Abstract
Background
Screening for celiac disease (CD) in children with diabetes is controversial because no studies have demonstrated metabolic complications in asymptomatic, seropositive subjects or beneficial effects of dietary intervention.
Objective
We hypothesized that seropositivity to celiac antigens is associated with decreased growth and bone mineralization in asymptomatic diabetic children.
Design/Methods
Asymptomatic diabetic children were screened for seropositivity to tissue transglutaminase. Villous atrophy was assessed by small bowel biopsy in a subset of seropositive subjects. We compared measures of growth and bone mineralization in 30 seropositive subjects, and 34 matched seronegative controls.
Results
Relative to seronegative controls, the seropositive subjects had reductions in insulin-like growth factor (IGF) binding protein 3 z scores (p < 0.05) and bone mineral density (BMD) z scores (p = 0.05). Weight, body mass index, IGF-I, and bone mineral apparent density (BMAD) z scores were marginally lower, but height z scores were comparable. Seropositive patients with severe villous atrophy had lower weight (−0.91 SDs), height (−1.1 SDs), BMD (−2.0 SDs), and BMAD (−2.0 SDs) z scores and significant increases in parathyroid hormone (all p < 0.05). Four patients with severe villous atrophy maintained strict gluten restriction for at least 12 months. Gluten restriction increased BMD and BMAD z scores.
Conclusions
High-titer seropositivity to celiac antigens is associated with reductions in weight and BMD in diabetic children, justifying screening of high-risk patients. Results suggest that biopsy is required to confirm the diagnosis and assess the severity of CD; those with severe villous atrophy are more likely to have growth failure and osteopenia. Gluten restriction may reverse these complications.
Keywords: bone mineralization, CD, diabetes, growth, screening
Celiac disease (CD) is a gastrointestinal disorder caused by sensitivity to the gliadin fraction of gluten proteins found in wheat, barley, and rye. In genetically prone individuals, exposure to gluten initiates an inflammatory response in the lining of the small bowel and development of circulating antibodies to tissue transglutaminase (TGA), to the endomysium, and/or to gliadin. Patients with classical or clinically overt CD usually complain of abdominal pain, bloating, and diarrhea. If untreated, the inflammation leads to chronic malabsorption of macronutrients as well as folate, fat-soluble vitamins, and iron. Complications may include growth failure, osteoporosis, hepatocellular dysfunction, anovulation, infertility and habitual miscarriage, neurologic dysfunction, and an increased risk of small bowel lymphoma (1-8). Currently, the only treatment is strict gluten restriction.
The prevalence of CD in children with type 1 diabetes (1.7–10%) (1, 9-11) exceeds greatly than that in the general population (0.5-0.8%) (12-14). Interestingly, most patients with diabetes have a ‘subclinical’ form of the illness; they are seropositive to celiac antigens and may have pathological changes of the small bowel villi but lack the abdominal symptoms seen in classical CD. Serologic screening of children with diabetes for CD is controversial because no studies have demonstrated metabolic complications in asymptomatic, seropositive subjects or beneficial effects of dietary intervention.
We hypothesized that seropositivity to celiac antigens is associated with decreased parameters of weight, height, and bone mineralization in asymptomatic children with type 1 diabetes. To that end, we compared baseline weight, height, and bone mineralization and serum insulin-like growth factor (IGF)-I, IGF-binding protein 3 (IGFBP-3), 25 hydroxy vitamin D (25 OHD), and parathyroid hormone (PTH) in seropositive children with diabetes with baseline values in seronegative diabetic controls. We analyzed parameters of weight gain and growth and bone mineralization in a subset of seropositive subjects who underwent small bowel biopsy to assess correlations with severity of villous pathology. Finally, we assessed the changes in growth and bone mineralization in four asymptomatic subjects who maintained for 1 yr or more a strict gluten-free diet.
Experimental subjects
Children and adolescents receiving care for diabetes at Duke University Medical Center and Children’s Hospital of Pittsburgh were screened for seropositivity to celiac antigens. The majority of seropositive and seronegative patients were randomly screened, although some were tested or rescreened because of (i) unexplained variability in glycemic control; (ii) recurrent or unexplained hypoglycemia; or (iii) decrease in weight or growth velocity that could not be explained entirely by glycemic instability or thyroid dysfunction. No patients complained of persistent abdominal pain, diarrhea, vomiting, or food intolerance prior to screening. Patients were screened with TGA antibodies and were classified as seropositive if TGA titers exceeded 0.05 units by radioimmunoassay (RIA) (15), performed in the laboratory of Dr George Eisenbarth at the Barbara Davis Center for Childhood Diabetes, or 30 units by enzyme-linked immunosorbent assay (ELISA) (Mayo Medical Laboratories, Rochester, MN, USA). The cut off RIA value of 0.05 corresponds to three times the highest value in 184 normal subjects (16). The 30-unit ELISA value is based on a study of 202 normal individuals, two of whom had values of 21 and 24 units, respectively (Mayo Medical Laboratories). Three patients were identified by high-titer positivity to endomysial antibodies.
Only the Duke Institutional Review Board approved small bowel biopsies as part of the protocol. Therefore, the subset of 11 seropositive patients studied at Duke University underwent small bowel biopsies; a diagnosis of CD was based on histological evidence of lymphocytic infiltration, blunted villous architecture, and/or crypt hyperplasia. Patients with type 1 diabetes and no detectable TGA were used to select seronegative controls. Seronegative controls were matched with seropositive patients according to age (±1 yr), gender, race, duration of diabetes (±1 yr), and mean hemoglobin A1c (HbA1c) (±1%) during the previous year. Patients with major acute or chronic illnesses other than diabetes or CD were excluded from the study; however, euthyroid patients taking supplemental thyroxine were eligible for enrollment. Informed consent was obtained from parents.
Methods
Thirty seropositive diabetic patients and 34 seronegative controls were analyzed after initial screening; 11 of the seropositive subjects underwent small bowel biopsy. The following data were obtained:
-
(i)
Baseline body weight, height, and body mass index (BMI);
-
(ii)
Baseline serum concentrations of IGF-I (by RIA), IGFBP-3 (by RIA), PTH (by immunochemiluminometric assay [ICMA]), and 25 OHD (by complete protein binding after column chromatography). All laboratory studies were performed by Esoterix (Calabasas Hills, CA, USA);
-
(iii)
Lumbar spine BMD and lumbar spine BMAD. BMD was assessed by dual-energy X-ray absorptiometry (Hologic at center #1 and Lunar at center #2). Daily quality control procedures with a QC spine phantom ensured proper calibration. BMAD was calculated on the Bone Mineral Density Applet website (http://www-stat-class.stanford.edu/pediatric-bones/).
Data analysis
Data are reported as absolute values or, when appropriate, as z scores based on population norms adjusted for age, gender, and race. The z scores for parameters of bone mineralization were estimated using population norms, adjusting for age and gender (BMD, BMAD, IGF-I, and IGFBP-3) and race (BMD and BMAD) when appropriate (17, 18). All data are presented as mean ± standard error. Significant differences among sample groups were tested by anova, followed by the Bonferroni test of comparisons. A p value ≤0.05 was considered statistically significant.
Results
Prevalence of celiac seropositivity in children with diabetes
Prior to and during the course of this study, a total of 612 pediatric patients (aged 2–20 yr) with type 1 diabetes were screened for seropositivity at Duke Children’s Hospital and the Children’s Hospital of Pittsburgh; 48 (7.8%) of these proved seropositive for TGA and/or endomysial antibodies. All seropositive patients except one were Caucasian (97%). Thirty of the seropositive subjects were eligible for and enrolled in the study. The main reason for exclusion was incomplete baseline data. They were matched with 34 seronegative controls according to age (±1 yr), gender, race, duration of diabetes (±1 yr), and mean HbA1c (±1%) during the previous year (Table 1).
Table 1.
Summary of patient characteristics
| TGA (+) (n = 30) | TGA (−) (n = 34) | p Value | |
|---|---|---|---|
| Age (yr) | 12.4 ± 0.78 | 12.5 ± 0.56 | 0.89 |
| Race (% Caucasian) | 97 | 100 | |
| Females (%) | 50 (n = 15) | 56 (n = 19) | 0.44 |
| Duration of type 1 diabetes (yr) |
7.1 ± 0.89 | 6.8 ± 0.74 | 0.81 |
| Average hemoglobin A1c (%) |
8.04 ± 0.19 | 8.05 ± 0.13 | 0.96 |
TGA, tissue transglutaminase.
Values are mean ± standard error.
Weight, height, growth factors, and bone mineralization in seropositive and seronegative subjects
Relative to seronegative type 1 diabetic controls, the seropositive subjects had significant reductions in IGFBP-3 (−1.2 ± 0.13 vs. −0.65 ± 0.17, p < 0.05) and BMD (−0.6 ± 0.23 vs. 0.05 ± 0.23, p = 0.05) z scores. Weight (0.39 ± 0.17 vs. 0.83 ± 0.16, p = 0.06), BMI (0.6 ± 0.15 vs. 0.94 ± 0.13, p = 0.08), IGF-I (−1.6 ± 0.22 vs. −1.0 ± 0.17, p = 0.07), and BMAD (−0.49 ± 0.26 vs. 0.06 ± 0.22, p = 0.11) z scores were marginally (but not significantly) lower in seropositive than in seronegative subjects, and height z scores were comparable (Table 2). Seronegative diabetic controls had lower serum IGF-I (−1.0 ± 0.17) and IGFBP-3 (−0.65 ± 0.17) z scores compared with published age-matched normal values despite higher than average body weight (0.83 ± 0.16) and BMI (0.94 ± 0.13) z scores (Table 2). Height, serum 25 OHD, PTH, BMD, and BMAD z scores were all within the normal range.
Table 2.
Summary of clinical and laboratory data in seropositive vs. seronegative children with type 1 diabetes
| TGA (+) | TGA (−) | P Value |
|
|---|---|---|---|
| Weight | 0.39 ± 0.17 | 0.83 ± 0.16 | 0.06 |
| Height | −0.15 ± 0.18 | 0.17 ± 0.16 | 0.17 |
| BMI | 0.6 ± 0.15 | 0.94 ± 0.13 | 0.08 |
| BMD | −0.6 ± 0.23 | 0.05 ± 0.23 | 0.05 |
| BMAD | −0.49 ± 0.26 | 0.06 ± 0.22 | 0.11 |
| IGF-I | −1.6 ± 0.22 | −1.0 ± 0.17 | 0.07 |
| IGFBP-3 | −1.2 ± 0.13 | −0.65 ± 0.17 | 0.03 |
| Parathyroid hormone (pg/mL) | 37.9 ± 5.1 | 33.3 ± 1.9 | 0.38 |
| 25 hydroxy vitamin D (ng/mL) | 24.96 ± 1.5 | 26.93 ± 1.3 | 0.32 |
BMAD, bone mineral apparent density; BMD, bone mineral density; BMI, body mass index; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein-3; TGA, tissue transglutaminase.
Weight, height, BMI, IGF-I, and IGFBP-3 reported as z scores normalized for age and gender. BMD and BMAD reported as z scores normalized for age, gender, and race. Values are mean ± standard error.
Correlations between bone mineralization and weight and height z scores, serum IGF-I, IGFBP-3, PTH, and 25 OHD
In the 64 seronegative and seropositive diabetic patients as a group and in seropositive patients alone, BMD z score correlated more strongly with weight z score (r = 0.57, p < 0.001) and BMI z score (r = 0.51, p < 0.001) than with all other parameters except BMAD z score (r = 0.85). BMD z score also correlated positively with height z score (r = 0.36, p < 0.004) but not with IGF-I z score, IGFBP-3 z score, PTH, or 25 OHD. BMAD z score, like BMD z score, correlated most strongly with weight z score (r = 0.36, p < 0.004) and BMI z score (r = 0.29, p < 0.02).
Relations between height and bone mineralization and severity of villous pathology
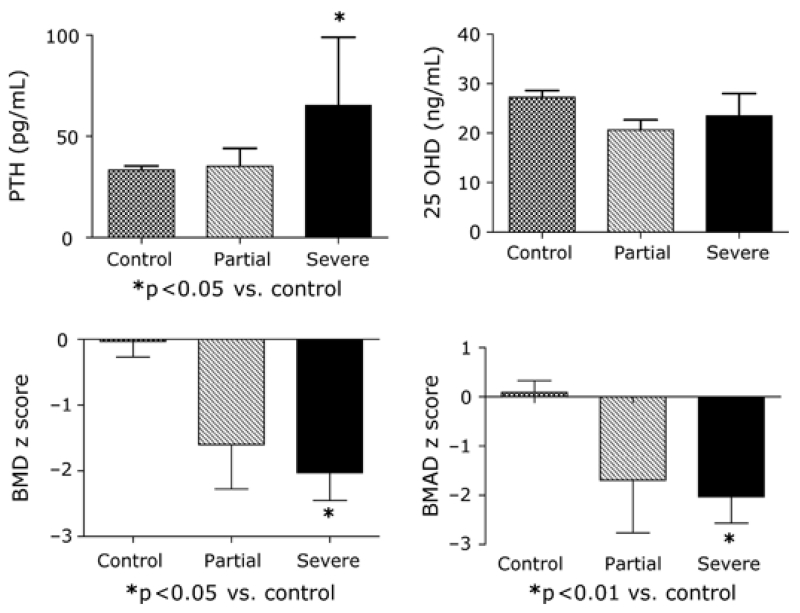
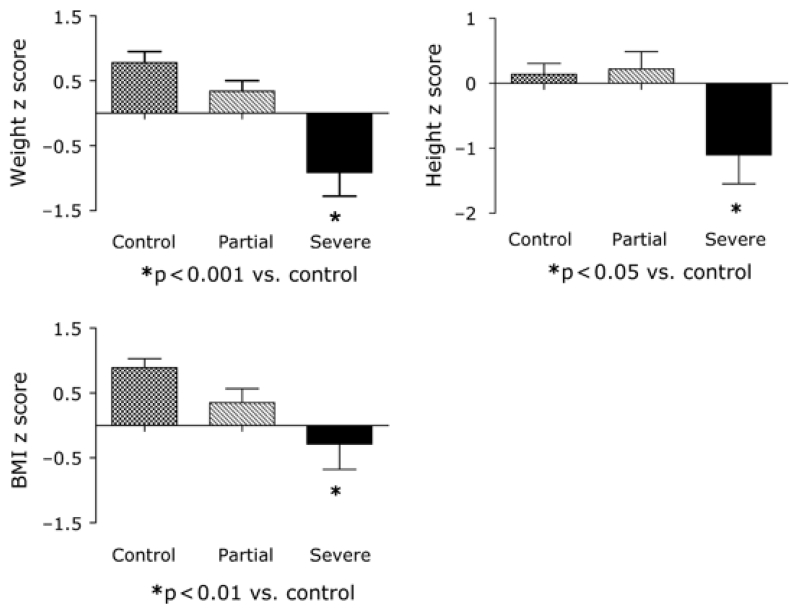
Eleven seropositive subjects underwent small bowel biopsy; of these, nine had findings diagnostic of CD. Six of those with histologic features of CD had TGA titers exceeding 150 units (ELISA); the other three had marked elevations (1:1280–1:6400) in endomysial antibody titres (Table 3). Five had severe villous atrophy (near total or total villous atrophy with crypt hyperplasia and increased intraepithelial lymphocytes), and four had partial villous atrophy (villous blunting with increased numbers of intraepithelial lymphocytes). Four of the five with severe villous atrophy and the four with partial villous atrophy had complete baseline studies. Relative to seronegative controls, the seropositive subjects with severe villous atrophy had significant reductions in weight (−0.91 ± 0.37 vs. 0.83 ± 0.16, p < 0.001), height (−1.12 ± 0.45 vs. 0.17 ± 0.16, p < 0.05), BMI (−0.29 ± 0.39 vs. 0.94 ± 0.13, p < 0.01), BMD (−2.04 ± 0.42 vs. 0.05 ± 0.23, p < 0.05), and BMAD (−2.03 ± 0.55 vs. 0.12 ± 0.22, p < 0.01) z scores and had increased levels of PTH (65.3 ± 33.6 vs. 33.3 ± 1.9 pg/mL, p < 0.05, Figs 1 and 2). In contrast, body weight, height, and PTH were normal in patients with partial villous atrophy, although BMD (−1.57 ± 0.68) and BMAD (−1.69 ± 1.06) z scores were marginally reduced. Serum IGF-I and IGFBP-3 z scores and 25 OHD levels did not differ significantly among the three groups.
Table 3.
Summary of antibody titers and HbA1c before and after gluten-free diet
| Subject | Antibody titer | Prediet HbA1c (%) |
Biopsy result |
Gluten-free diet |
Postdiet TGA titer |
Postdiet HbA1c (%) |
|---|---|---|---|---|---|---|
| 1 | Endomysial, 1:6400 |
7.8 | Severe | + | 9.7 | 7.6 |
| 2 | TGA < 250 | 8.3 | Severe | − | n/a | n/a |
| 3 | TGA < 250 | 9.7 | Partial | − | n/a | n/a |
| 4 | TGA < 250 | 7.8 | Severe | + | 9.8 | 7.5 |
| 5 | TGA = 159.2 | 8.1 | Partial | + | 49 | 10.8 |
| 6 | Endomysial, 1:1280 |
7.6 | Severe | + | 69 | 8.8 |
| 7 | Endomysial, 1:6400 |
8.3 | Severe | + | 75.5 | 8.2 |
| 8 | TGA < 250 | 12.3 | Severe | − | n/a | n/a |
| 9 | TGA < 250 | 6.6 | Partial | + | 18 | 9.4 |
| 10 | TGA = 107.5 | 10.9 | Negative | − | n/a | n/a |
| 11 | TGA = 80.5 | 8.3 | Negative | − | n/a | n/a |
HbA1c, hemoglobin A1c; TGA, tissue transglutaminase; n/a, not applicable.
Figure 1.
Bone mineral density (BMD) and bone mineral apparent density (BMAD) z scores, serum parathyroid hormone (PTH) and 25 hydroxy vitamin D (25 OHD) levels in seronegative (control, n = 34) and seropositive children whose biopsies showed partial (n = 4) or severe (n = 5) villous atrophy.
Figure 2.
Weight, height, and body mass index (BMI) z scores in seronegative (control, n = 34) and seropositive children whose biopsies showed partial (n = 4) or severe (n = 5) villous atrophy.
Effects of gluten restriction on growth and bone mineralization in subjects with subclinical CD
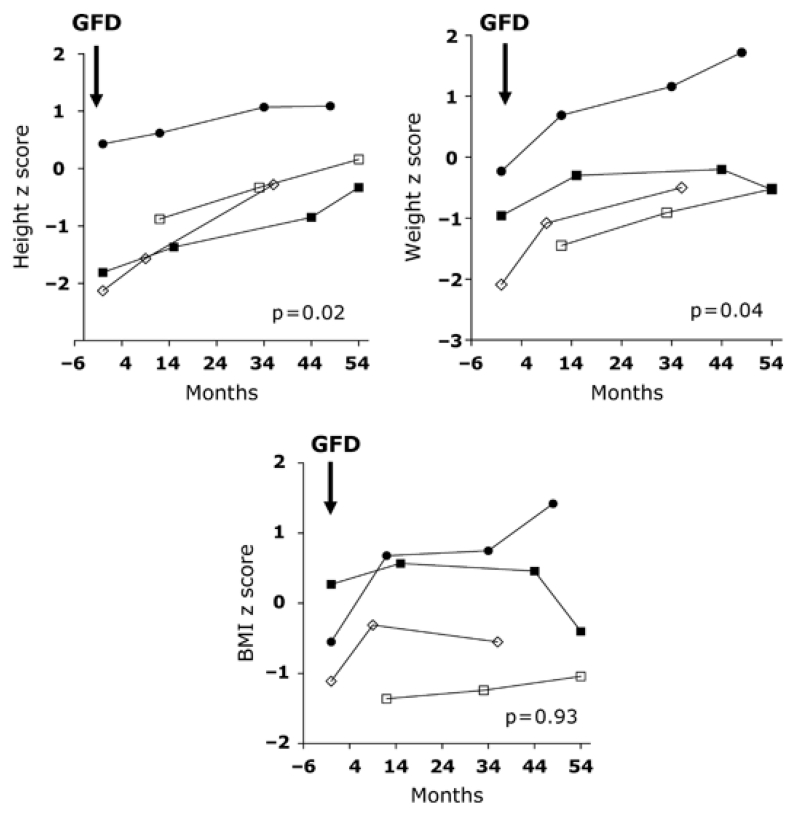
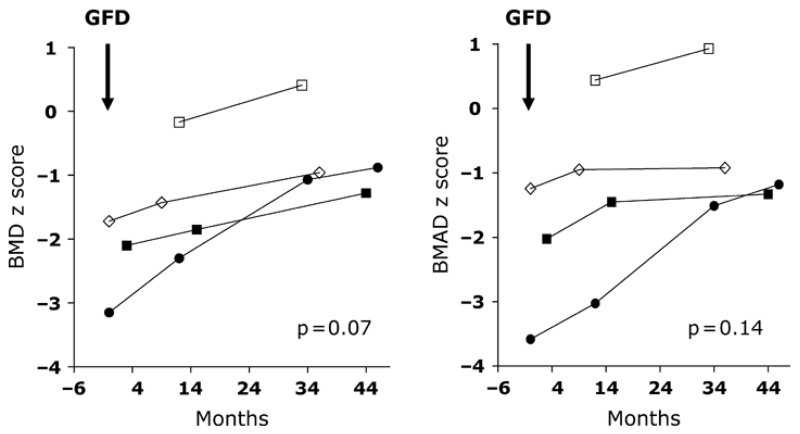
A gluten-free diet was recommended for all patients with histologic evidence of CD. Four patients with severe villous atrophy followed a strict gluten-free diet; adherence to the diet was confirmed by normalization or marked reduction in TGA titers (Table 3). After 12–48 months, there were increases in growth velocity with trends toward increases in height (from −1.1 to 0.16, p = 0.02), weight (from −1.18 to 0.16, p = 0.04), BMI (from −0.69 to −0.14, p = 0.93, Fig. 3), BMD (from −1.79 to 0.68, p = 0.07), and BMAD (from −1.6 to −0.63, p = 0.14, Fig. 4) z scores.
Figure 3.
Changes in height, weight, and body mass index (BMI) z scores in seropositive children (n = 4) with severe villous atrophy who followed a strict gluten-free diet (GFD).
Figure 4.
Changes in bone mineral density (BMD) and bone mineral apparent density (BMAD) z scores in seropositive children (n = 4) with severe villous atrophy who followed a strict gluten-free diet.
Final adult heights in seropositive and seronegative subjects
Final adult height was available for 10 seropositive patients and 10 seronegative controls. Final height was 3.1 ± 2.5 cm below mid-parental height in seropositive patients and 3.0 ± 2.8 cm below mid-parental height in seronegative patients. Seropositive patients who followed a gluten-free diet (n = 3) had final adult heights 1.1 ± 4.9 cm below mid-parental height, whereas seropositive patients who did not follow a gluten-free diet (n = 7) had final adult heights 4.3 ± 3.7 cm below mid-parental height.
Discussion
Previous studies have examined the rates of growth and bone mineralization in children with and without diabetes and adults with classical symptomatic CD (1, 19-21). Our study differs from previous studies in its focus on asymptomatic children with seropositivity to TGA. We elected to study children lacking the classic gastrointestinal symptoms because the great majority of children with diabetes with CD have a subclinical form of the illness (22) and would not have been diagnosed during routine pediatric care.
Relative to seronegative diabetic control subjects matched for gender, age, race, duration of diabetes, and mean HbA1c, our seropositive subjects had significant reductions in IGFBP-3 and BMD z scores and marginal reductions in weight, BMI, IGF-I, and BMAD z scores. Height z scores in the two groups, however, were comparable. BMD z scores correlated more strongly with weight and BMI z scores than with all other parameters except BMAD z scores (r = 0.85); thus, the major effect of celiac seropositivity on bone mineralization may be mediated through effects on nutrient absorption, which in turn modulates hepatic GH sensitivity (23-26) and IGF-1 and IGFBP-3 expression (27-29). Nevertheless, weight and BMI alone cannot be used to identify patients with bone demineralization because some patients with marked reductions in BMD and BMAD have weight and BMI z scores that fall within the normal range for age and gender; for example, the Duke patient with lowest BMD z score had ‘normal’ weight (−0.23) and BMI (−0.55) z scores prior to gluten restriction.
The results of previous investigations (16, 30, 31) and the findings of this study suggest that the great majority of subjects with diabetes with high-titer TGA or endomysial seropositivity (TGA > 150 units by ELISA and endomysial antibody titers ≥ 1:1280) have a form of subclinical CD with histological evidence of villous pathology. Nevertheless, 2 of 11 seropositive subjects in our study who underwent small bowel biopsy had no evidence of villous atrophy, and a chart review of Duke patients performed subsequent to completion of this study found that 3 of 14 additional seropositive children with diabetes had normal small bowel biopsies. Two of these subjects had very high TGA titers (>250 units by ELISA). Thus, seropositivity alone does not guarantee the presence of active villous inflammation detectable on biopsy; the latter is established by small bowel biopsy during a period of normal gluten intake.
The severity of villous pathology correlated with severity of growth failure and bone demineralization in the 11 seropositive subjects who underwent small bowel biopsy. The greatest reductions in weight, height, and bone mineralization and the highest levels of PTH were detected in seropositive subjects with severe villous atrophy. In contrast, body weight, height, and PTH were normal in patients with partial villous atrophy, although BMD and BMAD were reduced non-significantly in the small number of patients studied. Thus, patients with diabetes with subclinical CD are at highest risk for growth failure and bone demineralization if they have severe villous atrophy.
Previous studies showed that gluten restriction can reverse complications and may normalize bone mineralization in classical symptomatic CD (2, 5, 7, 32, 33). In our subset of biopsy-confirmed patients with severe villous atrophy, gluten restriction increased rates of linear growth and bone mineralization. This preliminary observation suggests that early intervention in asymptomatic patients with severe gastrointestinal inflammation may provide long-term benefits for growth and bone health. Our findings lend support to the position that children with diabetes should be screened for CD and treated if pathological changes are detected on small bowel biopsy. Gluten-free foods are increasingly available in local markets and on the internet but may be deemed less palatable by treated subjects in the USA. Also, dietary restriction presents challenges for children and families already stressed by type 1 diabetes; thus, long-term dietary compliance remains problematic for many.
This pilot study has a number of limitations including relatively small sample size and the use of different assays for measuring TGA. In addition, BMD and BMAD were assessed using different machinery (Hologic and Lunar) in the two study centers and the results were not corrected for bone age. Nevertheless, our findings support the hypothesis that seropositivity to TGA and asymptomatic CD may be associated with decreased weight gain and bone demineralization in children with type 1 diabetes. Our very preliminary findings suggest that gluten restriction may increase growth and bone mineralization in children with diabetes with severe villous atrophy even in the absence of gastrointestinal symptoms.
Acknowledgements
This study was supported by a postdoctoral fellowship grant (to E. A. and M. F.) from the Genentech Center for Clinical Research, T32 DK063686 (to J. W.-U.), RO1 DK 24021 (to D. B.) and the Children’s Hospital Of Pittsburgh GCRC MO1 RR 00084, and the Renziehausen fund. The authors thank Jean Litton and Linda Freytag for assistance with patient recruitment.
References
- 1.Kaspers S, Kordonouri O, Schober E, Grabert M, Hauffa BP, Holl RW. Anthropometry, metabolic control, and thyroid autoimmunity in type 1 diabetes with celiac disease: a multicenter survey. J Pediatr. 2004;145:790–795. doi: 10.1016/j.jpeds.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Demir H, Yuce A, Caglar M, et al. Cirrhosis in children with celiac disease. J Clin Gastroenterol. 2005;39:630–633. doi: 10.1097/01.mcg.0000170734.49725.53. [DOI] [PubMed] [Google Scholar]
- 3.Novacek G, Miehsler W, Wrba F, Ferenci P, Penner E, Vogelsang H. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur J Gastroenterol Hepatol. 1999;11:283–288. doi: 10.1097/00042737-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Cicarelli G, Della Rocca G, Amboni M, et al. Clinical and neurological abnormalities in adult celiac disease. Neurol Sci. 2003;24:311–317. doi: 10.1007/s10072-003-0181-4. [DOI] [PubMed] [Google Scholar]
- 5.Martinelli P, Troncone R, Paparo F, et al. Coeliac disease and unfavourable outcome of pregnancy. Gut. 2000;46:332–335. doi: 10.1136/gut.46.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129:454–463. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 7.Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128(Suppl. 1):S79–S86. doi: 10.1053/j.gastro.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Zelnik N, Pacht A, Obeid R, Lerner A. Range of neurologic disorders in patients with celiac disease. Pediatrics. 2004;113:1672–1676. doi: 10.1542/peds.113.6.1672. [DOI] [PubMed] [Google Scholar]
- 9.Peretti N, Bienvenu F, Bouvet C, et al. The temporal relationship between the onset of type 1 diabetes and celiac disease: a study based on immunoglobulin a antitransglutaminase screening. Pediatrics. 2004;113:e418–e422. doi: 10.1542/peds.113.5.e418. [DOI] [PubMed] [Google Scholar]
- 10.De Vitis I, Ghirlanda G, Gasbarrini G. Prevalence of coeliac disease in type I diabetes: a multicentre study. Acta Paediatr Suppl. 1996;412:56–57. doi: 10.1111/j.1651-2227.1996.tb14253.x. [DOI] [PubMed] [Google Scholar]
- 11.Doolan A, Donaghue K, Fairchild J, Wong M, Williams AJ. Use of HLA typing in diagnosing celiac disease in patients with type 1 diabetes. Diabetes Care. 2005;28:806–809. doi: 10.2337/diacare.28.4.806. [DOI] [PubMed] [Google Scholar]
- 12.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 13.Catassi C, Fabiani E, Ratsch IM, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 14.Kolho KL, Farkkila MA, Savilahti E. Undiagnosed coeliac disease is common in Finnish adults. Scand J Gastroenterol. 1998;33:1280–1283. doi: 10.1080/00365529850172368. [DOI] [PubMed] [Google Scholar]
- 15.Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143–148. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]
- 16.Hoffenberg EJ, Bao F, Eisenbarth GS, et al. Transglutaminase antibodies in children with a genetic risk for celiac disease. J Pediatr. 2000;137:356–360. doi: 10.1067/mpd.2000.107582. [DOI] [PubMed] [Google Scholar]
- 17.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 18.Van DerSluis IM, DeRidder MA, Boot AM, Krenning EP, DeMuinck Keizer-Schrama SM. Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch Dis Child. 2002;87:341–347. doi: 10.1136/adc.87.4.341. discussion 341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boersma B, Houwen RH, Blum WF, VanDoorn J, Wit JM. Catch-up growth and endocrine changes in childhood celiac disease. Endocrine changes during catch-up growth. Horm Res. 2002;58(Suppl. 1):57–65. doi: 10.1159/000064771. [DOI] [PubMed] [Google Scholar]
- 20.Barera G, Beccio S, Proverbio MC, Mora S. Longitudinal changes in bone metabolism and bone mineral content in children with celiac disease during consumption of a gluten-free diet. Am J Clin Nutr. 2004;79:148–154. doi: 10.1093/ajcn/79.1.148. [DOI] [PubMed] [Google Scholar]
- 21.Mora S, Weber G, Barera G, et al. Effect of gluten-free diet on bone mineral content in growing patients with celiac disease. Am J Clin Nutr. 1993;57:224–228. doi: 10.1093/ajcn/57.2.224. [DOI] [PubMed] [Google Scholar]
- 22.Cronin CC, Shanahan F. Insulin-dependent diabetes mellitus and coeliac disease. Lancet. 1997;349:1096–1097. doi: 10.1016/S0140-6736(96)09153-2. [DOI] [PubMed] [Google Scholar]
- 23.Mercado M, Molitch ME, Baumann G. Low plasma growth hormone binding protein in IDDM. Diabetes. 1992;41:605–609. doi: 10.2337/diab.41.5.605. [DOI] [PubMed] [Google Scholar]
- 24.Bereket A, Lang CH, Blethen SL, et al. Effect of insulin on the insulin-like growth factor system in children with new-onset insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1995;80:1312–1317. doi: 10.1210/jcem.80.4.7536205. [DOI] [PubMed] [Google Scholar]
- 25.Hanaire-Broutin H, Sallerin-Caute B, Poncet MF, et al. Effect of intraperitoneal insulin delivery on growth hormone binding protein, insulin-like growth factor (IGF)-I, and IGF-binding protein-3 in IDDM. Diabetologia. 1996;39:1498–1504. doi: 10.1007/s001250050604. [DOI] [PubMed] [Google Scholar]
- 26.Shishko PI, Dreval AV, Abugova IA, Zajarny IU, Goncharov VC. Insulin-like growth factors and binding proteins in patients with recent-onset type 1 (insulin-dependent) diabetes mellitus: influence of diabetes control and intraportal insulin infusion. Diabetes Res Clin Pract. 1994;25:1–12. doi: 10.1016/0168-8227(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 27.Clayton KL, Holly JM, Carlsson LM, et al. Loss of the normal relationships between growth hormone, growth hormone-binding protein and insulin-like growth factor-I in adolescents with insulin-dependent diabetes mellitus. Clin Endocrinol (Oxf) 1994;41:517–524. doi: 10.1111/j.1365-2265.1994.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 28.Fainstein Day P, Fagin JA, Vaglio RM, Litwak LE, Picasso MF, Gutman RA. Growth hormone-insulin-like growth factor-I axis in adult insulin-dependent diabetic patients: evidence for central hypersensitivity to growth hormone-releasing hormone and peripheral resistance to growth hormone. Horm Metab Res. 1998;30:737–742. doi: 10.1055/s-2007-978969. [DOI] [PubMed] [Google Scholar]
- 29.Thrailkill KM. Insulin-like growth factor-I in diabetes mellitus: its physiology, metabolic effects, and potential clinical utility. Diabetes Technol Ther. 2000;2:69–80. doi: 10.1089/152091599316775. [DOI] [PubMed] [Google Scholar]
- 30.Cerutti F, Bruno G, Chiarelli F, Lorini R, Meschi F, Sacchetti C. Younger age at onset and sex predict celiac disease in children and adolescents with type 1 diabetes: an Italian multicenter study. Diabetes Care. 2004;27:1294–1298. doi: 10.2337/diacare.27.6.1294. [DOI] [PubMed] [Google Scholar]
- 31.Barera G, Bonfanti R, Viscardi M, et al. Occurrence of celiac disease after onset of type 1 diabetes: a 6-year prospective longitudinal study. Pediatrics. 2002;109:833–838. doi: 10.1542/peds.109.5.833. [DOI] [PubMed] [Google Scholar]
- 32.Kavak US, Yuce A, Kocak N, et al. Bone mineral density in children with untreated and treated celiac disease. J Pediatr Gastroenterol Nutr. 2003;37:434–436. doi: 10.1097/00005176-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Mora S, Barera G, Beccio S, et al. A prospective, longitudinal study of the long-term effect of treatment on bone density in children with celiac disease. J Pediatr. 2001;139:516–521. doi: 10.1067/mpd.2001.116298. [DOI] [PubMed] [Google Scholar]