Abstract
Chronic myeloid leukemia (CML) in children and young adults is uncommon. Young patients have long life expectancies and low morbidity with hematopoietic cell transplantation (HCT). Prolonged tyrosine kinase inhibitor (TKI) use may cause significant morbidity. In addition indication for HCT in patients in first chronic phase is not established.
We hence retrospectively evaluated outcomes in 449 CML patients with early disease receiving myeloablative HCT reported to the CIBMTR. We analyzed various factors affecting outcome specifically the effect of age and pre-HCT TKI in pediatric patients (<18 yrs, n= 177) and young adults (18–29 years, n=272) with the goal of identifying prognostic factors.
Post-HCT probability of 5 y overall survival (OS) and leukemia free survival (LFS) were 75% and 59% respectively. OS and LFS were 76%, 57% in <18 yr and 74%, 60% in 18–29 yr group respectively by univariate analysis (p= 0.1 and 0.6). Five-year OS for human leukocyte antigen (HLA) matched sibling donor (MSD) and bone marrow (BM) stem cell source were 83% and 80% respectively. In multivariate analysis, there was no effect of age (<18 vs 18–29) or pre-HCT TKI therapy on OS, LFS, transplant related mortality (TRM) or relapse. Favorable factors for OS were MSD (p<0.001) and recent HCT (2003–2010) (p=0.04). LFS was superior with MSD (P<0.001), BM as graft source (P=0.001) and performance score >90 (P=0.03) compared to unrelated or mismatched, peripheral blood stem cells donors and recipients with lower performance scores. Older age was associated with increased incidence of chronic graft vs. host disease (cGVHD) (p=0.0002).
In the current era, HCT outcomes are similar in young patients and children with early CML, and best outcomes are achieved with BM grafts and MSD.
Keywords: CML, pediatrics
Introduction
Chronic myeloid leukemia (CML) in pediatrics and young adults is uncommon accounting for only about 3% of all leukemias and <10% of all reported CML.1 Since the introduction of tyrosine kinase inhibitors (TKI) (e.g. imatinib, dasatinib, nilotinib etc.), hematopoietic cell transplantation (HCT) in patients with CML, especially among those with early disease, has declined.2,3 HCT is usually only offered to selected patients with inadequate response or intolerance to TKI, and it is generally recommended to continue TKI indefinitely without HCT.3,4,5 Indications for HCT in children and young adults are extrapolated from adult studies and include advanced disease, presence of resistant mutation or failure to achieve complete cytological remission 1 year after initiation of TKI therapy. 6,7,8 Children and young adults with projected longer life expectancies are likely to receive prolonged TKI therapy during periods of active growth and sexual development, which may increase cumulative risk for known and yet unknown morbidities.9,10,11,12 Given that results of HCT are superior in children and younger adults compared to older adults, it may be assumed that HCT for chronic phase (CP) is beneficial by curing disease at lower cost, without need for prolonged TKIs, especially if an appropriate donor is available.12,13 We conducted a retrospective cohort study of patients 0–29 years of age with early CML (defined as first chronic phase (CP1) and clinical remission as captured in CBMTR forms) who received a myeloablative conditioning (MAC) allogeneic HCT. We reviewed outcomes of children <18 years and young adults (18–29 years) with the goal of identifying prognostic factors that affect HCT outcomes.14
Patients and Methods
Data source
The CIBMTR (Center for International Blood and Marrow Transplant Research) is a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. It comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits and patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on site audits of participating centers ensure data quality. Studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
The CIBMTR collects data at two levels: Transplant Essential Data (TED) level and Comprehensive Report Form (CRF) level. The TED-level data is an international accepted standard data set that contains a limited number of key variables for all consecutive transplant recipients. TED-level data, with some additional details of donor and graft characteristics, comprise the obligatory data submitted to the SCTOD (Stem Cell Therapeutic Outcomes Database). When a transplant is registered with the CIBMTR; a subset of patients are selected for CRF-level data through a weighted randomization scheme. The CRF-level captures additional patient, disease and treatment-related data. TED and CRF level data are collected pre-transplant, 100 days and six months post HCT, annually until year 6 post-transplant and biannually thereafter until death. Data for these analyses were retrieved from CIBMTR (TED and CRF) report forms.
Patient selection
We identified 2785 patients with CML who received allogeneic HCT between 2001 and 2010. We excluded from the analysis patients with age >29 years (n=1964); advanced and intermediate disease defined as accelerated phase, blast crisis and second chronic phase or greater (n=197); non-myeloablative or reduced intensity conditioning regimen (n=56); cord blood graft (n= 39); In vitro T cell depletion/CD34 selection (n= 36); lack of HLA matching data (n=27); additional missing data (n=16); and one patient who died on stem cell infusion day. Exclusion was due to either incomplete data or different projected course and outcomes based on different conditioning regimens and sources. The final study population consisted of a total of 449 patients 0–29 years that received myeloablative conditioning regimen in early CML.
Study definitions
Primary outcomes were overall survival (OS) and leukemia-free survival (LFS). For OS, patients were considered to have an event at the time of death from any cause. LFS was defined as time to the first of either relapse or death from any cause. For OS and LFS, patients alive in continuous complete remission were censored at last follow-up. Relapse was defined by submitting centers and included hematologic, molecular and cytogenetic relapse. Secondary outcomes included hematopoietic recovery as defined by time to neutrophils (ANC) >0.5 × 109/L (first of 3 consecutive days) and time to platelets ≥20 × 109/L (first of 3 consecutive days and no platelet transfusions 7 days prior), transplant related mortality (TRM), relapse, grade II–IV acute GVHD, and chronic GVHD (cGVHD).16,17 TRM is defined as death from any cause in the first 28 days post transplantation irrespective of relapse status. Death beyond day +28 was only considered transplant related if the disease was in remission. TRM was summarized by cumulative incidence estimate with relapse as competing risk. Post-HCT relapse was summarized by cumulative incidence estimate with TRM as the competing risk. For relapse and TRM, patients in continuous complete remission were censored at last follow-up. Donor and recipient HLA matching were defined using best available HLA-matching data.18
Data collection on late effects
Transplant centers reported late effects as part of follow-up CRFs that inquired whether an HCT recipient had developed any clinically significant organ impairment or disorder since the date of the last report. Specific questions focused on the following late effects: neurological (stroke/seizures), cardiovascular (myocardial infarction), gastrointestinal/hepatic (cirrhosis), genitourinary (gonadal dysfunction/infertility requiring hormone replacement, renal failure severe enough to warrant dialysis), musculoskeletal (avascular necrosis), special sensory (cataracts), and endocrine (growth hormone deficiency/growth disturbance, hypothyroidism) impairment. Responses to each item were dichotomous (yes/no) and followed by a question requesting the date of onset for each individual organ impairment or disorder. Other late effects were reported using an “other-specify” field that was also checked for the late effects of interest.
Statistical Analysis
Variables related to patient, disease, and transplant characteristics were summarized using descriptive statistics. Distribution of baseline characteristics among the two age groups (children versus young adults) was compared using chi-square test for categorical variables and Wilcoxon rank test for continuous variables. The probabilities of OS and LFS were calculated using the Kaplan-Meier method. Log-rank p-values were calculated to evaluate the overall differences of the Kaplan-Meier estimates. All other outcomes were summarized using the cumulative incidence function to take into account the effects of competing risks.19 Gray’s test p-values were then calculated to evaluate the overall differences across cumulative incidence estimates.20 Cox proportional hazards regression was performed for multivariate analysis for OS, LFS, relapse, TRM and GVHDs.19 Covariates being tested included: patient age, gender, Karnofsky/Lansky performance score at the time of transplantation, time from diagnosis to transplantation, TKI use pre-transplant, graft source, donor type, total body irradiation (TBI) in conditioning regimen, donor age, donor-recipient gender match, recipient cytomegalovirus (CMV) status, GVHD prophylaxis and anti T cell therapy used. All covariates were tested for inclusion via stepwise selection. Similarly, interactions between the main effect and significant covariates were tested. The proportional hazards assumption for each covariate was tested. If violated, the covariate was included as a time-dependent variable. When an interaction between the main effect and a covariate was found significant, the interaction was adjusted using stratified regression analysis on the significant covariate. Logistic regression was used for engraftment. All analyses were performed with SAS 9.3 (SAS Institute Inc.). Having a p-value of less than 0.05 was considered statistically significant.
Results
We report on 449 young patients with early CML <18 years (n=177) and 18–29 years (n=272) transplanted from 2001-10 in CP1 (n=428) and clinical remission (n=28) and followed for a median of 6 years (range 0.25–12y) (Table 1). About 50% patients did not receive TKIs pre-HCT. Patients received MAC and were equally distributed in the TBI versus chemotherapy only conditioning groups. Bone marrow (BM) grafts were more frequently used in pediatrics (p <0.001). Compared to 78% of pediatric patients, only 58% of young adults were transplanted within 1 year of diagnosis (p<0.001). Pediatric patients tended to be CMV negative and more frequently receive ATG/alemtuzumab compared to young adults.
Table 1.
Characteristics of pediatric and young adult patients (0–29 years) undergoing allogeneic Hematopoietic Cell Transplantation (HCT) for early CML between 2001 and 2010, grouped by age
| Variable | Age < 18 years N (%) |
Age 18–29 years N (%) |
P-value |
|---|---|---|---|
| Number of patients | 177 | 272 | |
| CP-1 | 164(93) | 257 | |
| Clinical remission | 13(7) | 15(6) | |
| Number of centers | 80 | 102 | |
| Age at transplant, median | 14 (1–18) | 24 (18–29) | <0.001 |
| Gender | 0.18 | ||
| Male | 101 (57) | 173 (63) | |
| Pre HCT Karnofsky/Lansky score | 0.43 | ||
| 90–100% | 150 (85) | 235 (86) | |
| Country | 0.02 | ||
| US | 101 (57) | 126 (46) | |
| Other countries | 76 (43) | 146 (54) | |
| TKI pre HCT | 0.04 | ||
| No TKI | 70 (40) | 141 (52) | |
| TKI + | 107 (60) | 131 (48) | |
| Graft type | <0.001 | ||
| Bone marrow | 138 (78) | 156 (57) | |
| Peripheral blood stem cell | 39 (22) | 116 (43) | |
| Type of donor | 0.24 | ||
| HLA Matched sibling donor | 82 (46) | 142 (52) | |
| HLA matched unrelated donor | 57 (32) | 89 (33) | |
| HLA Mismatched unrelated donor | 38 (22) | 41 (15) | |
| Conditioning regimen | 0.77 | ||
| Total Body Irradiation based | 62 (35) | 92 (34) | |
| Busulfan based | 115 (65) | 177 (65) | |
| Other | 0 | 3 (1) | |
| Year of transplant | 0.008 | ||
| 2001–2002 | 32(18) | 84(31) | |
| 2003–2004 | 63(36) | 83(31 | |
| 2005–2006 | 50 (28) | 79 (29) | |
| 2007–2008 | 17(10) | 16(6) | |
| 2009–2010 | 15(8) | 10(4)) | |
| Median unrelated donor age (years) (range) |
34 (19–59) | 33 (19–54) | 0.51 |
| Time from diagnosis to HCT | <0.001 | ||
| 0–6 months | 49 (28) | 65 (24) | |
| 6–12 months | 88 (50) | 92 (34) | |
| >= 12 months | 40 (23) | 115 (42) | |
| Donor-recipient sex match | 0.10 | ||
| M-M | 57 (32) | 112 (41) | |
| M-F | 45 (25) | 46 (17) | |
| F-M | 44 (25) | 61 (22) | |
| F-F | 31 (18) | 53 (19) | |
| Recipient CMV status | <0.001 | ||
| Negative | 109 (62) | 113 (41) | |
| Positive | 66 (37) | 154 (57) | |
| Unknown | 2 (1) | 5 (2) | |
| Donor-recipient ABO match | 0.86 | ||
| Matched | 94 (53) | 152 (56) | |
| Minor mismatch | 24 (14) | 40 (15) | |
| Major mismatch | 34 (19) | 51 (19) | |
| Bi-directional | 11 (6) | 13 (5) | |
| Unknown | 14 (8) | 16 (6) | |
| GVHD prophylaxis | 0.14 | ||
| FK506 based | 43 (24) | 84 (31) | |
| CSA based | 134 (76) | 188 (69) | |
| Serotherapy used | <0.001 | ||
| ATG | 43 (24) | 37 (14) | |
| Alemtuzumab | 14 (8) | 8 (3) | |
| No ATG or Alemtuzumab | 120 (68) | 228 (84) | |
| Post-HCT donor lymphocyte infusion | 0.45 | ||
| No | 161 (91) | 250 (92) | |
| Yes | 15 (8) | 22 (8) | |
| Unknown | 1 (<1) | 0 | |
| Post-HCT TKI (planned pre HCT) | 0.01 | ||
| No | 160 (90) | 259 (95) | |
| Yes | 16 (9) | 8 (3) | |
| Missing | 1 (<1) | 5 (2) | |
| Post-HCT TKI (not planned pre HCT) | 0.58 | ||
| No | 154 (87) | 239 (88) | |
| Yes | 22 (12) | 29 (11) | |
| Missing | 1 (<1) | 4 (1) | |
| Median follow-up of survivors (range), months |
72 (3–146) | 72 (3–148) |
TKI: tyrosine kinase inhibitors, ATG: Antithymocyte globulin, CP-1-Chronic phase 1
Overall Survival
The probability of overall survival for the whole cohort was 85% (95% CI, 81–88%), 78% (95% CI, 74–82%) and 75% (95% CI, 71–79%) at 1, 3 and 5 years after HCT, respectively, and was similar among recipients in the pediatric and the young adult group (Supplementary data).
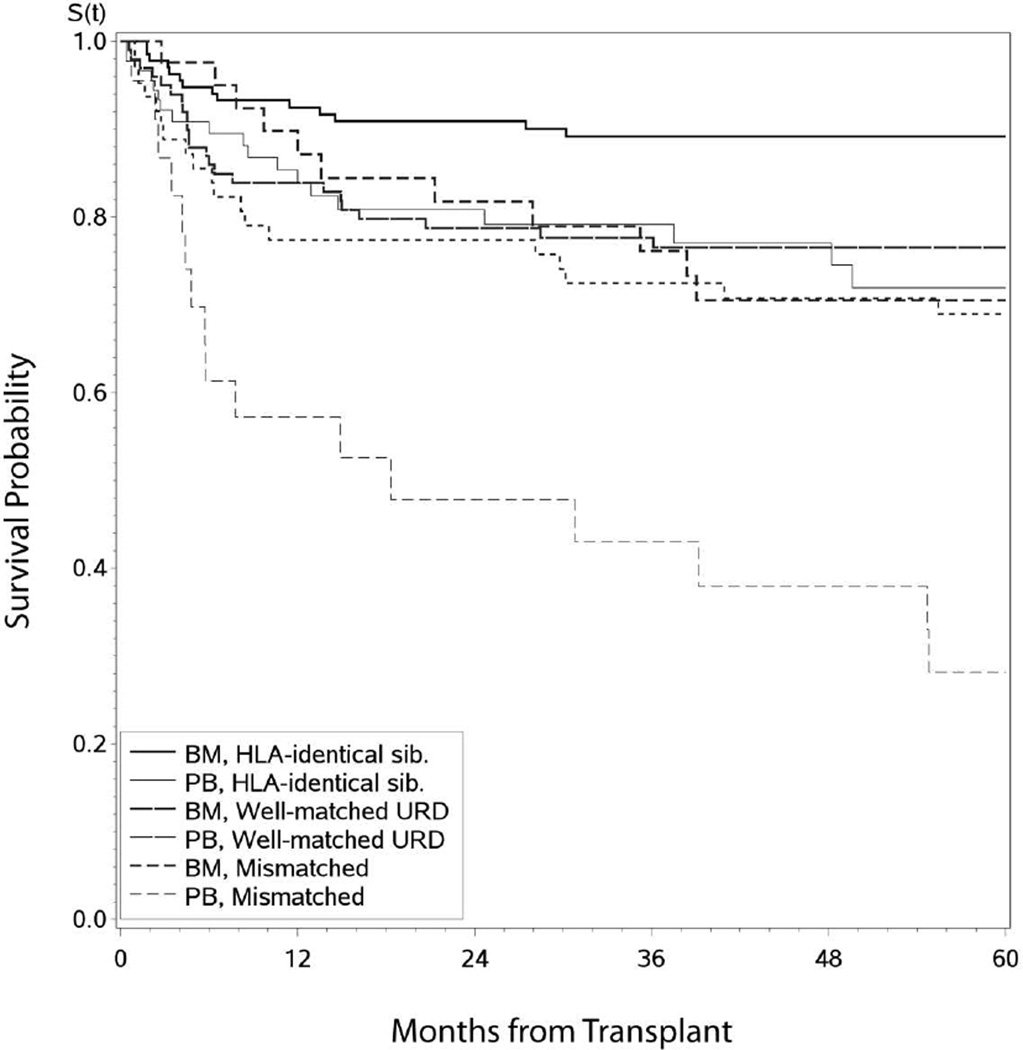
Univariate analysis showed 5 year OS was 83% (95% CI, 77–88%) in matched sibling donor (MSD) compared to 68% (95% CI, 61–74%) in unrelated donors (URD) (p=<0.001); 80% (95% CI, 75–85%) for BM grafts compared to 62% (95% CI, 53–71%) for peripheral blood stem cell (PBSC) grafts (p=0.001) (; Figure 1, supplementary data), with no effect of pre-HCT TKI use (). However on multivariate analysis, only use of unrelated donors (p<0.001) and HCTs before 2003 (p=0.04) increased the risk of long-term mortality (Table 2).
Figure 1.
Overall Survival in CML patients (n=449) transplanted in early disease (CP1, hematological remission) by donor type
Table 2.
Multivariate analysis for pediatric and young adult patients (0–29y) undergoing allogeneic HCT for CML between 2001 and 2010
| Neutrophil engraftment at 28 days | N | RR | 95% CI | p-value | Overall p-value |
|
|---|---|---|---|---|---|---|
| Age in years | ||||||
| < 18 | 177 | 1.00 | ||||
| 18–29 | 267 | 1.57 | 0.74 – 3.31 | 0.2367 | ||
| Graft source | ||||||
| BM | 291 | 1.00 | ||||
| PBSC | 153 | 3.87 | 1.31– 11.47 | 0.0145 | ||
| Gender | ||||||
| Male | 270 | 1.00 | ||||
| Female | 174 | 4.14 | 1.55–11.06 | 0.0046 | ||
| Platelet recovery @ 100 days | N | RR | 95% CI | p-value |
Overall p-value |
|
| Age in years | ||||||
| < 18 | 168 | 1.00 | ||||
| 18–29 | 258 | 4.87 | 1.54– 15.44 | 0.0072 | ||
| Donor type | ||||||
| HLA Matched unrelated donor | 139 | 1.00 | 0.007 | |||
| HLA Matched sibling donor | 216 | 5.65 | 1.15 – 27.83 | 0.0334 | ||
| HLA Mismatched unrelated donor | 71 | 0.46 | 0.16– 1.37 | 0.163 | ||
| Acute GVHD (Stratified by donor type) | N | RR | 95% CI | p-value |
Overall p-value |
|
| Age in years | ||||||
| < 18 | 176 | 1.00 | 0.73 | |||
| 18–29 | 272 | 0.94 | 0.67–1.33 | 0.7340 | ||
| Donor-Recipient sex match | ||||||
| M-M | 169 | 1.00 | 0.01 | |||
| F-F | 84 | 1.37 | 0.88– 2.13 | 0.1618 | ||
| F-M | 105 | 1.78 | 1.20–2.64 | 0.0042 | ||
| M-F | 90 | 1.07 | 0.68–1.70 | 0.7680 | ||
| Graft source | ||||||
| BM | 293 | 1.00 | ||||
| PB | 155 | 1.97 | 1.42– 2.72 | <0.0001 | ||
| Serotherapy | ||||||
| Neither | 346 | 1.00 | 0.01 | |||
| ATG | 80 | 0.70 | 0.46–1.08 | 0.1110 | ||
| Alemtuzumab | 22 | 0.24 | 0.09– 0.67 | 0.0064 | ||
| ATG vs. Alemtuzumab | 2.92 | 1.02– 8.37 | 0.0458 | |||
| Chronic GVHD (Stratified by donor type) | N | RR | 95% CI | p-value |
Overall p-value |
|
| Age in years | ||||||
| < 18 | 174 | 1.00 | ||||
| 18–29 | 269 | 1.84 | 1.34– 2.53 | 0.0002 | ||
| D-R sex match | ||||||
| M-M | 166 | 1.00 | 0.008 | |||
| F-F | 84 | 1.58 | 1.08– 2.30 | 0.0178 | ||
| F-M | 103 | 1.76 | 1.22– 2.54 | 0.0024 | ||
| M-F | 90 | 1.38 | 0.93– 2.04 | 0.1138 | ||
| Graft source | ||||||
| Bone Marrow | 291 | 1.00 | ||||
| Peripheral blood stem cell | 152 | 1.72 | 1.28– 2.32 | 0.0003 | ||
| Serotherapy | ||||||
| Neither | 344 | 1.00 | 0.02 | |||
| ATG | 79 | 0.66 | 0.44– 0.99 | 0.0461 | ||
| Alemtuzumab | 20 | 0.42 | 0.19– 0.92 | 0.0299 | ||
| ATG vs. Alemtuzumab | 1.58 | 0.69–3.62 | 0.28 | |||
| Relapse (Stratified by region) | N | RR | 95% CI | p-value |
Overall p-value |
|
| Age in years | ||||||
| < 18 | 174 | 1.00 | ||||
| 18–29 | 265 | 1.06 | 0.69– 1.64 | 0.7908 | ||
| GVHD prophylaxis | ||||||
| Tacrolimus based | 126 | 1.00 | ||||
| Cyclosporine based | 313 | 1.90 | 1.03– 3.48 | 0.0389 | ||
| Serotherapy | ||||||
| Neither | 338 | 1.00 | <0.0001 | |||
| ATG | 79 | 1.31 | 0.77– 2.23 | 0.3248 | ||
| Alemtuzumab | 22 | 6.22 | 3.09– 12.50 | <0.0001 | ||
| ATG vs. Alemtuzumab | 0.21 | 0.10 | 0.46 | 0.0001 | ||
| TRM (D+100) | N | RR | 95% CI | p-value |
Overall p-value |
|
| Age in years | ||||||
| < 18y | 177 | 1.00 | ||||
| 18–29y | 272 | 0.81 | 0.51–1.28 | 0.36 | ||
| Donor type | ||||||
| HLA Matched unrelated donor | 146 | 1.00 | ||||
| HLA Matched sibling donor | 224 | 0.43 | 0.25– 0.77 | 0.004 | <0.001 | |
| HLA Mismatched unrelated donor | 79 | 2.03 | 1.21– 3.40 | 0.008 | ||
| Graft source | ||||||
| BM | 294 | 1.00 | ||||
| PBSC | 155 | 1.96 | 1.26– 3.05 | 0.003 | ||
| Recipient CMV status | ||||||
| Negative | 222 | 1.00 | ||||
| Positive | 220 | 1.81 | 1.15– 2.86 | 0.011 | ||
| Year of transplant | ||||||
| 2001–2002 | 116 | 1.00 | ||||
| 2003–2004 | 146 | 0.49 | 0.28– 0.84 | 0.01 | 0.02 | |
| 2005–2006 | 129 | 0.67 | 0.39– 1.15 | 0.14 | ||
| 2007–2008 | 33 | 0.17 | 0.04– 0.70 | 0.01 | ||
| 2009–2010 | 25 | 0.45 | 0.14– 1.46 | 0.18 | ||
| LFS (Stratified by donor type) | N | RR | 95% CI | p-value |
Overall p-value |
|
| Age in years | ||||||
| < 18 | 174 | 1.00 | ||||
| 18–29 | 265 | 0.97 | 0.70– 1.33 | 0.8308 | ||
| Country | ||||||
| US | 225 | 1.00 | ||||
| Other | 214 | 1.88 | 1.33– 2.64 | 0.0003 | ||
| Graft source | ||||||
| Bone marrow | 288 | 1.00 | ||||
| Peripheral blood stem cell | 151 | 1.64 | 1.19– 2.27 | 0.0027 | ||
| Pre HCT Karnofsky/Lansky score | ||||||
| < 90 | 32 | 1.00 | ||||
| 90–100 | 377 | 1.09 | 0.61–1.94 | 0.7661 | 0.04 | |
| Missing | 30 | 2.18 | 1.02– 4.62 | 0.0430 | ||
| Serotherapy | ||||||
| Neither | 338 | 1.00 | ||||
| ATG | 79 | 1.22 | 0.80– 1.85 | 0.3553 | 0.0248 | |
| Alemtuzumab | 22 | 2.25 | 1.25– 4.03 | 0.0066 | ||
| ATG vs. Alemtuzumab | 0.54 | 0.30– 0.99 | 0.0474 | |||
| Overall Survival (Stratified by graft source) | N | RR | 95% CI | p-value |
Overall p-value |
|
| Age | ||||||
| < 18y | 177 | 1.00 | ||||
| 18–29y | 272 | 0.90 | 0.61 | 1.35 | 0.6178 | |
| Donor type | ||||||
| HLA Matched unrelated donor | 146 | 1.00 | ||||
| HLA Matched sibling donor | 224 | 0.61 | 0.38 | 0.98 | 0.0405 | <0.0001 |
| HLA Mismatched unrelated donor | 79 | 2.02 | 1.26 | 3.23 | 0.0035 | |
| Year of transplant | ||||||
| 2001–2002 | 116 | 1.00 | ||||
| 2003–2004 | 146 | 0.53 | 0.33 | 0.85 | 0.0087 | 0.04 |
| 2005–2006 | 129 | 0.60 | 0.36 | 0.99 | 0.0448 | |
| 2007–2008 | 33 | 0.44 | 0.18 | 1.04 | 0.0617 | |
| 2009–2010 | 25 | 0.49 | 0.18 | 1.37 | 0.6178 | |
TKI: tyrosine kinase inhibitors, ATG: Antithymocyte globulin, CI-Confidence Interval, RR-Relative risk. Higher RR indicates higher risk of treatment failure (death or relapse)
Leukemia-Free Survival
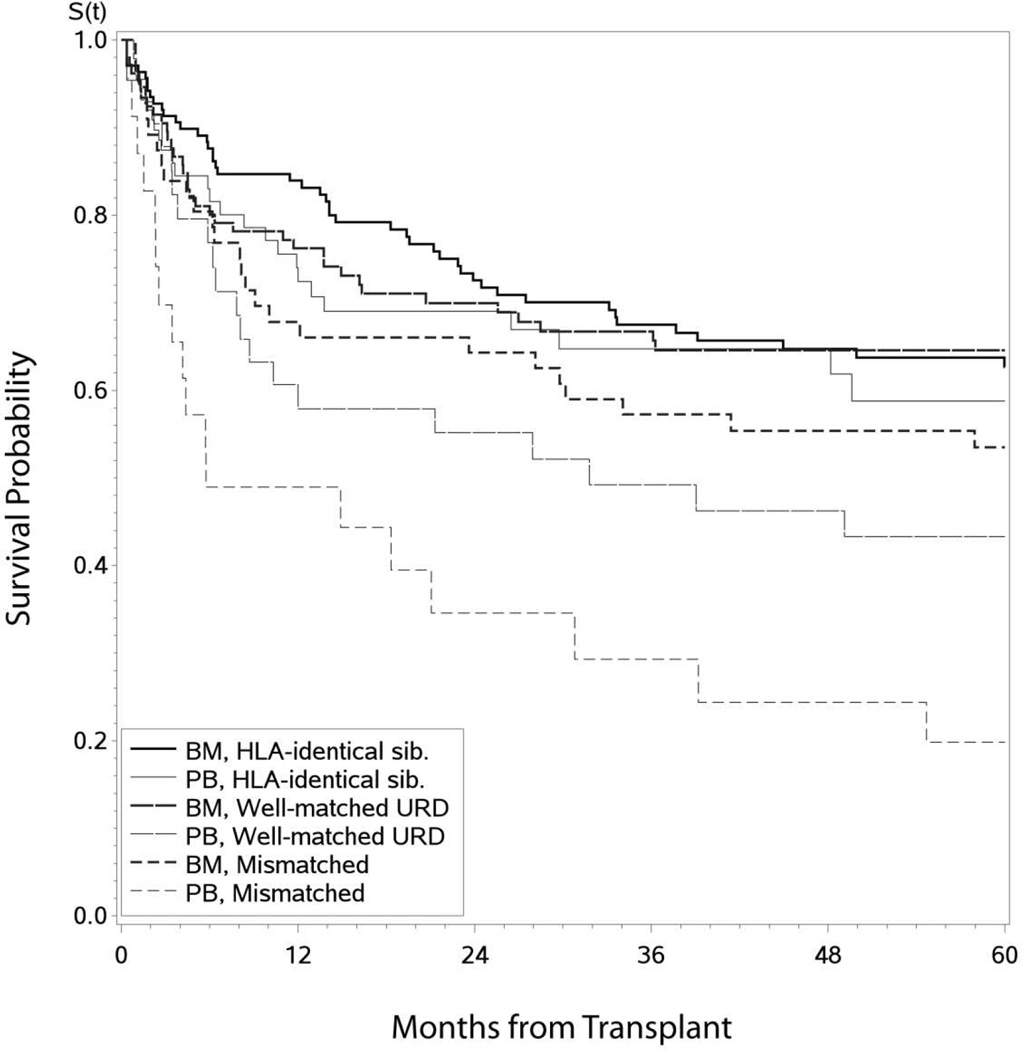
Leukemia-free survival rates for the entire cohort at 1, 3 and 5 years post-HCT were 76% (95% CI, 72–80%), 63% (95% CI, 58–68%) and 59% (95% CI 54–64%), respectively. On univariate analysis, neither age (p=0.63) nor donor type (p=0.11) or graft source (p=0.07) affected LFS (Supplementary data; Figure 2). Although pre-HCT TKI use was associated with superior LFS (p=0.03) on univariate analysis, this effect was lost on multivariate analysis. Only use of ATG/alemtuzumab (p=0.02), PBSC source (p=0.003), and transplant in non US centers (p=0.0003) were independent predictors of lower LFS (Table 2).
Figure 2.
Leukemia Free Survival in CML patients (n=449) transplanted in early disease (CP1, hematological remission) by donor type
Engraftment
There was no difference in neutrophil engraftment at day 28 between pediatric patients and young adults (p=0.23); however, as expected, more patients receiving peripheral blood as graft source engrafted by day 28 (p=0.01) (Table 2). In addition, female patients had a significantly increased incidence of neutrophil recovery (p=0.004). Young adult recipients had higher incidence of platelet recovery at day 100 (p=0.007) as did recipients of MSD grafts (p=0.007).
GVHD
Incidence of grade II–IV aGVHD was 36% (95% CI: 31–40%) and grade 3–4 aGVDH 17% (95% CI 13–20%) at 100 days post HCT. Incidence of cGVHD was 46% (95% CI: 42–51%), 51% (95%CI: 46–56%), and 52% (95% CI: 47–57%) at 1 year, 3 years and 5 years post HCT, respectively. Incidence of aGVHD was significantly reduced with bone marrow grafts (p<0.001), male donors to male recipients (p=0.01) and ATG/alemtuzumab use (p=0.01) but not affected by recipient age (p=0.73). Risk of cGVHD was higher in the young adult population (p=0.0002), female donors (p=0.008), PBSC source (p=0.0003), and without use of ATG/alemtuzumab (p=0.02) (Table 2). Extensive and limited cGVHD was seen in 21% and 15% of pediatric patients, respectively, as opposed to 41% and 14% of young adults, respectively.
Relapse
The cumulative incidence of relapse for the entire cohort was 11% (95% CI 8–14%) at 1 year, 18% (95% CI 15–22%) at 3 years and 21% (95% CI, 17–25%) at 5 years after HCT. As described by reporting centers, relapse (n=104) was hematological, cytogenetic and molecular in 41%, 21% and 22%, respectively, with 16% unknown. On multivariate analysis, risk of relapse was higher with use of cyclosporine (p=0.03) and alemtuzumab use (p<0.001) (Table 2). Relapse rates were unaffected by donor type, graft source, recipient age or pre-HCT TKI use (Table 2).
Transplant Related Mortality
The cumulative incidence of TRM for the entire cohort was 13% (95% CI, 10–17%) at 1 year, 18% (95%CI, 15–22%) at 3 years and 20% (95% CI, 16–24%) at 5 years post HCT. Risk of TRM increased with use of unrelated donors (p<0.001), PBSC (p=0.003), CMV seropositive recipients (p=0.01) and HCTs prior to 2003 (p=0.02); it was similar in the pediatric and young adult patients (p=0.36) (Table 2). Cause of deaths included GVHD (n=26), infections (n=12), end organ failure (n=17), idiopathic pneumonitis (n=8), graft failure (n=4) and others/unknown (n=17).
Transplant Variables and Outcomes
For the entire cohort of patients <29 years, there was no difference in OS, LFS, relapse or TRM with use of pre-HCT TKI therapy, time to HCT from diagnosis, donor age, donor recipient ABO mismatch or TBI-based conditioning regimens. The effect of post-HCT TKI (used in <15% of all patients for either prophylaxis or treatment) and DLI were not evaluated in this data set due to small numbers.
Late Effects
Among 144 pediatric patients with a median follow-up of 6 years, there were 16 cases of gonadal dysfunction, 12 avascular necrosis, 8 hypothyroidism, 7 growth deficiency, 6 diabetes mellitus, 6 new malignancies and 1 cardiac abnormality reported. Among the 201 young adult patients with similar follow-up, there were 10 cases of gonadal dysfunction, 10 cataracts, 3 hypothyroidism, 2 diabetes mellitus and 2 with avascular necrosis.
Discussion
Several reports have documented a decline in annual transplantation rates for CML, especially among patients in CP1.3,5,8 In the TKI era, HCT is recommended in CP1 patients if they are resistant or intolerant to TKI.21,22 Although it is the only established treatment to eliminate leukemic stem cells,23 HCT, especially with a myeloablative conditioning regimen, causes significant long term morbidities. Children and young adults are known to better tolerate HCT, in general, and they typically enjoy superior outcomes compared to older adults.12,24,25,26,27 Additionally, advances in supportive care, have led to significant reduction in the incidence and impact of HCT-related complications.25,26,27
Addressing specific prognostic factors for CP-CML and HCT for pediatrics and young adults is limited by the relatively small number of patients in single-center reports. 28,29,30 Given its unique clinical and biological features, CML in the younger age group needs to be better categorized.31 The CIBMTR database offers the advantage of a large number of patients with extensive data, which permit performance of multivariate analyses. We believe our study provides valuable information as data for HCT in children with early CML are scarce.
Our study of 449 pediatric and young adult patients with early disease (CP1 or hematologic remission) who underwent MAC allogeneic HCT during the past decade (2001–2010) is the largest review of young patients in the TKI era. The 5-year OS was 75% for all patients <29 years, and outcomes were similar in children and young adults although significantly more pediatric patients tended to receive pre HCT TKI, BM grafts and HCT within 1 year from diagnosis The differences may reflect standard treatment practice differences in the two cohorts. Factors contributing to better OS, as expected, include related donors (83%) and more recent HCT (2003–2010) due to lower TRM. Although BM donors had a 5-year OS of 80%, this was not significantly better than PBSC. This finding contrasts with the CIBMTR report of a cohort of unrelated donor leukemia patients in which a survival advantage for BM in CML-CP1 was described.32 Other reports in similar age group include an EBMT registry retrospective analysis that reported a 3-year OS of 75% with a sibling donor and 65% with a matched unrelated donor (MUD) in children with CP1; and German CML-IV study with an OS of 90% when patients were prospectively assigned to HCT for TKI intolerance or availability of MSD.33,34 TRM in this young cohort receiving MAC was comparable to previous reports and significantly higher in the unrelated donor (p<0.0001), PBSC (p=0.001), CMV positive donor (p=0.01) and HCTs before 2003 (p=0.02).30
Five-year LFS for our patient cohort was 59%, and similar to previously described studies; worse outcomes were reported with use of unrelated donors and PBSC source. 33,34,35 Unique to our analysis is the worse LFS seen with use of alemtuzumab but not in the ATG group when comparing with those that received none. LFS was significantly worse when comparing ATG with alemtuzumab, although the number of patients in this group was small. Worse LFS in our patients, especially compared to prospective studies, is likely due to heterogeneous patient/donor group in our retrospective analysis however could potentially be attributed to NK and additional immune cell depletion with Alemtuzumab that may impact GVL.36
Cumulative incidence of relapse at 5 years, 21% for the whole group, is higher than in other studies.37,38 This increased relapse may be due to current HCT recommendations that potentially select for a group with more aggressive disease than those on prolonged TKIs only, who are not traditionally referred for HCT although indications for HCT were not captured in CIBMTR database. Relapse was higher in the group receiving ATG/alemtuzumab and also with the use of cyclosporine over tacrolimus based GVHD prophylaxis. The latter finding has not been previously described and needs to be further evaluated; and is contrary to reports suggesting decreased graft-versus-leukemia (GVL) effect with tacrolimus.39 Alemtuzumab use is often paired with donor lymphocyte infusions to decrease risk of relapse, but we were unable to assess for this effect.40 While the curative effect of HCT in CML relies on the graft-versus-leukemia (GVL) effect, similar to other reports, donor source/matching did not play a role in decreasing relapse or LFS.41 The role of cGVHD in this group needs to be further studied. TBI was surprisingly used in about 35% of this young population, equally in both age cohorts although randomized comparison of Cy/TBI to BuCy in patients with CML reported comparable OS, LFS and maybe even decreased relapse.42,43 Similar to a recent CIBMTR CML study our results showed no advantage for TBI.44
We confirmed increased incidence of acute and chronic GVHD with PBSC, female donors and regimens without ATG/alemtuzumab. 45,46,47 The protective role of cGVHD in CML was recently reported in a CIBMTR analysis confirming earlier studies.48,49,50 Although this was not specifically analyzed in our study, there was no difference in relapse/LFS with PBSC donors and young adults, both of whom had increased incidence of cGVHD.
Similar to earlier CIBMTR reports, we did not show any impact of pre-HCT TKI use on post-HCT outcomes for early CML in young patients.30,48 Given that pre HCT TKI use likely interacts with era of HCT leading to change in reported outcomes, adjusted analysis was performed to confirm this finding. Imatinib is the only FDA approved TKI in pediatrics. The estimated LFS in pediatric patients with early CML treated with imatinib is 98% at 3 yrs with a complete hematologic response in 98%. 28 However, we would caution that a direct comparison between groups receiving HCT to those on TKI alone is flawed as direct comparison can be made only if indications for HCT were available. However, in the current era, when most patients receive TKI, this finding is less relevant.
Although there are significant differences in graft source, conditioning regimen and donor source used by age group (reflecting different practice patterns in pediatric versus young adult), there was no difference in clinical outcomes in the pediatric versus young adult age group, suggesting that these groups are relatively uniform biologically in terms of age, health status and underlying disease.
Late effects in CML HCT were similar to those described with other malignant neoplasms HCT survivors. The infrequent occurrence of these events, possibly, in part, due to the relatively short follow-up and inconsistent data capture and reporting precludes analysis of risk factors such as TBI. As described, gonadal dysfunction and growth failure were common whereas very few cardiac adverse events were noted.26 This study was restricted to MAC regimens, but several studies have described encouraging long term outcomes with RIC conditioning in CML, which is especially relevant in this age group.51,52,53 With increasing number of reports of side effects of TKI, such as growth abnormalities, cardiac events and infertility in this group of patients, it is important to better describe long-term effects of HCT to establish guidelines for treatment.54,55,56,57,58,59
We recognize the limitations of these analyses. Some outcomes of interest were unavailable, such as the indications for HCT in early CML (planned versus resistance to TKI/donor availability); molecular/cytogenetic disease status at the time of HCT and relapse; and BCR/ABL1 mutations. The retrospective study design also limited our ability to report comprehensively on late effects. While the best way to determine the benefit of HCT for children and young adults with early CML is to conduct a prospective randomized trial comparing upfront HCT and TKI therapy, it is unlikely to have sufficient number of patients for such a study.
In conclusion, children and young adults with early CML have similar outcomes with HCT. When indicated, HLA MSD and BM stem cell source lead to best outcomes with no benefit for radiation containing regimens. There is a need for prospective trials in this age group to better define guidelines for HCT, improving post HCT relapse rates without need for lifelong dependence on TKI.
Supplementary Material
-
-
Outcomes for hematopoietic stem cell transplant are similar in pediatric and young adult patients
-
-
OS and LFS superior with matched sibling donors and bone marrow as stem cell source
-
-
Pre HCT tyrosine kinase use did not alter outcomes in for hematopoietic stem cell transplant in pediatric and young adult patients
-
-
No role for irradiation in myeloablative conditioning regimen pre HCT
-
-
Role of hematopoietic stem cell transplant in pediatric and young adults in early CML needs to be better defined
Acknowledgments
Financial Disclosure: The authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, et al. SEER Cancer Statistical Review 1975–2012. Bethesda, MD: National Cancer Institute; 2015. Apr, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to SEER website. [Google Scholar]
- 2.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 3.Pavlu J, Szydlo RM, Goldman JM, Apperley JF. Three decades of transplantation for chronic myeloid leukemia: what have we learned? Blood. 2011;117(3):755–763. doi: 10.1182/blood-2010-08-301341. [DOI] [PubMed] [Google Scholar]
- 4.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2014 update on diagnosis, monitoring, and management. Am J Hematol. 2014;89(5):547–556. doi: 10.1002/ajh.23691. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien S, Radich JP, Abboud CN, et al. Chronic Myelogenous Leukemia, Version 1.2014. Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(11):1327–1340. doi: 10.6004/jnccn.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guidelines in Oncology; Chronic Myelogenous Leukemia. National Comprehensive Cancer Network. 2015 doi: 10.6004/jnccn.2009.0065. [DOI] [PubMed] [Google Scholar]
- 7.Baccarani M, Cortes J, Pane F, et al. Chronic Myeloid Leukemia: An Update of Concepts and Management Recommendations of European Leukemia Net. Journal of Clinical Oncology. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hijiya N, Millot F, Suttorp M. Chronic Myeloid Leukemia in Children: Clinical Findings, Management, and Unanswered Questions. Pediatr Clin North Am. 2015;62(1):107–119. doi: 10.1016/j.pcl.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Bansal D, Shava U, Varma N, Trehan A, Marwaha RK. Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr Blood Cancer. 2012;59(3):481–484. doi: 10.1002/pbc.23389. [DOI] [PubMed] [Google Scholar]
- 10.Giona F, Mariani S, Gnessi L, et al. Bone metabolism, growth rate and pubertal development in children with chronic myeloid leukemia treated with imatinib during puberty. Haematologica. 2013;98(3):e25–e27. doi: 10.3324/haematol.2012.067447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobernicht SL, Schweiger B, Zeitler P, et al. Acquired growth hormone deficiency in a girl with chronic myelogenous leukemia treated with tyrosine kinase inhibitor therapy. Pediatr Blood Cancer. 2011;56(4):671–673. doi: 10.1002/pbc.22945. [DOI] [PubMed] [Google Scholar]
- 12.Hijiya N, Schultz K, Metzler M, et al. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2015 Oct 28; doi: 10.1182/blood-2015-06-648667. ii: blood-2015-06-648667. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulsipher MA. Treatment of CML in pediatric patients: should imatinib mesylate (STI-571, Gleevec) or allogeneic hematopoietic cell transplant be front-line therapy? Pediatr Blood Cancer. 2004;43(5):523–533. doi: 10.1002/pbc.20062. [DOI] [PubMed] [Google Scholar]
- 14.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006 Sep 15;108(6):1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 15.US Department of health and Human Services, NIH. An analysis of National Cancer Institute’s investment in Pediatric Cancer Research. 2013 Sep; http://www.cancer.gov/types/childhood-cancers/research/pediatric-analysis.pdf.
- 16.Przepiorka D, Weisdorf D, Martin P, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 17.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980 Aug;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 18.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Gray R. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 21.Passweg JR, Baldomero H, Peters C, et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014;49:744–50. doi: 10.1038/bmt.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gratwohl A, Baldomero H, Gratwohl M, et al. Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a global observational study. Haematologica. 2013;98:1282–129. doi: 10.3324/haematol.2012.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez C, Gomez V, Tomás JF, et al. Relapse of chronic myeloid leukemia after allogeneic stem cell transplantation: outcome and prognostic factors: the Chronic Myeloid Leukemia Subcommittee of the GETH (Grupo Español de Trasplante Hemopoyético) Bone Marrow Transplant. 2005;36:301–306. doi: 10.1038/sj.bmt.1705063. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia S. Caring for the long-term survivor after allogeneic stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2014 Dec 5;2014(1):495–503. doi: 10.1182/asheducation-2014.1.495. [DOI] [PubMed] [Google Scholar]
- 26.Perkins JL, Kunin-Batson AS, Youngren NM, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer. 2007;49:958–963. doi: 10.1002/pbc.21207. [DOI] [PubMed] [Google Scholar]
- 27.Bernard F, Auquier P, Herrmann I, Contet A, et al. Health status of childhood leukemia survivors who received hematopoietic cell transplantation after BU or TBI: an LEA study. Bone Marrow Transplant. 2014 May;49(5):709–16. doi: 10.1038/bmt.2014.3. [DOI] [PubMed] [Google Scholar]
- 28.Millot F, Suttorp M, Guilhot J, et al. The International Registry for Chronic Myeloid Leukemia (CML) in Children and Adolescents (I-CML-Ped-Study): Objectives and preliminary results. Blood. 2012;120:3741. [Google Scholar]
- 29.Hamidieh AA, Ansari S, Darbandi B, et al. The treatment of children suffering from chronic myelogenous leukemia: a comparison of the result of treatment with Imatinib mesylate and allogeneic hematopoietic stem cell transplantation. Pediatric Transplantation. 2013;17(4):380–386. doi: 10.1111/petr.12074. [DOI] [PubMed] [Google Scholar]
- 30.Lee SE, Choi SY, Kim SH. Prognostic factors for outcomes of allogeneic stem cell transplantation in chronic phase chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Hematology. 2014 Mar;19(2):63–72. doi: 10.1179/1607845413Y.0000000100. [DOI] [PubMed] [Google Scholar]
- 31.Kalmanti L, Saussele S, Lauseker M, et al. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomize CML study IV. Ann Hematol. 2013;93(1):71–80. doi: 10.1007/s00277-013-1937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eapen M, Logan BR, Appelbaum FR, et al. Long-term survival after transplantation of unrelated donor peripheral blood or bone marrow hematopoietic cells for hematologic malignancy. Biol Blood Marrow Transplant. 2015 Jan;21(1):55–9. doi: 10.1016/j.bbmt.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gratwohl A, Brand R, Apperley J, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results: an analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2006;91(4):513–521. [PubMed] [Google Scholar]
- 34.Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010 Mar 11;115(10):1880–5. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 35.Muramatsu H, Kojima S, Yoshimi A, et al. Outcome of 125 children with chronic myelogenous leukemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Biol Blood Marrow Transplant. 2010;16(2):231–238. doi: 10.1016/j.bbmt.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Klangsinsirikul P, Carter G, Byrne J, et al. Campath-1G causes rapid depletion of circulating host dendritic cells (DCs) before allogeneic transplantation but does not delay donor DC reconstitution. Blood. 2002 Apr 1;99(7):2586–91. doi: 10.1182/blood.v99.7.2586. [DOI] [PubMed] [Google Scholar]
- 37.Suttorp M, Claviez A, Bader P, et al. Allogeneic stem cell transplantation for pediatric and adolescent patients with CML: results from the prospective trial CML-paed I. Klin Padiatr. 2009;221(6):351–7. doi: 10.1055/s-0029-1239529. [DOI] [PubMed] [Google Scholar]
- 38.Cwynarski K, Roberts IA, Iacobelli S, et al. Stem cell transplantation for chronic myeloid leukemia in children. Blood. 2003;102(4):1224–31. doi: 10.1182/blood-2002-12-3637. [DOI] [PubMed] [Google Scholar]
- 39.Goldman JM, Majhail NS, Klein JP, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28(11):1888–1895. doi: 10.1200/JCO.2009.26.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imado T, Iwasaki T, Kuroiwa T, et al. Effect of FK506 on donor T-cell functions that are responsible for graft-versus host disease and graft-versus-leukemia effect. Transplantation. 2004 Feb 15;77(3):391–8. doi: 10.1097/01.TP.0000111759.48240.F5. [DOI] [PubMed] [Google Scholar]
- 41.Copelan EA, Crilley PA, Szer J, et al. Late mortality and relapse following BuCy2 and HLA identical sibling marrow transplantation for chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15:851–855. doi: 10.1016/j.bbmt.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Clift R, Buckner C, Thomas E, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 84(1994), pp):2036–2043. [PubMed] [Google Scholar]
- 43.Devergie A, Blaise D, Attal M, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM) Blood. 85(1995):2263–2268. [PubMed] [Google Scholar]
- 44.Zuckerman T, Katz T, Haddad N, et al. Allogeneic stem cell transplantation for patients with chronic myeloid leukemia: risk stratified approach with a long-term follow-up. Am J Hematol. 2012;87(9):875–879. doi: 10.1002/ajh.23263. [DOI] [PubMed] [Google Scholar]
- 45.Poir´e X, Artz A, Larson RA, et al. Allogeneic stem cell transplantation with alemtuzumab-based conditioning for patients with advanced chronic myelogenous leukemia. Leuk Lymphoma. 2009;50(1):85–9. doi: 10.1080/10428190802626624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisdorf DJ, Nelson G, Lee SJ, et al. Sibling versus unrelated donor allogeneic hematopoietic cell transplantation for chronic myelogenous leukemia: refined HLA matching reveals more graft-versus-host disease but not less relapse. Biol Blood Marrow Transplant. 2009 Nov;15(11):1475–8. doi: 10.1016/j.bbmt.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copelan E, Avalos B, Ahn K, et al. Comparison of outcomes of allogeneic transplantation for chronic myeloid leukemia with cyclophosphamide in combination with intravenous busulfan, oral busulfan, or total body irradiation. Biol Blood Marrow Transplant. 2015 Mar;21(3):552–8. doi: 10.1016/j.bbmt.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyiadzis M, Arora M, Klein JP, et al. Impact of Chronic Graft-versus-Host Disease on Late Relapse and Survival on 7,489 Patients after Myeloablative Allogeneic Hematopoietic Cell Transplantation for Leukemia. Clin Cancer Res. 2015 May;21(9):2020–8. doi: 10.1158/1078-0432.CCR-14-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan K, Weiden P, Storb R, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–8. [PubMed] [Google Scholar]
- 50.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. [PubMed] [Google Scholar]
- 51.Khoury HJ, Kukreja M, Goldman JM. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012 Jun;47(6):810–6. doi: 10.1038/bmt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satwani P, Morris E, Bradley MB, et al. Reduced intensity and non-myeloablative allogeneic stem cell transplantation in children and adolescents with malignant and non-malignant diseases. Pediatr Blood Cancer. 2008 Jan;50(1):1–8. doi: 10.1002/pbc.21303. [DOI] [PubMed] [Google Scholar]
- 53.Crawley C, Szydlo R, Lalancette M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005 Nov 1;106(9):2969–76. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 54.Bansal D, Shava U, Varma N, Trehan A, Marwaha RK. Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr Blood Cancer. 2012;59(3):481–484. doi: 10.1002/pbc.23389. [DOI] [PubMed] [Google Scholar]
- 55.Giona F, Mariani S, Gnessi L, et al. Bone metabolism, growth rate and pubertal development in children with chronic myeloidleukemia treated with imatinib during puberty. Haematologica. 2013;98(3):e25–e27. doi: 10.3324/haematol.2012.067447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hobernicht SL, Schweiger B, Zeitler P, Wang M, Hunger SP. Acquired growth hormone deficiency in a girl with chronic myelogenous leukemia treated with tyrosine kinase inhibitor therapy. Pediatr Blood Cancer. 2011;56(4):671–673. doi: 10.1002/pbc.22945. [DOI] [PubMed] [Google Scholar]
- 57.Millot F, Guilhot J, Baruchel A, et al. Impact of early molecular response in children with chronic myeloid leukemia treated in the French Glivec phase 4 study. Blood. 2014;124(15):2408–2410. doi: 10.1182/blood-2014-05-578567. [DOI] [PubMed] [Google Scholar]
- 58.Apperley J. Issues of imatinib and pregnancy outcome. J Natl Compr Canc Netw. 2009;7(10):1050–1058. doi: 10.6004/jnccn.2009.0069. [DOI] [PubMed] [Google Scholar]
- 59.Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111(12):5505–5508. doi: 10.1182/blood-2007-10-114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.