Abstract
De novo CD5+ diffuse large B-cell lymphomas (DLBCL) are a distinct subgroup of DLBCL with poor prognosis. However the role of rituximab-containing therapy and salvage stem cell transplantation in this patients’ population remain to be defined. We retrospectively reviewed clinical features and outcomes of 102 patients with de novo CD5+ DLBCL treated with rituximab-containing therapy at 9 different institutions. By Hans’ criteria, 64 patients had activated B-cell (ABC) subtype, 24 germinal center B-cell (GCB) subtype, and 14 were not evaluated. No patients had a myc translocation. Eighty-three patients were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP), 7 with rituximab, etoposide, cyclophosphamide, doxorubicin, vincristine, prednisone (R-EPOCH) and 6 with R-CHOP with methotrexate, 3 g/m2. The overall response rate to frontline therapy was 85%. The 3-year progression free survival (PFS) and overall survival (OS) for all patients were 40% and 65%, respectively. The 3-year PFS for ABC- and GCB-subtypes was 34% and 45%, respectively. The 3-year OS for ABC- and GCB-subtypes was 62% and 67%, respectively. The median time to second treatment failure was 3 months and 1 month for ABC- and GCB-subtypes, respectively. Twenty of 28 (71%) transplanted patients with autologous, allogeneic, or both, relapsed. This study confirms the poor prognosis of de novo CD5+ DLBCL in a large multi-center cohort despite initial rituximab-containing chemotherapy and suggests that stem cell transplantation fails to salvage the majority of these patients. Approaches to prevent recurrence and/or novel therapies for relapsed disease are needed for this subgroup of DLBCL patients.
Keywords: CD5, DLBCL, rituximab, transplant, chemotherapy
Introduction
Approximately 5–10% of de novo diffuse large B-cell lymphomas (DLBCL) are CD5+ [1–3]. Compared with CD5− DLBCL, the majority of patients with de novo CD5+ DLBCL belong to the activated B cell (ABC) subtype, have a higher international prognostic index (IPI), more frequently have extranodal disease including central nervous system (CNS) involvement, and have significantly poorer outcome compared to CD5− DLBCL [2–12]. The 2 most recent studies of de novo CD5+ DLBCL included 337 and 48 patients, respectively [7,8]. Miyazaki K et al. reported on 184 patients receiving rituximab (R)-containing chemotherapy, and 153 receiving chemotherapy without R [7]. Complete response (CR) was achieved in 80% of patients treated with R-chemotherapy and in 66% in the chemotherapy group. The 2-year overall survival (OS) rate was 70% in the R-chemotherapy and 54% in the chemotherapy group. In the series from Xu-Monette ZY et al., 48 patients with de novo CD5+ DLBCL were treated with standard rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) with a 67% CR rate among 30 patients evaluated for response [8]. Median progression-free survival (PFS) was 21.3 months and median OS was 25.3 months. Herein, we report a retrospective multicenter study of 102 patients with de novo CD5+ DLBCL treated with rituximab-containing regimens and whose response was evaluated by positron emission tomography (PET)/computed tomography (CT). Patient-specific characteristics, selected pathology parameters (cell of origin by Hans’ immunohistochemical criteria [13] and the presence of myc translocations), type of treatment, and response to treatment were studied to determine association with PFS and OS.
Methods
Patients with previously untreated de novo CD5+ DLBCL defined as DLBCL expressing CD5 diagnosed between 2000 and to 2014 at 9 academic medical centers in the United States were included. Patients with primary CNS lymphoma and human immunodeficiency virus-related DLBCL were excluded. Institutional Review Board approval for this study was obtained at all participating sites. Tumors were defined as CD5+ if ≥ 30% of the tumor cells positively stained for CD5 by immunohistochemistry (IHC). The cutoff was chosen based on previous publications [6,13]. CD5+ DLBCL cases were classified as ABC or germinal center B-cell (GCB) DLBCL using Hans’ criteria [13]. Fluorescence in situ hybridization (FISH) using split signal probes for myc and bcl-2 was performed following institutional standards at each participating site.
Response to treatment was determined by PET/CT [14]. Associations between cell of origin (ABC vs. GCB) and clinical characteristics were assessed using Fisher’s exact test or the Wilcoxon rank sum test for categorical and continuous variables, respectively. PFS and OS durations were calculated as the time from diagnosis to the time of progression and/or death, censoring patients without event at 60 months. Time to second treatment failure was calculated from the start of second treatment to relapse or death. Time to stem cell transplant (SCT) failure was calculated from time of the first SCT to relapse or death due to any cause. PFS and OS curves were summarized using the Kaplan-Meier method and compared using the log-rank test. Proportional hazards models were employed to further evaluate the association of clinical characteristics with PFS and OS. Univariable models for each clinical variable were fit. Multivariable models were constructed using forward selection where variables entered based on statistical significance (p<0.05). Variables tested included IPI score, gender, presence of B symptoms, bulky disease, R-CHOP as 1st treatment, and ABC cell origin.
Results
In 102 patients, the median age was 62 (range 21–91) and 56% patients were male (Table 1). Eastern Cooperative Oncology Group (ECOG) performance status (PS) was ≥2 in 24% of the patients, 37% had B symptoms, 73% had stage III or IV disease, 28% had bone marrow involvement, 3% had CNS involvement, 61% had other extranodal sites of disease (Supplemental Table 1), and 16% had bulky disease > 5cm. IPI was >2 in 47% of the patients. By Hans’ immunohistochemical staining [13], 63% patients were ABC-type, 23% were GCB DLBCL, and 14% were not assessed. The median Ki67 was 80% (range 20–100%); EBER was negative in all patients; and 0 of 58 evaluated patients had myc and/or bcl-2 translocations.
Table 1.
Demographic and Clinical Characteristics of 102 Patients with CD5+ DLBCL
| Characteristic | Overall (n=102) | ABC (n=64) | GC (n=24) | p-value |
|---|---|---|---|---|
| Age | 0.98 | |||
|
| ||||
| Median | 62.5 | 63.5 | 64.5 | |
| Range | 21–91 | 28–91 | 21–87 | |
|
| ||||
| Gender, no. (%) | 0.25 | |||
|
| ||||
| Male | 57 (56) | 38 (59) | 11 (46) | |
| Female | 45 (44) | 26 (41) | 13 (54) | |
|
| ||||
| Performance status, no. (%) | 0.41 | |||
|
| ||||
| ECOG 0–1 | 75 (76) | 45 (70) | 19 (79) | |
| ECOG ≥2 | 24 (24) | 19 (30) | 5 (21) | |
| Missing | 3 | 0 | 0 | |
|
| ||||
| Stage at diagnosis, no. (%) | 0.58 | |||
|
| ||||
| I/II | 28 (27) | 15 (23) | 7 (29) | |
| III/IV | 74 (73) | 49 (77) | 17 (71) | |
|
| ||||
| Sites of extranodal involvement, no. (%) | ||||
|
| ||||
| CNS | 3 (3) | 2 (3) | 1 (4) | 1.00 |
| Bone Marrow | 27 (28) | 19 (30) | 4 (17) | 0.18 |
| Other* | 63 (61) | 45 (70) | 18 (75) | |
|
| ||||
| Bulky disease (>5 cm), no. (%) | 0.74 | |||
|
| ||||
| Yes | 15 (16) | 9 (15) | 4 (18) | |
| No | 80 (84) | 52 (85) | 18 (82) | |
| Missing | 7 | 3 | 2 | |
|
| ||||
| Presence of B symptoms, no. (%) | 0.42 | |||
|
| ||||
| Yes | 37 (37) | 27 (43) | 8 (33) | |
| No | 63 (63) | 36 (57) | 16 (67) | |
| Missing | 2 | 1 | 0 | |
|
| ||||
| LDH, no. (%) | 0.66 | |||
|
| ||||
| High | 49 (57) | 33 (60) | 12 (55) | |
| Normal | 37 (43) | 22 (40) | 10 (45) | |
| Missing | 16 | 9 | 2 | |
|
| ||||
| KI-67 (%) | 0.03 | |||
|
| ||||
| Median | 80 | 80 | 70 | |
| Range | 20–100 | 40–100 | 20–100 | |
| Missing | 28 | 12 | 6 | |
|
| ||||
| IPI, no. (%) | 0.46 | |||
|
| ||||
| 0–1 | 25 (28) | 12 (21) | 8 (36) | |
| 2 | 21 (24) | 14 (25) | 4 (18) | |
| 3 | 17 (19) | 11 (19) | 5 (23) | |
| 4–5 | 25 (28) | 20 (35) | 5 (23) | |
| Missing | 14 | 7 | 2 | |
|
| ||||
| Cytogenetic, no. (%) | 0.70 | |||
|
| ||||
| Complex Karyotype | 13 (24) | 11 (29) | 2 (18) | |
| Normal Karyotype | 41 (76) | 27 (71) | 9 (82) | |
| Missing | 48 | 26 | 13 | |
|
| ||||
| First line treatment, no. (%) | 0.48 | |||
|
| ||||
| R-CHOP | 83 (81) | 48 (75) | 21 (88) | |
| DA-R-EPOCH | 7 (7) | 5 (8) | 2 (8) | |
| R-CHOP+MTX | 6 (6) | 5 (8) | 1 (4) | |
| Other** | 6 (6) | 6 (9) | 0 (0) | |
|
| ||||
| XRT (to residual disease) | 0.03 | |||
|
| ||||
| Yes | 28 (27) | 14 (22) | 11 (46) | |
| No | 74 (73) | 50 (78) | 13 (54) | |
|
| ||||
| Total number of treatments (after first-line) | 0.12 | |||
|
| ||||
| Median | 1 | 2 | 1 | |
| Range | 1–11 | 1–6 | 1–4 | |
| Missing | 0 | 0 | 0 | |
|
| ||||
| Response to first line treatment, no. (%) | 0.61 | |||
|
| ||||
| CR | 76 (75) | 43 (67) | 20 (83) | |
| PR | 11 (11) | 8 (13) | 2 (8) | |
| SD | 1 (1) | 1 (2) | 0 (0) | |
| PD | 14 (14) | 12 (19) | 2 (8) | |
| Relapsed after first line treatment | 59 (68) | 40 (78) | 11 (50) | |
|
| ||||
| Second line treatment, no. (%) | 0.24 | |||
|
| ||||
| Yes | 49 (48) | 33 (52) | 9 (38) | |
| Relapsed after second line treatment | 38 (76) | 27 (79) | 8 (89) | |
See supplemental table 1.
R-CVP 3 patients; Hyperfractionated Cyclophosphamide 1 patient; R-VACOP-B 1 patient; R-CHOP alternating with high dose methotrexate + Cytarabine. Abbreviations: DA-R-EPOCH: dose adjusted R-EPOCH; MTX: methotrexate at 3 g/m2; XRT: radiation therapy; R: rituximab; VACOP-B: etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin; CVP: cyclophosphamide, vincristine, prednisone.
As front-line therapy, 83 patients received R-CHOP, 7 patients received rituximab with dose-adjusted etoposide, cyclophosphamide, doxorubicin, vincristine, prednisone (DA-R-EPOCH), 6 received R-CHOP with 3 g/m2 of methotrexate, and 6 patients received miscellaneous treatment detailed in Table 1. Twenty-eight patients received radiation therapy in addition to frontline chemotherapy due to residual disease after front-line chemotherapy. None of the patients underwent autologous SCT in first remission.
The overall response rate (ORR) (complete response, CR, plus partial response, PR) to frontline therapy was 86% for all patients (95% confidence interval, CI, 78–92), 80% (95% CI, 70–90) for ABC cases and 91% (95% CI, 81–100) for GCB cases (p=0.61, Table 1). Notably, of the 7 patients that received front-line DA-R-EPOCH, 6 achieved a CR and are currently disease free with a median follow up of 30 months (range 13–52). Among 102 patients and censoring patients without event at 60 months, 72% have progressed, 34% have died, and 28% of patients remain in remission after a median follow up of 40 months. Among 59 patients with documented relapse, 8 patients had CNS involvement at relapse, including 6 patients with ABC DLBCL. Forty-nine patients received second line therapy, and 41 of these relapsed. The median number of treatments given following first-line therapy was 1 (range 1–11).
Twenty patients underwent autologous SCT (Supplemental Table 2). By Hans’ immunohistochemical staining [13], 82% of these patients were ABC-type, 18% were GCB DLBCL, 90% received 1 line of therapy prior to SCT, 10% 2 lines. Eighty-nine percent of the patients achieved a CR with salvage chemotherapy prior to SCT. Of these 20 patients, 12 relapsed following SCT and 7 died. Four patients went directly to allogeneic SCT: all of them were ABC-type, 3 of them had 3 lines of therapy prior to SCT, and one had 4. Two patients achieved a PR prior to SCT, one had stable disease (SD) and one had progressive disease (PD). They all relapsed after transplant and died of disease. Four patients received both autologous and allogeneic SCT, 2 of them were ABC-type, the other two were unknown. Number of prior therapies and disease status pre-SCT for these 4 patients are listed in Supplemental Table 2. They all relapsed after SCT and died. For the 28 transplanted patients, median time to SCT failure calculated from time of the first SCT to relapse or death due to any cause was 4.9 months (95% CI: 3.2–9.0).
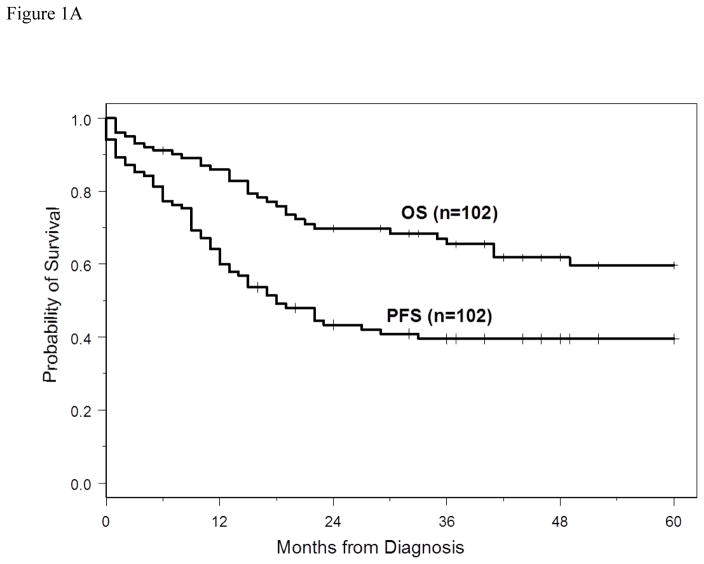
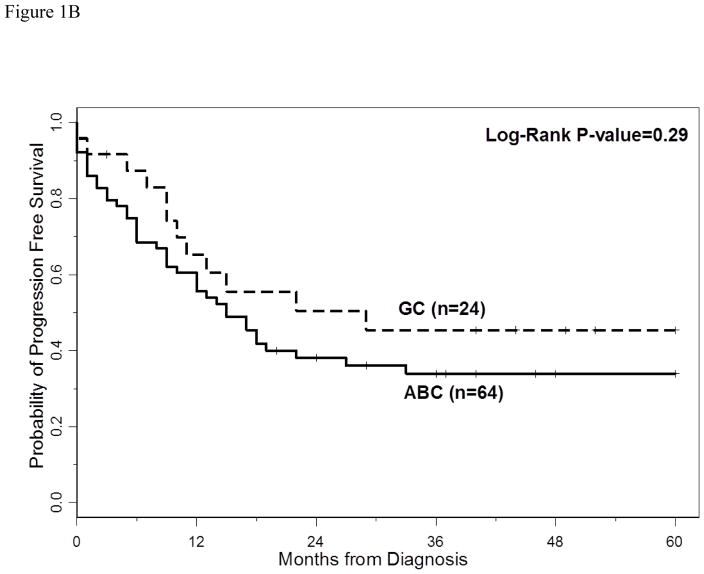
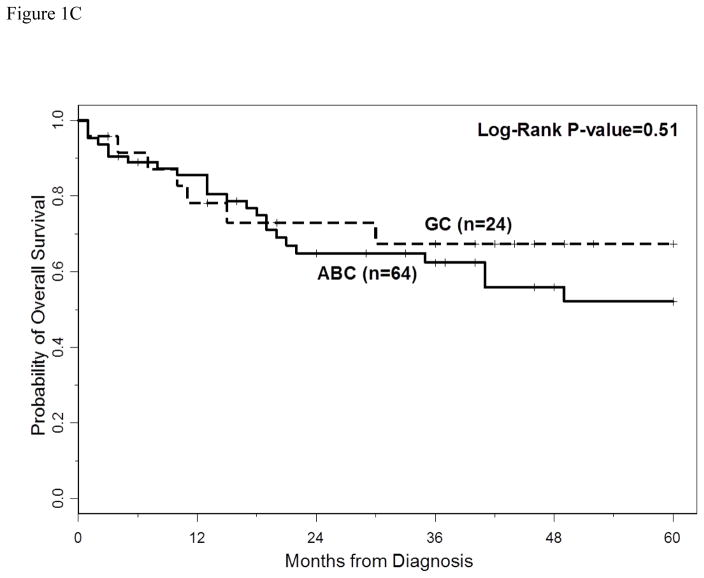
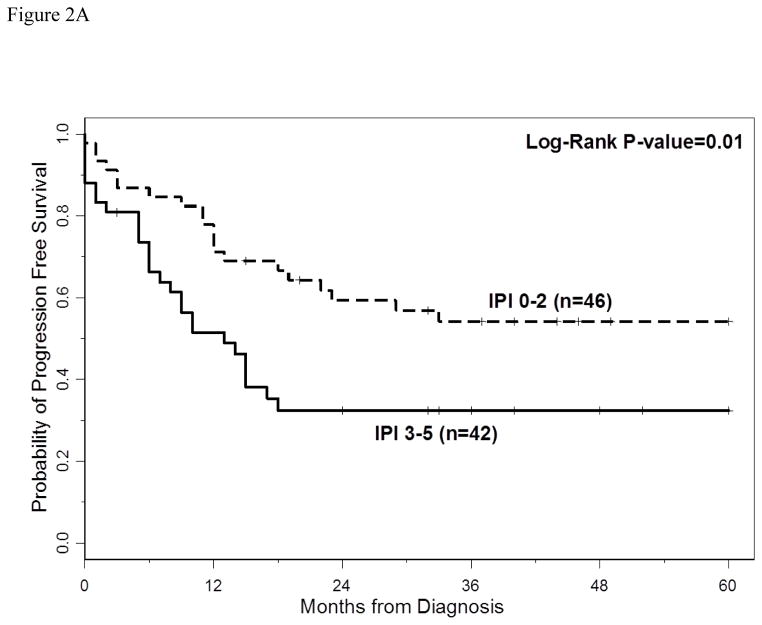
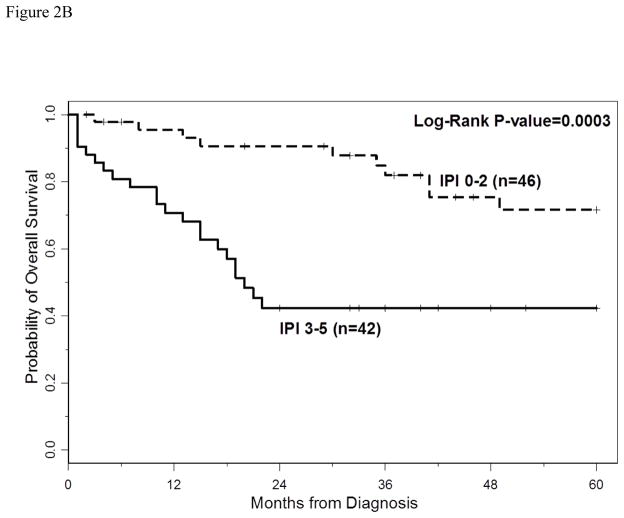
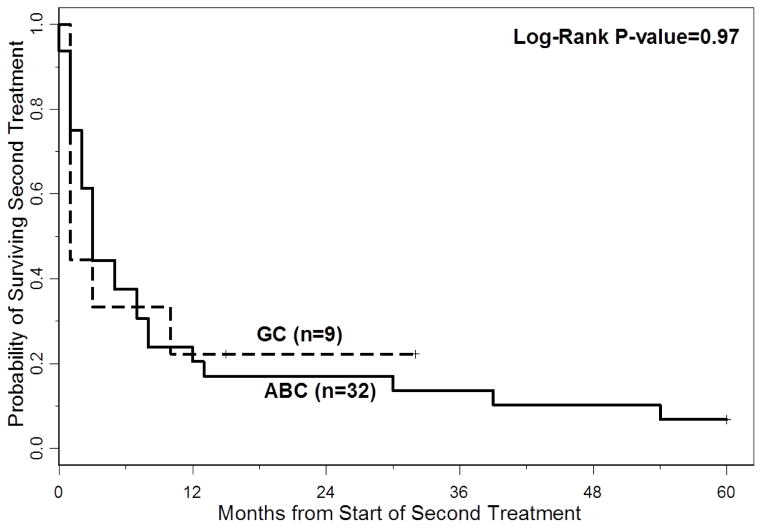
The 3-year PFS and OS for all patients were 40% (95% CI: 30%–49%) and 66% (95% CI: 54%–74%), respectively. The 5-year PFS and OS for all patients were 40% (95%CI: 30–49%) and 60% (95%CI: 47–70%), respectively. With a median follow-up of 40 months (range 2–60 months), the median PFS for all patients was 18 months (95% CI: 13–33 months) (Figure 1A), and median PFS for ABC and GCB CD5+ DLBCL was 15 months (95% CI: 9–27 months) and 29 months (95% CI: 10 months-not reached), respectively (p=0.29, Figure 1B). The median OS for all patients, ABC and GCB CD5+ DLBCL, was not reached at the time of analysis (Figure 1A and 1C). Patients with IPI > 2 had a significantly shorter PFS and OS compared to patients with IPI <2 (p=0.01 and p=0.0003, respectively, Figures 2A and 2B). The median time to second treatment failure was 3 months for ABC (95% CI: 2–7 months) and 1 month for GCB-CD5+ DLBCL (95% CI: 1- not reached) (p=0.97, Figure 3).
Figure 1.
Figure 1A: Progression free survival (PFS) and overall survival (OS) are illustrated for all patients. With a median follow-up of 40 months (range 2–60 months), the median PFS was 18 months (95% CI, 13–33 months), the median OS for all patients was not reached at the time of analysis.
Figure 1B: Progression free survival (PFS) is illustrated for patients with CD5+ DLBCL ABC-type versus GCB-type. The median PFS for ABC and GCB CD5+ DLBCL was 15 months (95% CI, 9–27 months) and 29 months (95% CI, 10 months-not reached), respectively. The 3 year PFS rate for ABC-type and GCB-type were 34% (95% CI 22–46) and 45% (95% CI 24–65), respectively.
Figure 1C: overall survival (OS) is illustrated for patients with CD5+ DLBCL ABC-type versus GCB-type. The median OS for ABC and GCB CD5+ DLBCL was not reached at the time of analysis. The 3 year OS rate for ABC-type and GCB-type were 62% (95% CI 48–74) and 67% (95% CI 43–83), respectively.
Figure 2.
Figure 2A: Progression free survival (PFS) is illustrated for patients with CD5+ DLBCL and IPI ≤2 versus IPI >2. Patients with IPI > 2 had a significant shorter PFS compared to patients with IPI <2 (p=0.01).
Figure 2B: Overall survival (OS) is illustrated for patients with CD5+ DLBCL and IPI ≤2 versus IPI >2. Patients with IPI > 2 had a significant shorter OS compared to patients with IPI <2 (p=0.0003).
Figure 3.
Time to 2nd treatment failure is illustrated for patients with CD5+ DLBCL ABC-type versus GCB-type. The median time to second treatment failure was 3 months for ABC-type (95% CI 2–7 months) and 1 month for GCB-CD5+ DLBCL (95% CI 1-not reached) (p=0.97).
In univariable analysis, stage III/IV (p=0.003), bone marrow involvement (p=0.003), CNS involvement (p=0.03), IPI >2 (p=0.001) and ECOG PS ≥2 (p=0.002) were associated with shorter PFS (Supplemental Table 3). Age (p=0.006), stage III/IV (p=0.003), bone marrow involvement (p<0.0001), CNS involvement (p=0.003), IPI >2 (p<0.0001), ECOG PS ≥2 (p<0.0001) and elevated LDH (p=0.02), were also significantly associated with shorter OS (Supplemental Table 4). When IPI was accounted for in the multivariable model, no other variables provided additional significant prognostic information.
Discussion
To our knowledge, this represents the largest series of de novo CD5+ DLBCL patients diagnosed and treated in Western countries with initial rituximab-containing chemotherapy, PET/CT-assessed response, with available cell of origin and myc-bcl2 translocation data. Despite multimodal approach, the vast majority of these patients either had primary refractory disease or relapsed after front-line chemotherapy. Of note, 4 patients did not receive front-line anthracycline containing regimen. Although this represents a suboptimal approach for DLBCL, it must be noted that CD5+ lymphomas are often seen in elderly patients where sometimes it is not appropriate or safe to give R-CHOP. Although these 4 patients were included in the initial analysis, a separate analysis of PFS and OS excluding those patients showed no significant difference in the poor OS and PFS seen when compared to the entire group. Additionally, of the patients that received second line therapy, the majority relapsed with a very short median time to second treatment failure (≤ 3 months) regardless of the cell of origin. Notably, of the 28 transplanted patients, 20 had documented relapse, and 16 died.
The 3-year PFS and OS for all patients were 40% (95% CI: 30%–49%) and 66% (95% CI: 54%–74%), respectively. Although stage III/IV, bone marrow involvement, CNS involvement, IPI >2 and ECOG PS ≥2 were associated with shorter PFS and OS in univariable analysis, only IPI >2 was independently associated with shorter PFS and OS in multivariable analysis. Interestingly, no statistically significant differences were detected in patients’ characteristics, ORR, PFS or OS in patients with ABC vs GCB CD5+ DLBCL suggesting that CD5+ DLBCL patients have poor outcome regardless of the cell of origin.
Although our study highlights the poor outcome of CD5+ in de novo DLBCL, it is challenging to draw definitive conclusions given the retrospective nature of the analysis, the lack of CD5 negative controls and the large number of patients without myc/bcl2 data. However, historically, patients with newly diagnosed DLBCL treated with standard R-CHOP or CHOP-like chemotherapy have a 2 year PFS ranging from 57 to 69% and a 3 year OS of 70–78% [9,15,16]. If we restrict data to myc (by FISH) negative DLBCL patients treated with front-line R-CHOP [17], CD5+ DLBCL patients from our series appear to have inferior 5 year PFS (40% versus 66%) and OS (60% versus 72%).
When examined by cell of origin, patients in our series with CD5+ GCB-DLBCL had 3 year PFS 45% and patients with CD5+ ABC-DLBCL had 3-year PFS of 34%. These outcomes are inferior to those published by Lenz et al. [18], where patients with GCB and ABC DLBCL treated with R-CHOP had a 3 year PFS of 74% and 40% respectively. However, it must also be noted that, in our study, cell of origin was assessed by Hans’ immunohistochemical staining and not gene expression profiling. Given the limited accuracy of this approach [19, 20], no definitive conclusion can be drawn with regard to the relationship between cell of origin and outcomes. In addition no agreement exists on the cut off to be used to define CD5 positivity in DLBCL and further studies are required to homogenously define this percentage in DLBCL. Autologous SCT has been shown to be an effective therapy as compared with salvage chemotherapy for patients with chemo-sensitive DLBCL in first relapse [21, 22]. In our series, 20 patients underwent autologous SCT and 12 relapsed confirming the poor outcomes observed with SCT in patients who relapse or progress after front-line rituximab-containing therapy. Additionally, in our series, all 8 patients who received allogeneic SCT also relapsed, raising the question of the utility of this treatment modality in CD5+ DLBCL.
Similar to other series summarized in Supplemental Table 5, our trial demonstrates that CD5+ DLBCL have a poor outcome [3–7]. In a series by Miyazaki et al where 168 patients received R-CHOP, the 2-year OS was 70% similar to 3-year OS of 66% observed in this trial. Likewise, data from Xu-Monette et al., reported a median PFS of 21.3 months in CD5+ DLBCL patients treated with R-CHOP, similar to median PFS of 18 months in this series [8]. In addition to outcomes data on CD5+ DLBCL patients treated with rituximab-containing chemotherapy, in our retrospective study, treatment response was assessed by PET/CT and correlated with cell of origin. Furthermore, unlike the two trials mentioned above, our study provided data on time to second treatment failure and transplant outcome.
In conclusion, this large multi-center retrospective evaluation of CD5+ DLBCL demonstrates poor outcomes in this sub-group of DLBCL regardless of cell of origin and despite initial responses to rituximab-containing chemotherapy. Furthermore, the lack of myc translocations in this patient population suggests that this is a different poor prognostic subgroup of DLBCL and that CD5 positivity should be routinely evaluated at diagnosis and utilized for risk stratification in future DLBCL clinical trials. Prospective studies are needed to determine the appropriate IHC cut-offs to better define which cases are CD5+ and correlate degree of CD5 expression with outcomes. Lastly, while the optimal front-line regimen for CD5+ DLBCL remains unclear, this multi-center series demonstrates that very few patients with CD5+ DLBCL have prolonged disease-free survivals following front-line chemo-immunotherapy or SCT and novel therapeutics should be incorporated into front-line treatment regimens or as maintenance therapy for this DLBCL subgroup. Although evaluated in a very limited number of patients, given the rate and duration of response, DA-R-EPOCH could be considered for future trials.
Supplementary Material
Footnotes
Authorship
Conception and design: Lapo Alinari, Alejadro Gru, Kristie A. Blum; Research performance: Lapo Alinari, Carl Quinion, Alejandro Gru; Provision of clinical data under approved IRB: Lapo Alinari, Alejandro Gru, Arletta Lozanski, Gerard Lozanski, Jacqueline Poston, Girish Venkataraman, Eunhye Oak, Friederike Kreisel, Steven I. Park, Stephanie Matthews, Jeremy S. Abramson, Hana Iris Lim, Peter Martin, Jonathon B. Cohen, Andrew Evens, Zeina Al-Mansour, Arun Singavi, Timothy S. Fenske, Kristie A. Blum; Data analysis and interpretation: Lapo Alinari, Ying Huang, KAB; Manuscript writing: Lapo Alinari, Kristie A. Blum; Final approval of manuscript: All authors.
Disclosure
The authors have no competing interests to disclose
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 2.Jain P, Fayad LE, Rosenwald A, et al. Recent advances in de novo CD5+ diffuse large B-cell lymphoma. Am J Hematol. 2013;88(9):798–802. doi: 10.1002/ajh.23467. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi M, Seto M, Okamoto M, et al. De novo CD5+ diffuse large B-cell lymphoma: a clinicopathologic study of 109 patients. Blood. 2002;99(3):815–821. doi: 10.1182/blood.v99.3.815. [DOI] [PubMed] [Google Scholar]
- 4.Ennishi D, Takeuchi K, Yokoyama M, et al. CD5 expression is potentially predictive of poor outcome among biomarkers in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP therapy. Ann Oncol. 2008;19:1921–1926. doi: 10.1093/annonc/mdn392. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi M, Nakamura N, Suzuki R, et al. De novo CD5+ diffuse large B-cell lymphoma: results of a detailed clinicopathological review in 120 patients. Haematologica. 2008;93(8):1195–1202. doi: 10.3324/haematol.12810. [DOI] [PubMed] [Google Scholar]
- 6.Niitsu N, Okamoto M, Tamaru JI, et al. Clinocopathologic characteristics and treatment outcome of the addition of rituximab to chemotherapy for CD5-positive in comparison with CD5-negative diffuse large B-cell lymphoma. Ann Oncol. 2010;21:2069–2074. doi: 10.1093/annonc/mdq057. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki K, Yamaguchi M, Suzuki R, et al. CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011;22(7):1601–1607. doi: 10.1093/annonc/mdq627. [DOI] [PubMed] [Google Scholar]
- 8.Xu-Monette ZY, Tu M, Jabbar JK, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget. 2015;6(8):5615–5633. doi: 10.18632/oncotarget.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 10.Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110(1):29–36. doi: 10.1182/blood-2007-01-041871. [DOI] [PubMed] [Google Scholar]
- 11.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22–32. doi: 10.1182/blood-2014-05-577189. [DOI] [PubMed] [Google Scholar]
- 12.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct type of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 13.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphomas. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 15.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 16.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 17.Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114(17):3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 18.Lenz G, Wright GW, Tolga Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci. 2008;105(36):13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vose JM. Relapsed diffuse large B-cell lymphoma: clinical utility of cell of origin. J Clin Oncol. 2011;29(31):4065–6. doi: 10.1200/JCO.2011.37.5733. [DOI] [PubMed] [Google Scholar]
- 20.Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol. 2011;29(31):4079–87. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- 21.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 22.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.