Standfirst
Tissue engineering and regenerative medicine have advanced rapidly towards the development of therapeutic solutions for musculoskeletal disorders. In 2012, breakthroughs have been made in the guidance of adult stem cell homing, the tissue regenerative activity of stem-cell-derived extracellular matrix has been tested, and novel, mechanically superior biomaterials have been fabricated.
More than two decades ago, the field of tissue engineering, built on a combinatorial platform of cells, scaffolds, and biofactors, was conceptualized as a means to functional restoration and regeneration of diseased or damaged tissues and organs, including those of the musculoskeletal system. More recently, a number of new concepts have been developed that promise to enhance the success of cell-based and biomaterial-based tissue engineering approaches. In 2012, a number of studies exemplified these new concepts and represent key advances in the approach to regenerative medicine.
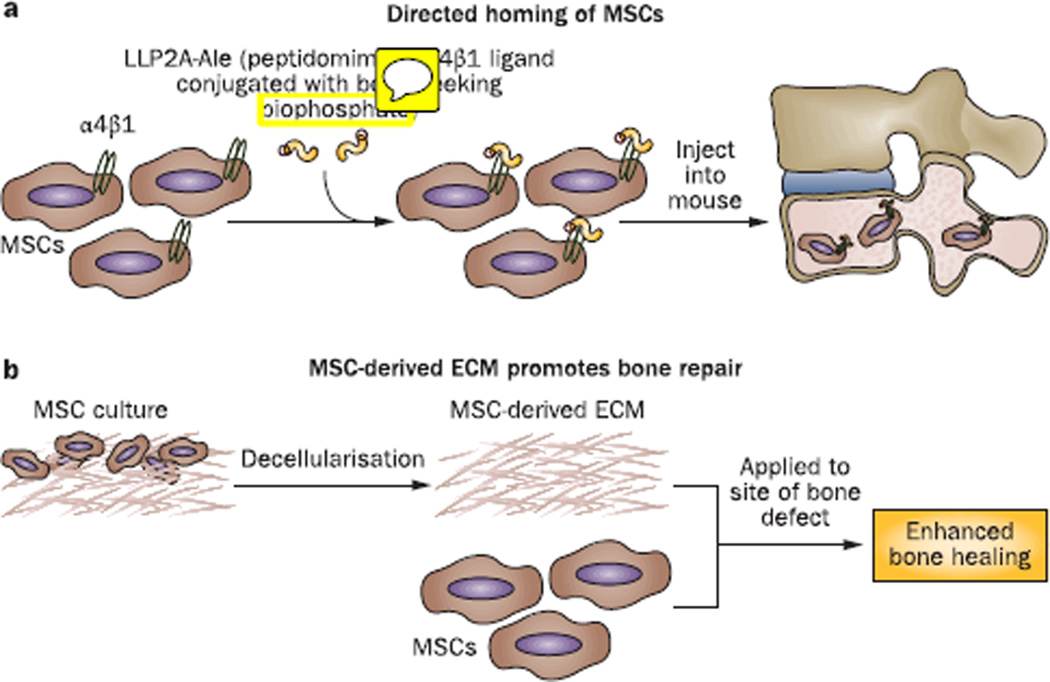
Adult mesenchymal stem cells (MSCs) represent the most widely investigated cell type for musculoskeletal tissue engineering;1 the concept that these cells have immunoregulatory, anti-inflammatory, pro-survival, and endothelial cell modulatory activities is increasingly considered in regenerative applications, in addition to their multilineage differentiation potential.2 In order to exert these activities in a way that will influence tissue regeneration, MSCs must first home to the target tissue and subsequently differentiate appropriately. In a 2012 study, Guan et al.3 developed a conjugate molecule comprising LLP2A, a peptidomimetic ligand for α4β1 integrin receptors that were found to be highly expressed on osteoprogenitor MSCs, and the bisphosphonate alendronate, which has a high affinity for bone. In vitro analyses showed that this LLP2A–alendronate conjugate (LLP2A-Ale) increased MSC migration and osteogenic differentiation.3 Intravenously xenotransplanted human MSCs (hMSCs) were detected adjacent to the periosteal, endocortical and trabecular bone surfaces in the lumbar vertebral bodies of severe combined immunodeficiency mice only after co-injection with LLP2A-Ale.3 After 3 weeks follow-up, the hMSCs were seen embedded within the bone matrix and were associated with increased bone formation.3 Interestingly, injection of LLP2A-Ale alone also augmented bone formation;3 the authors suggested that the ‘bone-seeking’ alendronate-conjugated α4β1 ligand could promote homing of endogenous MSCs to bone, because LLP2A alone—which would have nonspecific tissue distribution—was not osteogenic.3 More importantly, injection of LLP2A-Ale alone was found to prevent trabecular bone loss in immunocompetent mice with ovariectomy-induced osteopenia, resulting in increased osteoblast and mineralizing surfaces, as well as higher rate of bone formation for the total bone surface.3 These exciting findings present a potentially effective means of guiding MSCs to bone, and provide strong evidence that MSCs can act as functional osteoprogenitor cells in vivo. Moreover, the effectiveness of LLP2A-Ale alone in osteopenic mice suggests that endogenous MSCs could, in fact, be adequately recruited to bone surfaces using this molecule. Studies to test the effectiveness of this approach for the treatment of bone abnormalities, such as fractures and osteoporosis, are certainly warranted. This system should also be useful to examine whether the regulatory activities of recruited MSCs also influence cells resident in the bone to cooperatively enhance bone formation.
Tissue matrices have been recognized as rich depots of molecular signals, primarily growth factors sequestered by macromolecular components of the extracellular matrix (ECM), which regulate tissue-specific biological activities.4 Indeed, when used as scaffolds for seeded MSCs, hMSC-derived ECM preparations, obtained by decellularizing in vitro MSC cultures, promote cell attachment, spreading, migration, proliferation, and maintenance of response to differentiation signals.5 This concept formed the basis of a different approach to optimize delivery and maintenance of MSCs in a 2012 study by Zeitouni et al.6
Injection of hMSCs, pre-treated with GW9662 to suppress adipogenic activity via inhibition of peroxisome proliferator-activated receptor-γ, improved bone healing in a mouse calvarial defect xenograft model;6 however, the effect was limited to the early osteogenic phase, and was lost during bone remodeling due to unsatisfactory hMSC retention.6 Three weeks after GW9662-treated hMSCs were grafted together with hMSC-derived ECM, increased hMSC engraftment was detected compared with grafting with hMSCs alone, as well as reproducible and near-complete repair of calvarial bone defects,6 indicating that the hMSC-derived ECM acted as a retentive scaffold. In the treated mice, large clusters of engrafted cells were observed that were not associated with the remodeling bone surface, suggesting that the matrix-localized hMSCs provided an enabling microenvironment that promoted intrinsic bone formation within the graft. These findings strongly suggest that MSC-derived ECM should be considered as a potential adjunct component in the design of bioactive biomaterial scaffolds for tissue engineering.
Researchers are increasingly realizing that tissue or organ regeneration must recapitulate the biological mechanisms of embryonic development and tissue morphogenesis,7 not only at a molecular level, but also in terms of the spatiotemporal and physical aspects.8 This understanding has led to increasingly rational design of biomaterial scaffolds, the development of 3-dimensional tissue constructs, and their propagation in bioreactors that provide more native environments. Because of the weight-bearing mechanical requirements of musculoskeletal tissues, a preferred bioactive scaffold would consist of a mechanically strong biomaterial scaffold, such as a bioresorbable polymer, that appropriately incorporates decellularized ECM. Hydrogels have been popular biomaterials for tissue engineering, drug delivery and modeling of ECM because of the ease of manipulation and cell seeding prior to gelation, but are severely limited by their mechanical behavior, particularly their low stretchability.
Sun et al.9 recently reported a new synthesis protocol for producing highly stretchable and tough hydrogels. Gelation of hydrogels is normally mediated via either ionic cross-links, such as in alginate, or covalent cross-links, such as in polyacrylamide. Sun and co-workers9 created an alginate–polyacrylamide hybrid hydrogel, in which the two types of polymer network were intertwined and further cross-linked via covalent bonds between amine groups on polyacrylamide chains and carboxyl groups on alginate chains. The hybrid gels contained ~90% water, but could be stretched beyond 20 times their original length, and had high fracture energies of around 9,000 J/m2 compared to 10–250 J/m2 for alginate or polyacrylamide gels;9 even when notches were created in the hydrogel structure, the stretchability was 17 times.9 The authors attributed the large improvement in hydrogel mechanical properties to two factors: crack bridging by covalent cross-links and hysteresis provided by the network of ionic cross-links. A nascent engineered or regenerated tissue initially produced by seeded cells is unlikely to exhibit, on its own, the physical properties of the mature tissue; thus, the biomaterial scaffold has the burden of meeting the physicomechanical requirements. In this respect, the principle of combining weak and strong cross-links between brittle constituents to produce a tough, stretchable material should be of high utility. Such an approach should minimize physical damage of the nascent engineered tissue construct and enhance its eventual maturation and integration with the host tissue.
Although ionic cross-links, mediated by divalent cations for example, are generally biocompatible, free radicals produced during chemical covalent cross-linking are cytotoxic and the reaction can also be exothermic. Photo-induced covalent cross-linking might represent a milder alternative,10 but is generally slower. To be adopted for cell-based tissue engineering, these issues will need to be resolved to make hybrid hydrogels biocompatible, enabling their evaluation in direct cell-seeding protocols.
Tissue engineering and regenerative medicine are highly active research disciplines that have seen rapid, complementary convergence of life science, engineering, and clinical medicine, illustrated by the advances in MSC grafting and biomechanical scaffold design made in 2012 described here. The translational nature of this discipline dictates that issues such as biocompatibility, scaling up, and safety must be considered, to enable preclinical and clinical trials that will lead to therapeutic product development. for musculoskeletal repair and regeneration.
Figure 1.
New approaches to enhance MSC-mediated tissue repair. a | Guided homing of MSCs to bone surface using an integrin-binding peptidomimetic ligand conjugated with the ‘bone-seeking’ bisphosphonate, alendronate (LLP2A-Ale). b | MSC-derived ECM co-administered with MSCs to enhance MSC retention and healing of bone defects Abbreviations: ECM, extracellular matrix; MSC, mesenchymal stem cells.
Key Advances.
Bisphosphonate-directed, integrin-dependent homing of mesenchymal stem cells (MSC) to bone improved bone formation and retention in mice;6 this approach could be used in patients with bone abnormalities
MSC-derived extracellular matrix has been used successfully as a bioactive scaffold to enhance bone repair in vivo,7 illustrating the potential utility of decellularized matrices in tissue engineering
Ionic and covalent cross-links between mechanically weak components improve hydrogel stretch and stiffness;9 these materials could be useful scaffolds for engineering of weight-bearing musculoskeletal tissues
Acknowledgments
Supported in part by grants from the Commonwealth of Pennsylvania Department of Health (SAP 4100050913), National Institutes of Health (NS080758), and U.S. Department of Defense (W81XWH-10-1-0850).
Biography
“Rocky S. Tuan, Ph.D., is the founding director of the Center for Cellular and Molecular Engineering in the Department of Orthopaedic Surgery at University of Pittsburgh School of Medicine, Pittsburgh, PA, USA. His research interests cover the development, growth, aging, and disease of skeletal tissues, and stem cell-based skeletal tissue engineering.”
References
- 1.Nöth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Adv. Drug Deliv. Rev. 2010;62:765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Why are MSCs therapeutic? New data: new insight. J. Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan M, et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 2012;18:456–462. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Lin H, Yang G, Tan J, Tuan RS. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33:4480–4489. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Zeitouni S, et al. Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci. Transl. Med. 2012;4:132ra55. doi: 10.1126/scitranslmed.3003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng. Part B Rev. 2009;15:381–394. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- 8.Guilak F, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JY, et al. Highly stretchable and tough hydrogels. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H, et al. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials. 2013;34:331–339. doi: 10.1016/j.biomaterials.2012.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]