One of the more common complaints of patients with vestibular disorders is gaze instability while walking.1 Gaze instability has also been called movement-induced dizziness, visual blurring, or oscillopsia.2–4,5 Oscillopisa during active head movements from an abnormal VOR may occur from an abnormal gain, abnormal timing (phase shift) between the eye and head movement, or from a directional mismatch between the head and eye rotation vectors.5 Everyday head movements have been reported to occur at frequencies above 1 Hz.2
The primary function of the vestibulo-ocular reflex (VOR) is to maintain gaze stability during head movement.1, 2, 6–8 Loss of vestibular function produces instability of gaze, which is characteristically worse during walking.1, 5
Visual acuity declines if eye movements cannot compensate for changes in head motion.6 As little as 2–4 degrees per second of retinal slip will degrade visual acuity.1, 9–11 Therefore, the probable cause of gaze instability during head movement is the inability of the VOR to maintain gaze.3, 4, 12
Fast random head perturbations can cause decreases in eye velocity in individuals with vestibular loss while pursuing a target.13 Functioning of the VOR may be inferred from dynamic visual acuity testing (DVAT).4, 8, 9, 14–16 Tests of dynamic acuity appear to be reliable indicators of vestibular pathology and rehabilitative success in patients with peripheral vestibular pathologies.9, 11, 14
Previous work has established computerized DVAT as a test for identifying unilateral and bilateral hypofunction and to demonstrate changes in dynamic visual acuity over the course of rehabilitation.14,11,17,16 The DVAT uses progressively smaller targets in order to determine the smallest optotype that a person can visualize during static and active head movements.14, 18 The DVAT has been used to demonstrate changes in fixation abilities at higher head frequencies after a course of vestibular rehabilitation.3, 10, 15, 16,19 A limitation of the DVAT is that changes in optotype size may differentially affect persons with visual comorbidities, including persons with cataracts, macular degeneration, or diabetic retinopathy.18
The gaze stabilization test (GST) requires the maintenance of fixation on a fixed target size during active head movement.18, 20 The GST was designed to quantify how quickly the subject can move their head and still maintain an easily seen target in focus. One problem with the computerized DVAT is that by ranging the acuity, eye abnormalities like refraction error, macular degeneration, or diabetic retinopathy begin to affect the results for smaller targets especially in older adults. The GST maintains a constant optotype size and presents the optotype at unpredictable intervals. Although the GST is an active head rotation test, the unpredictable nature of the direction of head movement and timing of target presentation minimizes the ability to generate a compensatory or anticipatory saccade which would allow for fixation.
The purpose of the present study was to: 1) determine if there was a difference in GST scores between older and younger subjects without vestibular disease, 2) determine if there were differences in GST scores between persons with and without vestibular dysfunction and 3) to describe the sensitivity and specificity of a computerized test that records gaze stabilization during active head movement in the pitch and yaw planes for the identification of persons with vestibular disease.
Materials and Methods
Subjects
Fifty-seven subjects agreed to participate in the study. The study was approved by the …………. Institutional Review Board. Three different groups participated: 20 young control subjects (20–40 years old), 20 older control subjects (60–80 years old), and 17 patients with vestibular disease. The mean age of the healthy young group was 27 ± 4.4 (range 21–34), the healthy older subjects mean age was 70 ± 5.3 (range 61–80), and the patient group mean age was 63 ± 19 (range 24–79).
Vestibular function was assessed in all participants and included ocular motor screening, caloric, positional, and rotational chair testing. Patient diagnoses included 12 with peripheral vestibular disorders (5 right and 7 left unilateral peripheral hypofunction), one with benign paroxysmal positional vertigo (BPPV), one with a peripheral vestibular disorder with past history of BPPV (negative Dix-Hallpike when seen), two with bilateral vestibular hypofunction, and one person with multisensory disequilibrium. Thirteen subjects has abnormal rotational chair findings, 8 abnormal caloric test results, 6 had abnormal static positional testing, and one had an abnormal occulomotor test result. All control subjects and people with vestibular disorders were examined by a neuro-otologist. Ten subjects used bifocals, 7 wore contacts, 4 used trifocals, and 4 wore regular lenses during testing. In addition, one subject wore progressive lenses and one wore monocular contact lenses.
All control subjects achieved a score of ≥24 on the Mini Mental State Exam (MMSE).21 Control subjects were excluded if they had any abnormality on the vestibular function tests or a MMSE score of <24. Patients were excluded if their MMSE was < 24.
Instrumentation
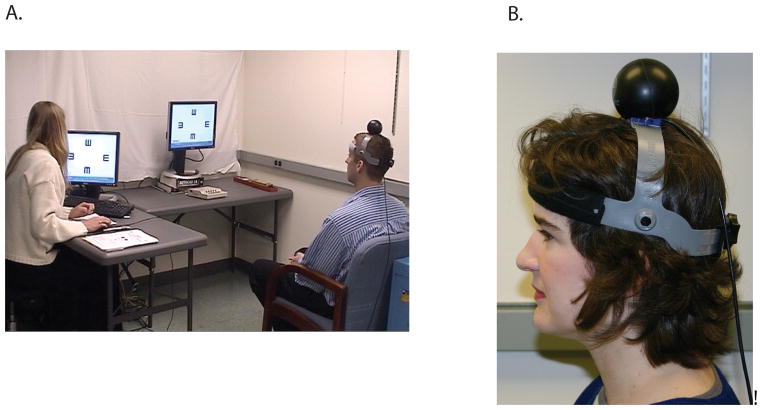
The gaze stabilization test (GST) provides information regarding the functional capacity of the vestibulo-ocular reflex (VOR). The device records the maximum active head velocity at which a person can stabilize gaze. The stimulus is a vertical or horizontal head movement at controlled, criterion velocities while viewing a visual optotype (letter E) (Figures 1A and B). The expected response is accurate recognition of the optotype orientation at a given velocity. Goebel et al18 have reported the GST to be highly reliable across three trials (ICC=0.91) in persons with unilateral vestibular disorders.
Figure 1.
A and B. A. The subject views a screen and attempts to identify the orientation of the optotype displayed in the center of the screen. B. The subject wears a head tracker during active head movement in the pitch or yaw plane while attempting to visualize the optotype that is displayed on the screen.
The inVision System™ from NeuroCom was used to project the computer-generated optotype E to quantify a participant’s ability to maintain stable gaze while actively moving their head. A head sensor (an InterSense Inertia Cube3, 3-axis integrating gyro, Figure 1B) was used to simultaneously record velocity and direction of head movements during testing. The head sensor was mounted on a headband and was secured on the head using an adjustable strap (Figure 1B).
Test Protocol
Testing was performed in a well-lit room at a viewing distance of 1.5 m. A white sheet was draped behind the computer screen to minimized potentially distracting visual clutter. Static visual acuity was determined with the subject seated. The optotype size was determined by individually determined static visual acuity calculated by the computer software. Subjects were asked to view the computer screen and the “E” optotype was projected. The E optotype was presented with a delay of 1 second in one of four orientations: up, down, left, or right. Subjects reported the direction of the “E” and the optotype size was adjusted based on the accuracy of the response. Each optotype size was projected a maximum of 5 times for a maximum on screen time of 100 msec, as subjects with three out of five presentations accurately reporting the correct orientation triggered a response from the computer to generate a smaller optotype and those with 3 out of 5 errors received an increase in optotype size. When the subject stabilized at 3 correct responses out of 5 but was unable to see the target 3 out of 5 times with a smaller optotype, static visual acuity was determined.
To determine gaze stability during active head movement, a headband with an InterSense Inertia Cube 2, 3-axis, integrating gyro was placed on the participant’s head. The sensor detected simultaneous velocity and direction of head movement. Subjects performed practice trials to become familiar with the test using the inVision training protocol that provided both the participant and the operator feedback on the subject’s performance about maintaining or exceeding the requested head velocity. Training with visual feedback was continued until performance reached a stable head velocity. Stable performance was determined when the participant demonstrated that they were able to move their head in the desired direction and velocity while correctly identifying the orientation of the optotype. If the subject did not maintain the required velocity for at least 40 msec, the optotype did not appear. There was a random delay in the presentation of the optotype and all optotype presentations occurred for ≤75 msec.
All subjects were randomly assigned the initial head movement direction (up/down versus right/left). The optotype size for testing was fixed at 0.2 logMAR about the subject’s determined static visual acuity based on the 1.5 m distance of the eye to the target on the flat screen of the computer. Testing began once the participant consistently generated head movement at the desired head velocity, which was greater than 2 Hz with less than 20 degrees excursion in all directions from midline.18 Initial head velocity was 40°/s and was progressed in 10°/s increments with an upper limit velocity of 200°/s. Once the required velocity was achieved, the computer randomly generated the optotype and the subject was asked afterwards to identify the direction of the optotype. If the participant maintained the required head velocity for less than 40msec, the trial was interrupted (the optotype would disappear) and the participant would repeat the trial. Trials were scored as an error if the participant incorrectly identified the orientation of the optotype and were repeated until three total errors were made for each direction of movement. The average head velocity was recorded as the mean of the head velocity over the velocity window in which the optotype appeared.
Data analysis
Multivariate analysis of variance (MANOVA) was used to compare the GST maximum velocity in four directions (pitch up/down and yaw right/left) between age groups in healthy subjects (20–24 and 60–80 year olds) and between the healthy and patient group. Differences in mean GST velocity in any direction between healthy old and young subject groups, and between healthy and patient groups were judged significant at p < 0.05. Analysis of the mean differences in GST velocity between the healthy and patient group was conducted with and without patients with a diagnosis of BPPV, due to previous reports having only recruited persons with unilateral hypofunction18,15 and uncertainty as to whether people with BPPV would unduly influence the findings. Significant group differences were described in terms of effect size (ES).22
The screening/diagnostic properties of GST maximum velocity in each direction for identification of subjects with vestibular disease were described by plotting a receiver operating characteristic (ROC, sensitivity vs. 1- specificity) curve. The areas under the resulting ROC curves (AUC) were evaluated for significance (at p < 0.05) against a curve area of 0.50, the level of no discrimination of patient vs. control subjects. Any GST velocity direction with an AUC found significantly greater than 0.50 was evaluated for the velocity values that maximized sensitivity, specificity and the likelihood ratio (sensitivity/1-specificity) for identification of subjects with vestibular disease.
The likelihood ratio (LR) combines the sensitivity and specificity into one value that allows the application of Bayes’ theorem to diagnostic identification of persons with vestibular disease at a given GST velocity.23 According to Bayes’ theorem, post-test diagnostic probability of vestibular disease will be increased at a given GST velocity over the pre-test level by a factor related to the magnitude of the LR. The LR thus provides an estimate of the gain in diagnostic certainty of vestibular disease that GST velocity provides over the pre-test condition.
Results
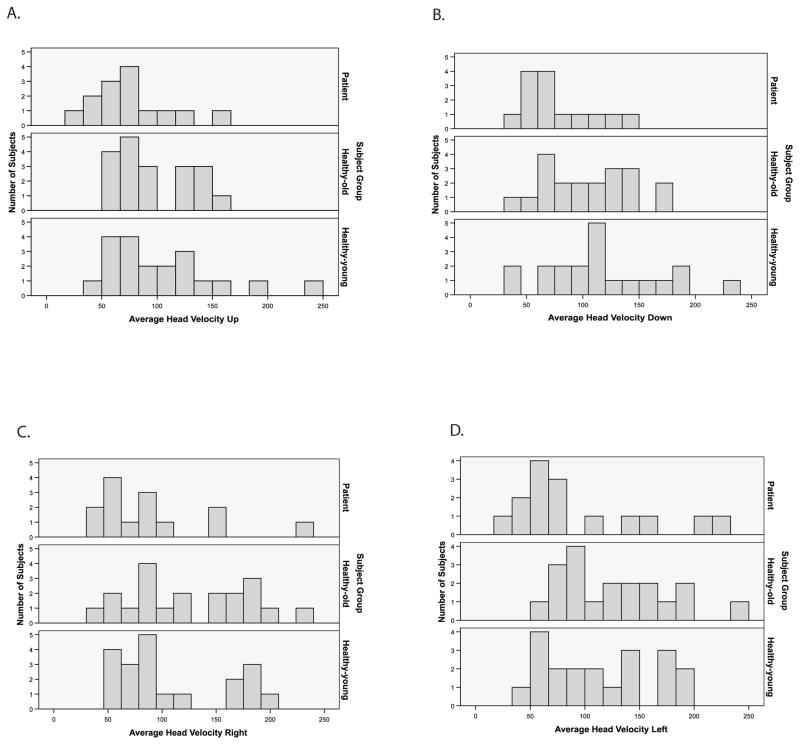
Two patients (both women with unilateral hypofunction, ages 74 and 75) were unable to attain the minimum head velocity and could not perform any testing. Once testing commenced, three of the subjects (one older control subject and 2 patients) were unable to complete the testing in all four directions. Overall, 4 of 17 patients (24%) were unable to complete all of the test conditions in the pitch and yaw planes. All patient data is displayed in Figure 2A–D.
Figure 2.
A–D. Histograms of GST average velocity by subject group in the up/down and right/left directions.
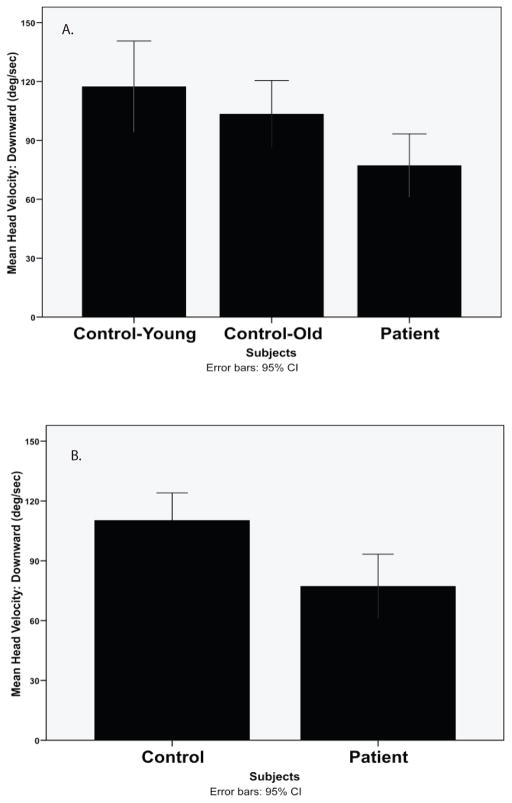
Gaze stability test results are displayed in the Table. Among healthy subjects there was no significant difference in GST velocity between old and young subject groups in any tested direction. Subjects with vestibular disease demonstrated a significantly slower GST velocity in the downward direction than healthy subjects when the data were pooled (MANOVA, F1,54 = 6.64, p < 0.02) (Figure 3). The effect size for the difference in GST velocity in the downward direction between the healthy and patient groups was 0.73 (Figure 3). No significant differences were noted in the other head movements tested: Left ES = 0.40 (p = 0.18), right ES = 0.53 (p = 0.08), and upward ES = 0.57 (p <0.06).
Table.
Gaze stabilization test (GST) average head velocity (mean ± standard deviation, 95% confidence interval (CI, degrees/sec) for healthy control subjects and patients in yaw and pitch plane directions.
| GST velocity (degrees/second) by head direction | ||||
|---|---|---|---|---|
| Subject group | Left (95% CI) | Right (95% CI) | Down (95% CI) | Up (95% CI) |
| Healthy-young (n = 20) | 114 ± 51 (90–137) | 107 ± 52 (83–131) | 117 ± 50 (94–141) | 104 ± 51 (81–128) |
| Healthy-old (n = 20) | 126 ± 51* (102–151) | 127 ± 56 (100–153) | 105 ± 38 (87–122) | 97 ± 35* (80–114) |
| Patient (n = 15) | 93 ± 62 (59–127) | 90 ± 54† (59–121) | 76 ± 30† (58–93) | 76 ± 38† (54–98) |
One control subject did not complete
One patient did not complete
Figure 3.
A and B. Mean and 95% confidence intervals for gaze stability testing velocities (deg/sec) in the downard direction for A. control-young subjects (age 20–40), control-old (age 60–80), patients and B. All control subjects and patients (difference significant p < 0.05).
When patients with BBPV were eliminated from the analyses, significant differences in GST velocity were found between the healthy and patient groups in the downward direction (MANOVA F1,48 = 4.53, p = 0.04, ES = 0.77). No significant differences between healthy and non-BPPV patients were noted in the other head movements tested: Left ES = 0.55 (p > 0.15), right ES = 0.71 (p = 0.06), upward ES = 0.65 (p <0.08).
The mean scores for all head velocities were lower for patients versus the healthy group, but the high standard deviations in all categories may have limited the effect size difference. Lack of power may have affected the results. Group differences between patients and healthy adults would have reached significance with an additional six patients at 80% power.
Analysis of the ROC curve (Figure 4) for identification of subjects with vestibular disease indicated that average downward GST velocity demonstrated the largest AUC [AUC = 0.73, 95% confidence interval (CI) 0.59 – 0.88, p < 0.01]. Analysis of the point coordinates of the downward velocity ROC curve illustrated that at a cut-point of velocity less than 61°/s, downward GST velocity was 44% sensitive and 90% specific for identification of subjects with vestibular disease. The likelihood ratio for identification of a subject with vestibular disease was maximized at average downward velocity of < 61 deg/sec with LR = 4.4; 95% CI=1.5–13.3. Screening properties of the GST for vestibular disorders would be optimal at a downward GST velocity of 108°/s (sensitivity = 80%). Screening at this value, however, would be associated with a relatively high false positive rate (49%).
Figure 4.
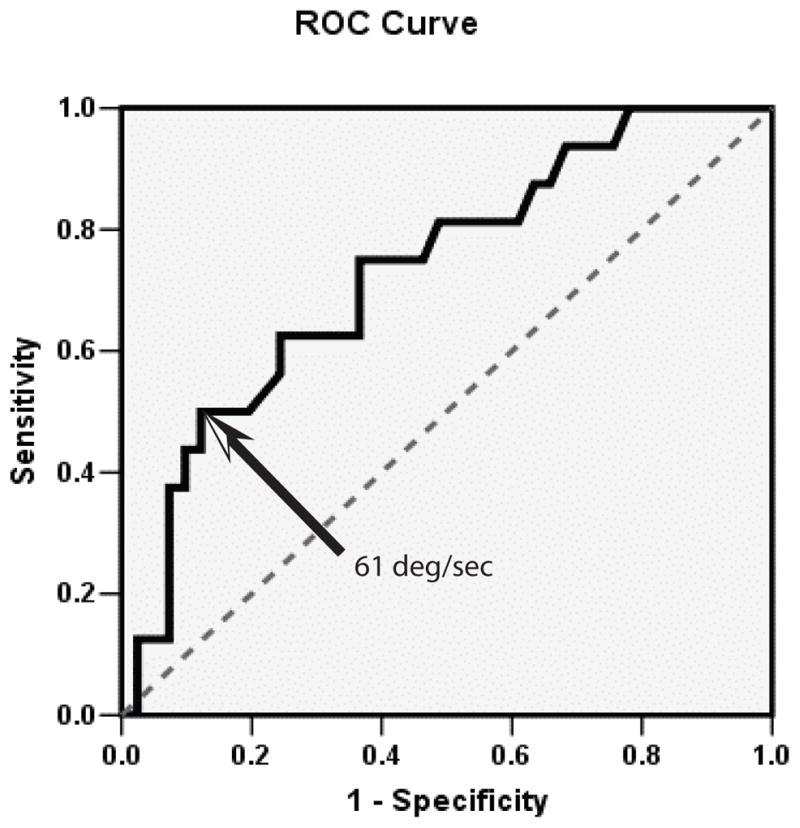
The Receiver operator curve identified maximum likelihood ratio (4.5, 95% CI=1.5–13.3) for differentiating people with and without vestibular disorders at a downward gaze velocity of <61°/s
Discussion
There was a difference in GST average velocity in the downward direction between patients with vestibular disorders and control subjects. It was surprising that the optimal discrimination between patients and control subjects occurred in the pitch plane, as pitch plane results have not previously been reported to our knowledge. It is possible that some of these patients had involvement of the otolith organ, as vestibular evoked myogenic potentials were not recorded in these patients, which may have made patients more likely to have difficulty in the pitch plane. Goebel et al18 reported that they performed the entire GST protocol, yet did not report on pitch plane movements. The GST mean maximum velocities in the Goebel et al paper in patients with unilateral hypofunction to the affected side were 84°/s and to the unaffected side were 112°/s.18 Coffey et al20 reported on GST results in the yaw plane. Scores for right/left yaw head movement for all three of Coffey et al’s groups were similar to Goebel et al’s18 results.
Yaw plane velocity scores in our study were more similar to Badaracco et al’s work,15 as their distance from the screen to the subject was 1.5 meters also. Badaracci et al’s patients with chronic dizziness had GST velocities to the right and left of 68°/s and 64°/s respectively whereas our scores were 80°/s and 84°/s, with an outlier removed.1 Badaracco et al’s sample included patients with chronic dizziness with a mix of diagnoses including peripheral hypofunction (unilateral and bilateral) plus central vestibular abnormalities.1 The present study consisted of patients with various peripheral vestibular diagnoses, who may have been less disabled. Screen to eye distance affects GST velocity, with longer screen to eye distances revealing faster GST velocity scores.
Four of the 17 patients could not complete any or parts of the test protocol. The test provoked occasional nausea, headaches, and one patient could not understand the directions, thus lowering the n of the patient group. Modifications to the test procedures have been designed so that the subject does not have to undergo as many trials, making it easier to complete the test protocol. Nausea, headaches, and fatigue may have affected the results of the patients in the current study.
The use of eyewear could have affected maximum head velocity results, with 14 of the 57 subjects wearing either bifocals or trifocals. Goebel et al18 reported testing their sample of persons with unilateral vestibular hypofunction with “best corrected vision”, but did not report an effect on GST due to visual correction. Others have not reported whether their subjects wore corrective eyewear,15 thus the effect of visual correction is unknown at this time.
The mean GST head velocities of this study are very different from those reported by Goebel et al.18 Goebel et al18 tested their subjects at 3.05m at a logMAR of 0.3. The present study tested subjects at 1.5m at logMAR 0.2. With the lower logMAR used in this study, the optotype becomes smaller, the velocity decreases, and the variability increases. Our mean range of GST yaw velocities for all groups was 90–127°/s, whereas that of Goebel et al18 was 84–149°/s.
Subjects who moved their head in the pitch plane at < 61°/s were more likely to be a patient. However, one can not rule out that they are a patient if their velocities were greater than 61°/s. Goebel et al18 have reported that the GST can distinguish between patients with unilateral hypofunction and healthy control subjects. In this study, a diverse group of patients with vestibular disorders were tested, which may be why only one direction (pitch plane) was able to differentiate between patients and young control subjects. The small patient data sample also contributed to the large variances seen in the data.
Dynamic visual acuity has been shown to improve in persons with unilateral and bilateral peripheral hypofunction after undergoing an exercise program to stimulate use of the VOR.3, 16 The concept of testing the visual system with active head movement while the target size is fixed is functional and may be of use as a future rehabilitation outcome. Subjects also had less ability to predict with a saccadic eye movement as attention to the computer screen where the target was to be displayed was critical for the subject to be able to view the target at the required head velocity.18 Goebel et al. suggests that the GST has potential as a future measure to predict functional disability in persons with vestibular disorders during activities such as driving a car, running or engaging in athletic activities.18
Nashner (personal communication, 2007)24 recently tested 45 professional football athletes who were “position” players (those who catch the ball). Nashner’s mean average head velocity (tested at 4 m, .02 LogMAR) was 246°/s ± 43 with a range of 154–357°/s.24 Their average head velocity difference to the right and left was 30°/s.24 In the past, it was assumed that the VOR gain at higher frequencies is actually quite stable up to a few hundred degrees per second.25. With the mean velocity calculated within a window of 100 m/s, it is highly unlikely that head deceleration or acceleration could have affected Nashner’s findings or those of this study. Nashner’s data suggest that there is the possibility of training the VOR to be more effective at faster head velocities. The athlete’s ability to pursue targets and their static visual acuities were similar to untrained adults, yet the athletes average head velocities were far superior to the non-athlete group. The athletes may have “trained” their VOR to work more effectively or conversely may have had a highly developed VOR, which enabled them to be highly successful at sporting activities. Nashner’s velocities were much higher than the highest mean velocity of any group in the present study (127°/s versus 246°/s).24 The mean head velocities overall were approximately 34% lower than the average velocity of the professional athletes in the patients and about 45% lower in the young control subjects.
The inability to visualize a target unless the head is still or moving slowly is common in persons with vestibular disorders.9 Persons with vestibular disorders often limit head movement in order to minimize oscillopsia.26 Most of the patients in this study moved more slowly than the control subjects, although there were 2 patient outliers (one with a diagnosis of BPPV and the other with a diagnosis of peripheral vestibular hypofunction) who moved very quickly, which increased the variance in our sample.
Various authors have noted a relationship between dynamic visual acuity and age,4, 26, 27 yet there was not a significant difference noted based on age in this study. There was an outlier in the older adult group who was able to move their head very quickly, which may have resulted in raising the mean of the older group.
Herdman et al10 reported that dynamic visual acuity testing that was unpredictably applied was affected by increasing age in healthy subjects and in persons with unilateral hypofunction. The head movements in this study were active and self-generated also. In Herdman et al’s work, the DVA test scores that were performed actively worsened with age, but were not statistically significant.14 Our subject ages were not distributed across the life span, as we had a gap in ages between 40 and 60, making inferences about age within the patient group impossible.
Conclusion
The GST appears to have promise for differentiating patients and healthy control subjects. Quantifying function of the VOR may help the clinician to determine if there are VOR deficits without having to perform expensive vestibular laboratory testing. Use of the GST as a possible rehabilitation outcome to quantify VOR change over the course of intervention is planned in future work.
Acknowledgments
This project was supported in part by funding from the National Institutes of Health via K23 DC005384 and P30 AG024827 (Pittsburgh Claude D. Pepper Center for Older Americans Independence Center).
Contributor Information
Miranda R. Pritcher, University of Pittsburgh Department of Audiology.
Susan L. Whitney, University of Pittsburgh Departments of Physical Therapy and Otolarynogology.
Gregory F. Marchetti, Duquesne University, Department of Physical Therapy and the University of Pittsburgh, Department of Otolaryngology.
Joseph M. Furman, University of Pittsburgh Department of Otolaryngology and Physical Therapy.
References
- 1.Grossman GE, Leigh RJ. Instability of gaze during locomotion in patients with deficient vestibular function. AnnNeurol. 1990;27(5):528–32. doi: 10.1002/ana.410270512. [DOI] [PubMed] [Google Scholar]
- 2.Bhansali SA, Stockwell CW, Bojrab DI. Oscillopsia in patients with loss of vestibular function. Otolaryngol Head Neck Surg. 1993;109(1):120–5. doi: 10.1177/019459989310900122. [DOI] [PubMed] [Google Scholar]
- 3.Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129(8):819–24. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- 4.Schubert MC, Herdman SJ, Tusa RJ. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol. 2002;23(3):372–7. doi: 10.1097/00129492-200205000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Leigh RJ, Zee DS. The Neurology of Eye Movements. 3. New York: Oxford University Press; 1999. [Google Scholar]
- 6.Demer JL, Crane BT, Tian JR, Wiest G. New tests of vestibular function. Ann N Y Acad Sci. 2001;942:428–45. doi: 10.1111/j.1749-6632.2001.tb03764.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee MH, Durnford SJ, Crowley JS, Rupert AH. Visual vestibular interaction in the dynamic visual acuity test during voluntary head rotation. Aviat Space Environ Med. 1997;68(2):111–7. [PubMed] [Google Scholar]
- 8.Tian JR, Shubayev I, Demer JL. Dynamic visual acuity during transient and sinusoidal yaw rotation in normal and unilaterally vestibulopathic humans. Exp Brain Res. 2001;137(1):12–25. doi: 10.1007/s002210000640. [DOI] [PubMed] [Google Scholar]
- 9.Demer JL, Honrubia V, Baloh RW. Dynamic visual acuity: a test for oscillopsia and vestibulo-ocular reflex function. Am J Otol. 1994;15(3):340–7. [PubMed] [Google Scholar]
- 10.Herdman SJ, Schubert MC, Tusa RJ. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg. 2001;127(10):1205–10. doi: 10.1001/archotol.127.10.1205. [DOI] [PubMed] [Google Scholar]
- 11.Tian JR, Shubayev I, Demer JL. Dynamic visual acuity during passive and self-generated transient head rotation in normal and unilaterally vestibulopathic humans. Exp Brain Res. 2002;142(4):486–95. doi: 10.1007/s00221-001-0959-7. [DOI] [PubMed] [Google Scholar]
- 12.Tian JR, Shubayev I, Baloh RW, Demer JL. Impairments in the initial horizontal vestibulo-ocular reflex of older humans. Exp Brain Res. 2001;137(3–4):309–22. doi: 10.1007/s002210000671. [DOI] [PubMed] [Google Scholar]
- 13.Huebner WP, Leigh RJ, Seidman SH, Billian C. An investigation of horizontal combined eye-head tracking in patients with abnormal vestibular and smooth pursuit eye movements. J Neurol Sci. 1993;116(2):152–64. doi: 10.1016/0022-510x(93)90320-x. [DOI] [PubMed] [Google Scholar]
- 14.Herdman SJ, Tusa RJ, Blatt P, Suzuki A, Venuto PJ, Roberts D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol. 1998;19(6):790–6. [PubMed] [Google Scholar]
- 15.Badaracco C, Labini FS, Meli A, De Angelis E, Tufarelli D. Vestibular Rehabilitation Outcomes in Chronic Vertiginous Patients Through Computerized Dynamic Visual Acuity and Gaze Stabilization Test. Otol Neurotol. 2007;26:809–13. doi: 10.1097/MAO.0b013e3180cab73f. [DOI] [PubMed] [Google Scholar]
- 16.Herdman SJ, Hall CD, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2007;133(4):383–9. doi: 10.1001/archotol.133.4.383. [DOI] [PubMed] [Google Scholar]
- 17.Schubert MC, Migliaccio AA, Della Santina CC. Dynamic visual acuity during passive head thrusts in canal planes. J Assoc Res Otolaryngol. 2006;7(4):329–38. doi: 10.1007/s10162-006-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goebel JA, Tungsiripat N, Sinks B, Carmody J. Gaze stabilization test: a new clinical test of unilateral vestibular dysfunction. Otol Neurotol. 2007;28(1):68–73. doi: 10.1097/01.mao.0000244351.42201.a7. [DOI] [PubMed] [Google Scholar]
- 19.Schubert MC, Migliaccio AA, Clendaniel RA, Allak A, Carey JP. Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil. 2008;89(3):500–7. doi: 10.1016/j.apmr.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffey B, Richards L, Olmschenk S, Peters J. Preliminary normative data for a new device to measure dynamic visual acuity. Optom Vis Sci. 2004;81:S127. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A Practical Method for Grading the Cognitive State of Patients for the Clinician. Journal of Psychiatry. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for behavioral sciences. 2. Hillsdale, NJ: Lawerence Earlbaum Associates; 1988. [Google Scholar]
- 23.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17(8):646–9. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nashner LM. Gaze stabilization test scores of professional athletes. Clackamus, OR: 2007. [Google Scholar]
- 25.Paige GD. Nonlinearity and asymmetry in the human vestibulo-ocular reflex. Acta Otolaryngol. 1989;108(1–2):1–8. doi: 10.3109/00016488909107385. [DOI] [PubMed] [Google Scholar]
- 26.Herdman SJ. Role of vestibular adaptation in vestibular rehabilitation. OtolaryngolHead Neck Surg. 1998;119(1):49–54. doi: 10.1016/S0194-5998(98)70195-0. [DOI] [PubMed] [Google Scholar]
- 27.Long GM, Crambert RF. The nature and basis of age-related changes in dynamic visual acuity. Psychol Aging. 1990;5(1):138–43. doi: 10.1037//0882-7974.5.1.138. [DOI] [PubMed] [Google Scholar]