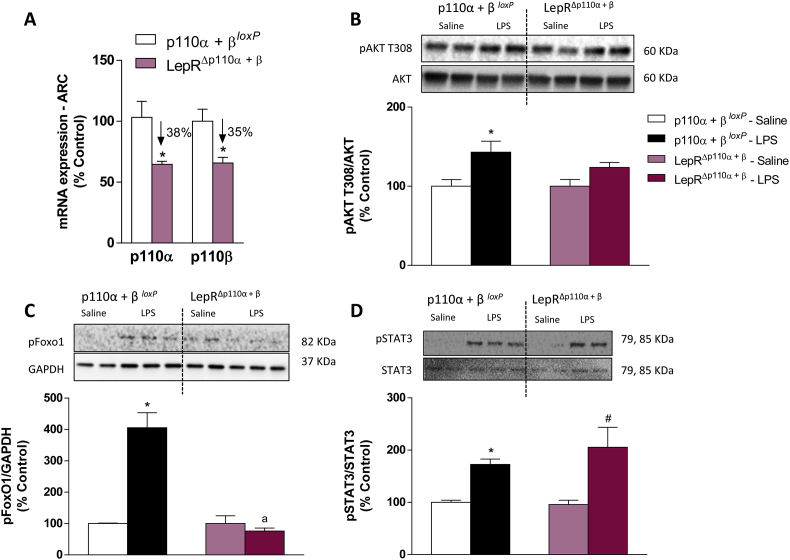
We therefore assessed the possible contribution of the PI3K catalytic subunit p110β in LPS-induced hypophagia performing p110α and p110β subunits double deletion in LepR cells. A 38% reduction in p110α and a 35% reduction in p110β mRNA expression in ARC punches of LepRΔp110α+β mice were observed respective to p110α + βloxP littermate controls (Figure 7A). As expected, LPS increased the AKT phosphorylation in control p110α + βloxP. However, mice with the double deletion did not show changes in AKT phosphorylation in response to LPS (Figure 7B). No alteration in phospho-mTOR expression was found among groups (data not shown). FoxO1 phosphorylation was increased in p110α + βloxP controls, but not in LepRΔp110α+β mice (Figure 7C). LPS-induced STAT3 phosphorylation was equivalent between groups (Figure 7D).
Figure 7.
Validation of deletion of PI3K p110α and p110β catalytic subunits in LepR cells and LPS-induced protein phosphorylation in the mediobasal hypothalamus. A: Percentage of relative mRNA expression in punches from the hypothalamic arcuate nucleus (ARC) of intact p110α + β loxP and LepRΔp110α+β mice. Percentage of pAKT (B), pFoxO1 (C) and pSTAT3 (D) expression in the mediobasal hypothalamus, 2 h after saline or LPS (100 μg/kg, ip) injection (n = 5 - 6/group). 2-tailed Student's t test (A) and Two-way ANOVA followed by Tukey's post hoc test (B–D) were performed. Data are expressed as mean ± SEM. (n = 8/group). *p < 0.05: p110α + βloxPvs LepRΔp110α+β and p110α + βloxP Saline vs p110α + βloxP LPS; a p < 0.05: p110α + βloxP LPS vs LepRΔp110α+β LPS; #p < 0.05: LepRΔp110α+β Saline vs LepRΔp110α+β LPS.
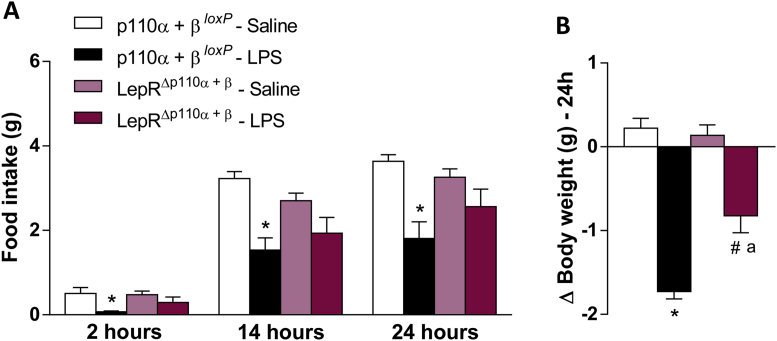
Interestingly, LPS hypophagic effect was blunted in mice with deletion of both catalytic subunits p110α and p110β, whereas only a small decrease in body weight was noticed compared to control mice (Figure 8A–B), indicating that p110β isoform in LepR neurons is required for the endotoxemic hypophagia.
Figure 8.
PI3K p110β catalytic subunit is required for LPS-induced hypophagia and weight loss. Cumulative food intake 2, 14 and 24 h (A) and 24 h changes in body weight (B) in p110α + β loxP and LepRΔp110α+β mice, after saline or LPS (100 μg/kg, ip) injection. Two-way ANOVA followed by Tukey's post hoc test was performed. Data are expressed as mean ± SEM. (n = 8/group). *p < 0.05: p110α + βloxP Saline vs p110α + βloxP LPS; a p < 0.05: p110α + βloxP LPS vs LepRΔp110α+β LPS; #p < 0.05: LepRΔp110α+β Saline vs LepRΔp110α+β LPS.
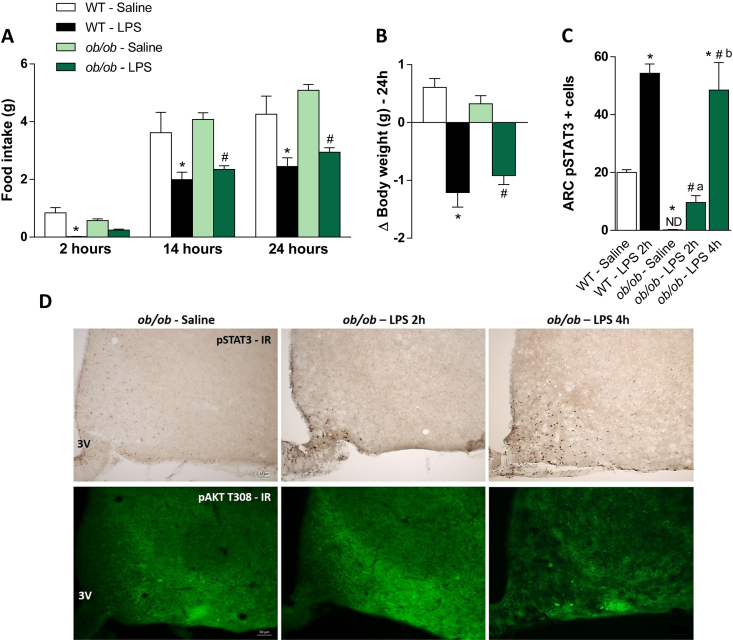
3.6. LPS-induced acute reduction in food intake is blunted in leptin-deficient ob/ob mice
Studies have shown that LPS increases leptin levels [5], [6], [7]. In orther to gain further insights into the contribution of leptin to LPS-induced hypophagia and weight loss, we next analyzed the food consumption and weight gain, as well as the STAT3 and AKT phosphorylation, in response to LPS in leptin-deficient mice (ob/ob). We observed that LPS failed to acutely (after 2 h) reduce food intake in ob/ob mice, but it did reduce food intake after 14 and 24 h. LPS treatment reduced the body weight similarly in wild type and ob/ob mice (Figure 9A–B). Because of lack of an acute response to LPS, we were interested to know whether LPS-induced pSTAT3 and pAKT were intact in ob/ob mice. In the ARC, very few pSTAT3 positive cells were detected 2 h after LPS in ob/ob mice and the number of pSTAT3 positive cells was significantly reduced in comparison with LPS-treated wild types at this time point (Figure 9C–D), suggesting that in the ARC, STAT3 phosphorylation in response to LPS depends at least in part on leptin signaling. Interestingly, we found an increased number of pSTAT3 positive cells in the ARC of ob/ob mice 4 h after LPS, compared with the initial 2 h. The number of cells expressing LPS-induced pSTAT3 at 4 h in ob/ob mice was higher than that in wild types treated with saline, and similar to the number found in wild types treated with LPS perfused 2 h after injection (Figure 9C–D), suggesting a delayed pSTAT3 induction by LPS in leptin-deficient mice. However, we observed very few LPS-induced pAKT in the ARC following 2 and 4 h of LPS treatment suggesting that LPS-induced pAKT requires leptin signaling.
Figure 9.
Acute LPS-induced hypophagia is abolished in leptin-deficient mice. Cumulative food intake 2, 14 and 24 h food intake (A), 24 h changes in body weight (B) and number of pSTAT3 positive cells (C) in hypothalamic arcuate nucleus (ARC) in wild type (WT) and leptin-deficient mice (ob/ob) treated with saline or LPS (100 μg/kg, ip), 2 and 4 h after injection. In the bottom panel (D), representative photomicrographs showing pSTAT3 expression (brown dots) or pAKT expression (green) in the ARC 2 and 4 h after saline or LPS injection, in WT and ob/ob mice. 3V = third ventricle. Scale bar: 50 μm. Two-way ANOVA followed by Tukey's post hoc test was performed. Data are expressed as mean ± SEM. (n = 6/group for food intake and BW measurements and n = 3/group for immunostaining). *p < 0.05: WT Saline vs WT LPS; #p < 0.05: ob/ob Saline vs ob/ob LPS; a p < 0.05: WT LPS 2 h vs ob/ob LPS 2 h; b p < 0.05: ob/ob LPS 2 h vs ob/ob LPS 4 h.
4. Discussion
Using pharmacological PI3K inhibitor and mouse models of selective deletion of the PI3K catalytic subunits p110α and p110β in LepR cells, we found that PI3K signaling is necessary for the acute suppression of food intake and weight loss during LPS challenges. Our findings further demonstrate that the p110β subunit, but not the p110α, in LepR expressing cells is required for the acute hypothagic response of endotoxemic mice. The role of leptin in LPS-induced AKT phosphorylation and acute hypophagia was further supported by the absence of acute (2 h) LPS-induced reduction of food intake and lack of LPS-induced AKT phosphorylation in the ARC of leptin-deficient mice. However, we found that leptin-induced pSTAT3 is not required for hypophagia in the initial stages of endotoxemia, as leptin-deficient mice showed a later food intake reduction in parallel to a delayed pSTAT3 expression in response to LPS, likely induced by inflammatory cytokines other than leptin.
As part of an effective immune response, bacterial infection induces acute hypophagia and weight loss, which initially promotes host survival given the lower energy availability to the pathogens [30]. The hypophagic effects of endotoxin are primarily mediated by the actions of interleukin (IL)-1, tumor necrosis factor (TNF)-α and other cytokines generated to counteract the infection [31]. A persistent hypophagic effect, however, is harmful to the organism. Hence, a useful therapy against infection could be the selective inhibition of the hypophagic response while preserving the actions of cytokines on pathogen removal. Consistent with acute bacterical infection, in this study, LPS-treated mice were lethargic in the first 2–4 h after injection. We have previously observed that rats treated with LPS (100 μg/kg, ip) show increased circulating levels of TNF-α [2] and present fever from 1 to 5 h after injection [32]. These findings are in agreement with data reported by Pohl and coworkers [33], [34] in rats. In the present study, we did not measure these parameters, but Lawrence et al. [35] demonstrated that the same dose of LPS as was used in our study increased the core body temperature and the cytokine levels in parallel to a reduction in food intake in mice. We observed before that LPS increased O2 consumption and energy expenditure, but it did not change the respiratory quotient in rats, indicating that LPS does not affect the utilization of carbohydrates as fuel [32].
LPS stimulates the secretion of the adipokine leptin [5], [6], [7]. In a recent article, Pohl and coworkers [34] showed that leptin modulates the fever response and cytokine levels induced by LPS. Treatment with leptin antibody prevents LPS-induced hypophagia and weight loss [36], in agreement with our findings that leptin is a downstream mediator in the endotoxemic hypophagia. In this regard, a recent report defining the LepR neuron transcriptome [37] described the gene expression of IL and TNF receptors in LepR neuronal population, evidencing that leptin responsive cells also might be responsive to molecules generated in the presence of LPS and are likely to share intracellular signaling pathways. Systemic inflammatory response to LPS alters the intestinal function [38]. Recent studies by Rajala et al. [39] and Sandoval [40] reported that leptin regulates antimicrobial peptide-encoding genes in the gut epithelium, demonstrating that the leptin receptor signaling has a direct role in modulating the microbiota composition. In view of these findings, it should be taken into account that LPS and the subsequent secretion of leptin can act in peripheral sites that express LepR not investigated in our study, such as intestine, and this action might have an impact on energy homeostasis during endotoxemia.
Leptin-induced hypophagia is mediated by Janus kinase 2 (JAK2)/STAT3 pathway [10], [11], which is also activated by LPS in rodents [6], [41]. Our findings are in agreement with this and further demonstrate that LPS induces pSTAT3 in a subset of LepR neurons of the ARC. Because roughly half of LPS-induced pSTAT3 cells of ARC are LepR cells, LPS may induce pSTAT3 in response to ligands other than leptin. Our data indicate that LPS-induced inhibition of food intake is stronger and sustained, compared with leptin-induced inhibition in wild type naïve mice. Exogenous leptin injection following LPS treatment was not able to potentiate the hypophagic effect promoted by LPS per se, suggesting a ceiling effect of LPS in the inhibition of food consumption. At present, LPS-induced hypophagia in wild type mice paralleled an increase in the anorexigenic POMC and no alteration in the orexigenic NPY and AgRP mRNA expression in the hypothalamus.
Leptin promotes hypothalamic astrogenesis during development [42] and it has been shown that leptin signaling in astrocytes regulates the neuronal leptin-induced STAT3 phosphorylation [43]. As previous studies have shown that LepR is expressed in astrocytes [42], [43], our mouse model may express Cre in astrocytes as well. It is possible that the deletion of PI3K may also have occurred in astrocytes, and the effects seen in this study could be due to astrocytic response. However, in our experimental design we were not able to dissociate the effects of neurons from those of astrocytes. The participation of astrocytes in modulating food intake during endotoxemia should be addressed in future studies.
Acute suppression of food intake induced by leptin is prevented by pharmacological PI3K inhibition [12], [13]. Interestingly, activation of Toll like receptor 4 (TLR4) by LPS stimulates host immune response through the PI3K/AKT pathway [23], [44]. The major PI3K/AKT downstream substrate is FoxO1, the activity of which is inhibited through its phosphorylation by PI3K/AKT, resulting in its nuclear exclusion and attenuation of the inhibition of targeted gene expression [45]. In the ARC neurons, FoxO1 located in the nucleus was shown to inhibit POMC expression, while prevented AgRP inhibition, in response to leptin [46]. In vitro studies in microglial cells showed that LPS-induced IL-6 and TNF-α secretion were abolished by the FoxO1-specific siRNA [47]. In view of these findings, we postulated that leptin could contribute to the acute LPS-induced hypophagia via activation of PI3K/AKT pathway in the hypothalamus. PI3K signaling is known to be the major stimulator of AKT phosphorylation at T308 residue, while the mammalian target of rapamycin complex 2 (mTORC2) mainly regulates AKT phosphorylation at S473 [48]. In our study, LPS had no effect on mTOR and S473 AKT phosphorylation (data not shown), but it increased the AKT phosphorylation at T308 residue in ARC neurons. We found a high colocalization of pAKT T308 with LepR cells, suggesting a recruitment of PI3K pathways and potential crosstalk between leptin and LPS in PI3K activation during endotoxemia. Using a pharmacological approach, we observed that the PI3K pathway plays a role in the LPS-induced hypophagia and weight loss, given that the PI3K inhibitor prevented the LPS effects on food intake and body weight.
Since central injection of PI3K inhibitor may reach different neuronal populations, we were interested in knowing whether PI3K signaling, specifically in LepR neurons, is required for LPS-induced hypophagia. Our study revealed that both p110α and p110β subunits in LepR cells are required for the endotoxemic hypophagia and weight loss. Because lack of p110α alone had no effect in LPS suppression of food intake and weight loss, our findings indicate that the p110β subunit plays a major role. Interestingly however, deletion of p110α or of both p110α and p110β impaired the ability of LPS to phosphorylate AKT at T308 and its downstream target FoxO1, suggesting that p110 catalytic subunits in LepR neurons play a role in AKT and FoxO1 phosphorylation by the endotoxin. Although this may appear inconsistent with the physiological findings, another piece of data should be highlighted. The LepRΔp110α, but not the LepRΔp110α+β mice, showed a potentiation of LPS-induced STAT3 phosphorylation. The molecular mechanism underlying this effect is not clear but high levels of pSTAT3 may contribute to the preserved hypophagic effect in LepRΔp110α mice.
Studies using conditional p110α and p110β null mice revealed that these subunits have different contributions to the regulation of neuronal function [19], [20]. Leptin differentially modulates POMC and NPY/AgRP neurons in the ARC via PI3K-dependent mechanisms; i.e., it depolarizes POMC and hyperpolarizes NPY/AgRP neurons [49], [50], [51]. Inactivating either p110α or p110β in POMC and AgRP neurons, Al-Qassab and coworkers [20] demonstrated a dominant role for p110β in energy homeostasis. POMC p110β null mice show hyperphagia and central leptin unresponsiveness, while POMC p110α null mice displayed a normal central response to leptin. Our model of double deletion prevents the compensatory effects of one subunit over the other and provides a mechanistic insight that the p110β subunit is required for the hypophagia during acute inflammatory challenges.
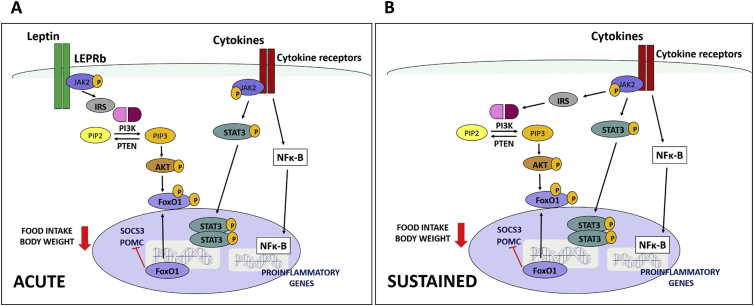
We found that in ob/ob mice, LPS failed to reduce food intake only in the first 2 h of lights off. This delay of LPS to suppress food intake in leptin-deficient mice is in agreement with data from Faggioni and coworkers [52] who showed that LPS administration stimulated TNF-α secretion and reduced food intake 24–72 h after treatment in ob/ob mice. However, no measurement of food intake in shorter intervals before 24 h was described. Because LPS-induced pSTAT3 in the ARC of ob/ob mice was delayed, not abrogated, leptin signaling via pSTAT3 is not required for endotoxemic suppression of food intake. However, given that ob/ob mice did not show increased pAKT T308 in response to LPS, leptin might be required for LPS-induced AKT phosphorylation, and this effect is likely to play a role in the acute, but not sustained, endotoxemic hypophagia. Our findings indicate that leptin mediation of the LPS-induced hypophagia is an acute effect that is required for sustained suppression of food intake, and the mechanisms of this effect might be explained by STAT3 phosphorylation induced by other inflammatory cytokines over the course of the endotoxemia (Figure 10).
Figure 10.
Proposed model for PI3K and STAT3 regulation of food intake and body weight in hypothalamic cells during endotoxemia. LPS stimulates the secretion of leptin and other cytokines in peripheral tissues. Leptin binds to its receptor in hypothalamic neurons and acutely activates the PI3K-FoxO1 pathway. Simultaneously, cytokines bind to their receptors and trigger the activation of PI3K-FoxO1 and JAK-STAT3 pathways (A). The final step in both pathways is the stimulation of the transcription of POMC, which in turn promotes reduction of food intake and body weight. Over the course of the endotoxemia, the cytokines promote a sustained activation of the JAK-STAT3 pathway, contributing to a persistent reduction of food consumption (B).
5. Conclusions
Our results contribute to the understanding of the acute hypophagia induced by endotoxin. PI3K/AKT pathway associated with TLR4 action on the immune system now is found to be equally important for the acute metabolic control during infection/inflammation. The PI3K pathway via p110β catalytic subunit in LepR neurons exerts a crucial role in the suppression of feeding in endotoxemic states. Given that in response to long term exposure to the endotoxin, the host organism develops tolerance to the pathogen, which is characterized by desensitization of several responses including cytokine secretion, fever and reduction of food intake [2], [6], [53]. We believe that the inhibition of acute hypophagia (mediated in part by PI3K in LepR expressing cells) benefits the organism, preventing excessive undernutrition during the acute-phase response to bacterial infections.
Acknowledgments
We would like to thank Susan J. Allen and Maria Valci A. S. Silva for their technical support. This work was supported by the National Institutes of Health (R01-HD-069702 to CFE), FAPESP: 2013/03915-0 (Sao Paulo Research Foundation, fellowship to BCB and RR) and CNPq (Brazilian National Council for Scientific and Technological Development, fellowship to BCB).
Contributor Information
Beatriz C. Borges, Email: beatrizd@med.umich.edu, borgesbc@gmail.com.
David Garcia-Galiano, Email: dgarciag@med.umich.edu.
Rodrigo Rorato, Email: rcrorato@rfi.fmrp.usp.br.
Lucila L.K. Elias, Email: llelias@fmrp.usp.br.
Carol F. Elias, Email: cfelias@med.umich.edu.
Conflict of interest
None declared.
References
- 1.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behavior and Immunity. 2003;17(1):13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 2.Borges B.C., Antunes-Rodrigues J., Castro M., Bittencourt J.C., Elias C.F., Elias L.L. Expression of hypothalamic neuropeptides and the desensitization of pituitary-adrenal axis and hypophagia in the endotoxin tolerance. Hormones and Behavior. 2007;52:508–519. doi: 10.1016/j.yhbeh.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Yue Y., Wang Y., Li D., Song Z., Jiao H., Lin H. A central role for the mammalian target of rapamycin in LPS-induced anorexia in mice. Journal of Endocrinology. 2015;224(1):37–47. doi: 10.1530/JOE-14-0523. [DOI] [PubMed] [Google Scholar]
- 4.Fischer C.W., Elfving B., Lund S., Wegener G. Behavioral and systemic consequences of long-term inflammatory challenge. Journal of Neuroimmunology. 2015;15(288):40–46. doi: 10.1016/j.jneuroim.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Grunfeld C., Zhao C., Fuller J., Pollack A., Moser A., Friedman J. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. Journal of Clinical Investigation. 1996;97(9):2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges B.C., Rorato R., Avraham Y., da Silva L.E., Castro M., Vorobiav L. Leptin resistance and desensitization of hypophagia during prolonged inflammatory challenge. American Journal of Physiology, Endocrinology and Metabolism. 2011;300:858–869. doi: 10.1152/ajpendo.00558.2010. [DOI] [PubMed] [Google Scholar]
- 7.Kurosawa N., Shimizu K., Seki K. The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1β-induced elevated leptin levels in mice. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4084-x. [DOI] [PubMed] [Google Scholar]
- 8.Considine R.V., Sinha M.K., Heiman M.L., Kriauciunas A., Stephens T.W., Nyce M.R. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England Journal of Medicine. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz M.W., Woods S.C., Porte D., Jr., Seeley R.J., Baskin D.G. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 10.Bates S.H., Dundon T.A., Seifert M., Carlson M., Maratos-Flier E., Myers M.G., Jr. LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53(12):3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 11.Buettner C., Pocai A., Muse E.D., Etgen A.M., Myers M.G., Jr., Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metabolism. 2006;4(1):49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niswender K.D., Morton G.J., Stearns W.H., Rhodes C.J., Myers M.G., Jr., Schwartz M.W. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 13.Rahmouni K., Haynes W.G., Morgan D.A., Mark A.L. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41(3 Pt2):763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 14.Xu A.W., Kaelin C.B., Takeda K., Akira S., Schwartz M.W., Barsh G.S. PI3K integrates the action of insulin and leptin on hypothalamic neurons. Journal of Clinical Investigation. 2005;115(4):951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon O., Kim K.W., Kim M.S. Leptin signalling pathways in hypothalamic neurons. Cellular and Molecular Life Sciences. 2016 doi: 10.1007/s00018-016-2133-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niswender K.D., Gallis B., Blevins J.E., Corson M.A., Schwartz M.W., Baskin D.G. Immunocytochemical detection of phosphatidylinositol 3-kinase activation by insulin and leptin. Journal of Histochemistry & Cytochemistry. 2003;51(3):275–283. doi: 10.1177/002215540305100302. [DOI] [PubMed] [Google Scholar]
- 17.Brachmann S.M., Ueki K., Engelman J.A., Kahn R.C., Cantley L.C. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Molecular Cell Biology. 2005;25(5):1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foukas L.C., Claret M., Pearce W., Okkenhaug K., Meek S., Peskett E. Critical role for the p110[alpha] phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 19.Ciraolo E., Iezzi M., Marone R., Marengo S., Curcio C., Costa C. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Science Signaling. 2008;1(36) doi: 10.1126/scisignal.1161577. ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Qassab H., Smith M.A., Irvine E.E., Guillermet-Guibert J., Claret M., Choudhury A.I. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metabolism. 2009;10(5):343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J.W., Xu Y., Preitner F., Fukuda M., Cho Y.R., Luo J. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150(11):4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams K.W., Sohn J.-W., Donato J., Lee C.E., Zhao J.J., Elmquist J.K. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. Journal of Neuroscience. 2011;31:13147–13156. doi: 10.1523/JNEUROSCI.2602-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luyendyk J.P., Schabbauer G.A., Tencati M., Holscher T., Pawlinski R., Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. Journal of Immunology. 2007;180(6):4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J.J., Cheng H., Jia S., Wang L., Gjoerup O.V., Mikami A. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proceedings of National Academy of Sciences U S A. 2006;103(44):16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia S., Roberts T.M., Zhao J.J. Should individual PI3 kinase isoforms be targeted in cancer? Current Opinion in Cell Biology. 2009;21(2):199–208. doi: 10.1016/j.ceb.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFalco J., Tomishima M., Liu H., Zhao C., Cai X., Marth J.D. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291(5513):2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 27.Scott M.M., Lachey J.L., Sternson S.M., Lee C.E., Elias C.F., Friedman J.M. Leptin targets in the mouse brain. Journal of Comparative Neurology. 2009;514(5):518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin K.B.J., Paxinos G. Academic Press; 2008. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 29.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Johnson R.W. Immune and endocrine regulation of food intake in sick animals. Domestic Animal Endocrinology. 1998;15:309–319. doi: 10.1016/s0739-7240(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 31.Langhans W. Bacterial products and the control of ingestive behavior: clinical implications. Nutrition. 1996;12(5):303–315. doi: 10.1016/s0899-9007(96)80052-9. [DOI] [PubMed] [Google Scholar]
- 32.Borges B.C., Rorato R., Uchoa E.T., Marangon P., da Silva G.S., de Paula F.J. High-fat diet induces site-specific unresponsiveness to LPS-stimulated STAT3 activation in the hypothalamus. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2014;306(1):R34–R44. doi: 10.1152/ajpregu.00147.2013. [DOI] [PubMed] [Google Scholar]
- 33.Pohl J., Woodside B., Luheshi G.N. Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in dietinduced obese rats. Endocrinology. 2009;150:4901–4910. doi: 10.1210/en.2009-0526. [DOI] [PubMed] [Google Scholar]
- 34.Pohl J., Woodside B., Luheshi G.N. Leptin modulates the late fever response to LPS in diet-induced obese animals. Brain, Behavior and Immunology. 2014;42:41–47. doi: 10.1016/j.bbi.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence C.B., Brough D., Knight E.M. Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Disease, Models & Mechanics. 2012;5(5):649–659. doi: 10.1242/dmm.009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachot C., Poole S., Luheshi G.N. Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. Journal of Physiology. 2004;15:263–272. doi: 10.1113/jphysiol.2004.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allison M.B., Patterson C.M., Krashes M.J., Lowell B.B., Myers M.G., Jr., Olson D.P. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Molecular Metabolism. 2015;4(4):299–309. doi: 10.1016/j.molmet.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z., Zhang X., Zhou H., Liu W., Li J. Exogenous s-nitrosoglutathione attenuates inflammatory response and intestinal epithelial barrier injury in endotoxemic rats. Journal of Trauma and Acute Care Surgery. 2016 doi: 10.1097/TA.0000000000001008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Rajala M.W., Patterson C.M., Opp J.S., Foltin S.K., Young V.B., Myers M.G., Jr. Leptin acts independently of food intake to modulate gut microbial composition in male mice. Endocrinology. 2014;155(3):748–757. doi: 10.1210/en.2013-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandoval D. Old dog, new trick: a direct role for leptin in regulating microbiota composition. Endocrinology. 2014;155(3):653–655. doi: 10.1210/en.2014-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borges B.C., Rorato R., Uchoa E.T., Marangon P.B., Elias C.F., Antunes-Rodrigues J. Protein tyrosine phosphatase-1B contributes to LPS-induced leptin resistance in male rats. American Journal of Physiology, Endocrinology and Metabolism. 2015;308:E40–E50. doi: 10.1152/ajpendo.00094.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rottkamp D.M., Rudenko I.A., Maier M.T., Roshanbin S., Yulyaningsih E., Perez L. Leptin potentiates astrogenesis in the developing hypothalamus. Molecular Metabolism. 2015;4(11):881–889. doi: 10.1016/j.molmet.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Hsuchou H., He Y., Kastin A.J., Pan W. Role of astrocytes in leptin signaling. Journal of Molecular Neurosciences. 2015;56(4):829–839. doi: 10.1007/s12031-015-0518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.S., Nauseef W.M., Moeenrezakhanlou A., Sly L.M., Noubir S., Leidal K.G. Monocyte p110alpha phosphatidylinositol 3-kinase regulates phagocytosis, the phagocyte oxidase, and cytokine production. Journal of Leukocyte Biology. 2007;81(6):1548–1561. doi: 10.1189/jlb.0906564. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto M., Accili D. All roads lead to FoxO. Cell Metabolism. 2005;1:215–216. doi: 10.1016/j.cmet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Kitamura T., Feng Y., Kitamura Y.I., Chua S.C., Jr., Xu A.W., Barsh G.S. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nature Medicine. 2006;12(5):534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 47.Dong H., Zhang X., Dai X., Lu S., Gui B., Jin W. Lithium ameliorates lipopolysaccharide-induced microglial activation via inhibition of toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1 pathway. Journal of Neuroinflammation. 2014;11(1):140. doi: 10.1186/s12974-014-0140-4. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Workman P., de Bono J. Targeted therapeutics for cancer treatment: major progress towards personalised molecular medicine. Current Opinion in Pharmacology. 2008;(4):359–362. doi: 10.1016/j.coph.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 49.van den Top M., Lee K., Whyment A.D., Blanks A.M., Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature Neuroscience. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 50.Hill J.W., Williams K.W., Ye C., Luo J., Balthasar N., Coppari R. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. Journal of Clinical Investigation. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu J., Fang Y., Rønnekleiv O.K., Kelly M.J. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. Journal of Neuroscience. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faggioni R., Fuller J., Moser A., Feingold K.R., Grunfeld C. LPS-induced anorexia in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 1997;273:R181–R186. doi: 10.1152/ajpregu.1997.273.1.R181. [DOI] [PubMed] [Google Scholar]
- 53.Ziegler-Heitbrock H.W. Molecular mechanism in tolerance to lipopolysaccharide. Journal of Inflammation. 1995;45:13–26. [PubMed] [Google Scholar]