Abstract
Purpose
The impact of pharmacist-assisted management (PAM) of pharmacotherapy for patients with human immunodeficiency virus (HIV) infection was investigated.
Methods
A retrospective cohort analysis was conducted to evaluate antiretroviral therapy (ART) outcomes in treatment-naive patients initiated on ART at an HIV clinic. Eligible patients enrolled in the clinic during the period 1999–2013 were classified into two groups: those referred to a clinic-based HIV pharmacist for initiation of ART (the PAM group) and those managed by a primary care provider (the control group). The primary study objective was the median time to viral suppression; secondary objectives included the durability of response to the first ART regimen. Relative hazards for the events of interest were estimated using a marginal structural Cox proportional hazards model and Kaplan–Meier curves, with inverse probability weights used to control for selection and confounding bias.
Results
Patients referred for PAM services (n = 819) typically had higher baseline viral loads and lower CD4+ cell counts than those in the control group (n = 436). The likelihood of viral suppression during the first two years after ART initiation was significantly higher in the PAM group versus the control group (hazard ratio, 1.37; 95% confidence interval, 1.18–1.59; p < 0.0001). The median durability of the first ART regimen was 100 months in the PAM group versus 44 months in the control group (p > 0.05).
Conclusion
In treatment-naive patients, suppression of HIV viral load occurred earlier when pharmacists assisted with initiating ART than when ART was initiated without that assistance.
The goals of treating patients with human immunodeficiency virus (HIV) infection are to reduce HIV-related morbidity and mortality, restore and preserve immunologic function, achieve maximal and durable suppression of the plasma HIV viral load, and prevent HIV transmission.1 Currently, durable viral suppression requires strict adherence to lifelong antiretroviral therapy (ART), which consists of at least three active antiretroviral agents. Consistent adherence to ART is a predictor of viral suppression; other predictors include a more potent ART regimen, a lower baseline plasma viral load, a higher baseline CD4+ cell count, and a rapid reduction in the viral load in response to treatment.2-5
Several studies have examined the impact of pharmacists’ interventions on the treatment of HIV-infected patients, with most focusing on improving adherence in nonadherent patients.6 A recent systematic review of the impact of HIV clinical pharmacists on HIV treatment outcomes found that, in the majority of studies, involvement by an HIV pharmacist was significantly associated with improvements in ART adherence and higher rates of viral load suppression.6
We conducted a study to further investigate the impact of pharmacists’ interventions in this area, specifically with regard to initiating ART in treatment-naive HIV-infected patients at a large academic, multidisciplinary clinic where clinical pharmacists function as part of a collaborative practice protocol. Our primary objective was to compare times to viral suppression after initiation of ART in two groups of treatment-naive patients: those receiving pharmacist-assisted management (PAM) services (the intervention group) and those not receiving PAM services (the control group). Secondary objectives were to compare the median times to the first regimen change in the two groups and to describe changes in prescribing patterns and utilization of PAM services over time.
Methods
Study setting and model of care
Our investigation took place within an adult HIV primary care clinic located in southern California that currently serves approximately 3000 patients. The clinic has three dedicated pharmacists who operate an antiretroviral management clinic (established in 1987). Each pharmacist has specialized training and credentials (conferred by the American Academy of HIV Medicine) in the management of HIV pharmaco-therapy. The pharmacists maintain their own patient schedule and are able to provide a variety of patient care services as part of a physician–pharmacist collaborative practice protocol. Patients are referred by their primary care provider (PCP) to the pharmacists for any of several interventions or issues, including initiation and change of ART, management of drug-related adverse effects, medication adherence problems, suspected drug resistance, and difficulties with medication coverage or acquisition. If a patient is not referred to a pharmacist, ART is managed by the primary HIV clinician (a physician, nurse practitioner, or physician assistant). The pharmacists are able to order laboratory tests to monitor the safety and effectiveness of ART initiation and modification and order medications to help manage common ART adverse effects, such as nausea and diarrhea, under the collaborative practice protocol. Patients continue to receive follow-up care from pharmacists until the issue for which they were originally referred is resolved, at which time they are discharged back to their PCP. Additionally, since the pharmacists are physically located within the clinic, they are available as a drug therapy resource for the clinic staff at any time. The pharmacists also assess the appropriateness of resistance testing, coordinate the evaluation and implementation of new ART regimens in patients with viral failure, and manage the resistance database.
Study design
This study was a retrospective, observational cohort study of treatment-naive HIV-positive patients who were initiated on ART between January 1999 and March 2013. Eligible patients were identified using the clinic's computerized databases. Data were extracted from an electronic health record (EHR) system. The following variables were ascertained: date of first pharmacist intervention, date of prescribing of first ART regimen, initial ART regimen prescribed, baseline HIV plasma viral load and CD4+ lymphocyte count, date of first undetectable viral load (i.e., ≤400 HIV RNA copies/mm3), last observed viral load, concurrent disease states (e.g., hepatitis B or C), type of PCP (physician or midlevel practitioner) at baseline (i.e., prior to initiation of ART), HIV transmission risk factors, and demographic characteristics.
Study participants
Patients included in the study were at least 18 years of age. Eligible patients had confirmed HIV infection, were treatment naive at study entry, and were initiated on ART at the study clinic. Patients were excluded if the pretreatment viral load was not documented or was undetectable or if no post-treatment viral load was documented. Patients initiated on ART while pregnant were also excluded from the study. The study was approved by the University of California San Diego human research protection program.
Study outcomes
The primary endpoint of the study was the time from initiation of the first antiretroviral regimen to an undetectable HIV plasma viral load. Secondary endpoints were the time to the first regimen discontinuation or change, prescribing patterns over time by drug class, and the proportion of patients referred for PAM by calendar year.
Statistical analyses
Marginal structural Cox proportional hazards models were used to estimate the time to the first undetectable viral load and the time to the first regimen discontinuation or change. Patients were followed from the date of ART initiation for 24 months or until their first measured undetectable viral load (the primary outcome) or their first ART regimen switch (a secondary outcome). Patient data were censored if the patient was lost to follow-up, defined as 12 months without a viral load measurement. We assigned study observation weights to control for three potential sources of confounding and selection bias: (1) prognostic imbalances in baseline characteristics (controlled for with treatment weights), (2) differential study dropout (controlled for with censoring weights), and (3) differential frequency of viral load monitoring (controlled for with viral monitoring weights). To control for potential confounding of the intervention–outcome relationship, we defined treatment weights that were the inverse probability of treatment conditional on baseline viral load, baseline CD4+ cell count, baseline hepatitis B and C infection status, age at ART initiation, race, transmission group, and time period of ART initiation (1999–2001, 2002–04, 2005–08, or 2009–12).7,8 Continuous variables were modeled flexibly with restricted quadratic splines.9 We included patients who did not have a measured baseline hepatitis B and C infection status and assumed that absence of this information was taken into account for treatment and monitoring decisions by the physicians as if it were simply another patient characteristic. To control for possible nondifferential censoring, we further defined censoring weights that were the cumulative inverse probability of not being censored conditional on baseline viral load, baseline CD4+ cell count, age at ART initiation, race, transmission group, and time period of ART initiation, as well as treatment group and the number of months since treatment initiation, modeled flexibly with polynomial terms.10 Treatment weights were estimated with a logistic model, and censoring weights were estimated with a pooled logistic model, with time stratified into months since treatment initiation; these weights were stabilized by the marginal probability of treatment and censoring, respectively, and multiplied together to obtain weights for the marginal structural Cox proportional hazards model. The estimated treatment weights had a mean of 1.00 (range, 0.40–3.75). The estimated censoring weights for time to viral suppression had a mean of 1.00 (range, 0.12–5.68). The estimated censoring weights for time to first regimen switch had a mean of 1.00 (range, 0.65–2.72).
Finally, to account for differential monitoring for viral suppression by treatment group, we estimated viral monitoring weights for the probability of having a viral load measurement (or not) in a given month (with the probability taken to be that corresponding to the actual pattern of patient observation).11 The weights were estimated using pooled logistic regression and were conditional on all baseline covariates, the number of months since treatment initiation, and the number of months since the most recent viral load measurement, each modeled flexibly with polynomial terms. The weights were stabilized by the marginal probability of having a measured viral load (or not) in a given month. They were then multiplied cumulatively across time, by patient, and the final monitoring weights were multiplied by the treatment and censoring weights. The estimated monitoring weights for viral suppression had a mean of 0.99 (range, 0.02–9.31), and the final estimated weights had a mean of 0.98 (range, 0.04–14.00). The estimated monitoring weights for regimen switch had a mean of 1.02 (range, 0.06–43.48), and the final estimated weights had a mean of 1.02 (range, 0.03–76.52).
The marginal structural Cox proportional hazards model fitted with the final weight estimates of the effect of PAM, as well as the effect of being seen as often as the average patient was seen for viral load monitoring at the clinic, with the assumption that there was no direct effect of viral load monitoring on the time to viral suppression (or, alternatively, on regimen switch). To relax this assumption, we fitted one more marginal structural Cox proportional hazards model that included an indicator for having had a visit within the prior three months and an interaction between the visit indicator and the treatment group.11 We report the hazard ratio (HR) and present adjusted Kaplan–Meier survival curves for the time to viral suppression. We report the median time on the initial ART regimen (i.e., the median time to ART regimen switch) for each treatment group, which was estimated from adjusted Kaplan–Meier survival curves for the time to switch.
Initial regimen selection by antiretroviral drug class was descriptively examined by period of clinic entry and by PAM referral category. Trends in referral for PAM were examined by year of clinic entry. Propensity to refer for PAM according to clinician category (physician versus midlevel practitioner) was examined using logistic regression.
The a priori level of statistical significance for all comparisons and hypothesis tests was 0.05.
Results
Population
A total of 1758 treatment-naive patients were identified, of whom 383 were excluded from the analyses due to an undocumented (n = 379) or undetectable (n = 4) pretreatment viral load. An additional 120 patients were excluded due to an undocumented viral load after initiation of ART, leaving 1255 patients evaluable for viral suppression, 819 (65%) of whom received PAM services. Table 1 summarizes the characteristics of evaluable patients by study group. On average, PAM patients had somewhat more advanced HIV disease than non-PAM patients, as reflected in the former group's slightly higher pretreatment viral plasma load (mean, 4.9 log10 copies/mm3 versus 4.7 log10 copies/mm3) and lower CD4+ cell count (mean, 275 cells/mm3 versus 380/mm3). In addition, PAM patients were more likely to be coinfected with hepatitis B or C and to have acquired HIV infection through injection drug use. On average, PAM patients had more frequent viral load monitoring than non-PAM patients during the first six months of treatment but not during the second six months. Over the study period, data on 45 patients (4%) were censored because the patients were lost to follow-up. Censoring before viral suppression was more common among non-PAM patients (n = 30, 7%) than among PAM patients (n = 15, 2%). Three hundred twenty-four patients (26%) were censored before the end of follow-up for regimen switching. Censoring prior to regimen switching was more common among non-PAM patients (n = 127, 29%) than among PAM patients (n = 197, 24%).
Table 1.
Characteristics of Patients Receiving and Not Receiving Pharmacist-Assisted Management (PAM) of Antiretroviral Therapya
| Characteristic | PAM (n = 819) | No PAM (n = 436) | p b |
|---|---|---|---|
| Median (IQR) age at ART initiation, yr | 37.7 (31.7-44.8) | 35.3 (27.9-44.0) | 0.0001 |
| Sex, no. (%) | 0.858 | ||
| Male | 728 (89) | 389 (89) | |
| Female | 91 (11) | 47 (11) | |
| Median (IQR) baseline HIV viral load, log10 copies/mm3 | 4.9 (4.5-5.4) | 4.7 (4.1-5.3) | <0.00001 |
| Median (IQR) CD4+ cell count, cells/mm3 | 234 (68-419) | 371 (147-539) | <0.00001 |
| Presence of HIV risk factors, no. (%) | 0.056 | ||
| MSM, not IDU | 512 (63) | 288 (66) | |
| IDU | 243 (30) | 104 (24) | |
| Other | 64 (8) | 44 (10) | |
| Result of first test for HBsAg, no. (%) | 0.952 | ||
| Negative | 726 (89) | 389 (89) | |
| Positive | 40 (5) | 20 (5) | |
| Result missing | 53 (6) | 27 (6) | |
| Result of first test for HCV Ab, no. (%) | 0.034 | ||
| Negative | 707 (86) | 393 (90) | |
| Positive | 93 (11) | 30 (7) | |
| Result missing | 19 (2) | 13 (3) | |
| Race or ethnicity, no. (%) | 0.038 | ||
| White | 341 (42) | 215 (49) | |
| Hispanic | 304 (37) | 146 (33) | |
| Black | 117 (14) | 56 (13) | |
| Other | 57 (7) | 19 (4) | |
| Primary care provider, no. (%) | <0.00001 | ||
| Physician | 395 (48) | 244 (56) | |
| Midlevel practitioner | 399 (49) | 110 (25) | |
| Not recorded | 25 (3) | 82 (19) | |
| Median (IQR) no. viral load measurements | |||
| During months 0-6 | 3 (2-4) | 2 (1-3) | <0.00001 |
| During months 7-12 | 1 (0-2) | 1 (0-2) | 0.2695 |
IQR = interquartile range, ART = antiretroviral therapy, HIV = human immunodeficiency virus, MSM = men having sex with men, IDU = injection drug use, HBsAg = hepatitis B surface antigen, HCV Ab = hepatitis C virus antibody.
Comparisons conducted with the Wilcoxon rank-sum test for medians and the chi-square test for categorical results.
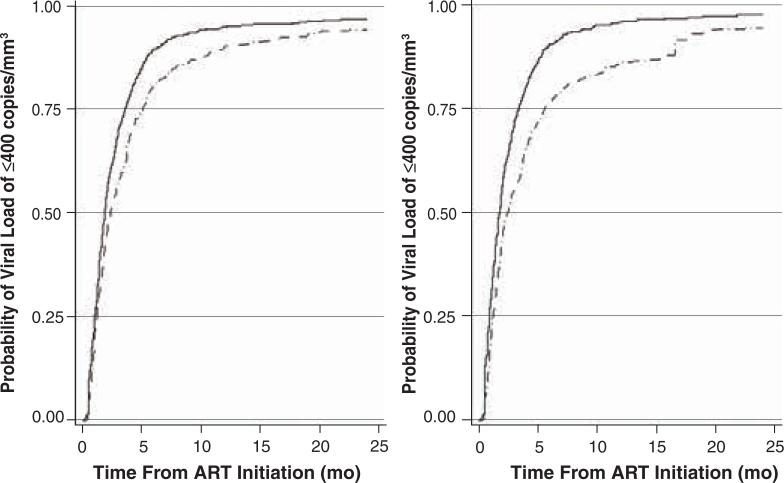
Primary endpoint
Figure 1 graphically depicts unadjusted and adjusted Kaplan–Meier estimates of the time to an undetectable HIV viral load (≤400 copies/mm3) by study group. In a Cox model fitted using inverse probability-of-treatment and censoring weights but not adjusted for differential monitoring frequency between the two groups, the PAM group achieved viral suppression 1.41 times faster than the non-PAM group (95% confidence interval [CI], 1.22–1.63; p < 0.0001). After adjusting for viral load monitoring, the adjusted HR for the joint effect of PAM and a standard visit schedule (i.e., a schedule consistent with the average visit frequency for the clinic) was 1.37 (95% CI, 1.18–1.59). The interaction between the PAM group and the indicator of having had a visit in the prior three months (a marker of monitoring frequency) was not significant (p = 0.88).
Figure 1.
Unadjusted (left) and adjusted (right) Kaplan–Meier curves of the estimated time to undetectable HIV viral load (≤400 RNA copies/mm3) in patients for whom initiation of antiretroviral therapy (ART) occurred with pharmacist assistance (solid line, n = 819) or without such assistance (broken line, n = 436).
Secondary endpoints
Time to first regimen change
After adjusting for confounding, censoring, and differential monitoring frequency, the median durations of the first regimen were 100 and 44 months for the PAM and non-PAM groups, respectively. The cumulative rates of regimen change for the two groups did not diverge until around the fourth year after ART initiation. The differences in the rate of switch were not statistically significant in the marginal structural Cox proportional hazards model that adjusted for confounding of the treatment–outcome relationship, censoring, and differential follow-up patterns between the groups (adjusted HR, 0.98; 95% CI, 0.74–1.30).
Changes in prescribing patterns
Table 2 summarizes the distribution of drug classes used in the initial antiretroviral regimens by study group and period of entry into treatment. Early in the study period and up to 2008, the majority of regimens initiated in the PAM group were protease inhibitor (PI) based; in the non-PAM group, nonnucleoside reverse transcriptase inhibitor (NNRTI)–based regimens were preferred until 2005. From 2009 through 2013, prescribing patterns in the PAM and non-PAM groups were similar.
Table 2.
Drug Classes in Initial Regimens for Patients Receiving and Not Receiving Pharmacist-Assisted Management (PAM) of Antiretroviral Therapy in HIV Clinic (n = 1255)a
| Proportion of Initial Regimens Containing Drug in Indicated Class |
||||
|---|---|---|---|---|
| Drug Class | Clinic Entry, 1999-2001 | Clinic Entry, 2002-04 | Clinical Entry, 2005-08 | Clinic Entry, 2009-13 |
| Patients With PAM | ||||
| NNRTI | 0.44 | 0.29 | 0.42 | 0.52 |
| PI | 0.52 | 0.63 | 0.57 | 0.42 |
| INSTI | 0 | 0 | 0.01 | 0.09 |
| Patients Without PAM | ||||
| NNRTI | 0.51 | 0.54 | 0.52 | 0.47 |
| PI | 0.46 | 0.36 | 0.43 | 0.50 |
| INSTI | 0.01 | 0 | 0.03 | 0.04 |
HIV = human immunodeficiency virus, NNRTI = nonnucleoside reverse transcriptase inhibitor, PI = protease inhibitor, INSTI = integrase strand transfer inhibitor.
Table 3 shows rates of patient referral for PAM by year of clinic entry. From 1999 through 2006, referrals for PAM averaged 69%. During the period 2007–11, there was a downward trend in referrals for PAM for initiation of ART in treatment-naive patients. During the final years of the study period (2012–13), the referral rate again increased. Midlevel practitioners were twice as likely as physicians to refer patients for PAM (odds ratio, 2.24; 95% CI, 1.72–2.92). However, the effect of PAM was not modified by clinician category.
Table 3.
Referrals for Pharmacist-Assisted Management (PAM) of Antiretroviral Therapy
| Year of Clinic Entry | Fraction (%) Patients Referreda |
|---|---|
| 1999 | 44/70 (63) |
| 2000 | 59/82 (72) |
| 2001 | 62/82 (76) |
| 2002 | 60/78 (77) |
| 2003 | 60/96 (63) |
| 2004 | 62/88 (70) |
| 2005 | 41/68 (60) |
| 2006 | 55/81 (68) |
| 2007 | 41/72 (57) |
| 2008 | 39/72 (54) |
| 2009 | 66/101 (65) |
| 2010 | 64/115 (56) |
| 2011 | 68/117 (58) |
| 2012 | 91/125 (73) |
| 2013 | 7/8 (88) |
| Total | 819/1255 (65) |
p = 0.006 for comparison of fractions receiving and not receiving PAM over time (Pearson χ2 [14 d.f.] = 31.008).
Discussion
In both the unadjusted and the adjusted analyses, patients with pharmacist-assisted ART management achieved more rapid viral suppression than patients managed without such assistance. Our results validated pharmacist activities (within the scope of a physician–pharmacist collaborative practice protocol) to help optimize patient outcomes and provide an example of successful long-term use of a recently described model of care.12 Particularly in the context of the concept of “treatment as prevention” of further HIV transmission, achieving viral suppression earlier may have clinically important implications.
When differences in baseline characteristics were accounted for, we found that patients referred for PAM for ART initiation typically achieved viral suppression more rapidly than patients not receiving PAM services. PAM also was associated with more frequent monitoring of the HIV viral load during the first six months of ART, suggesting more frequent patient follow-up. This may be a component of PAM that is an important mediator of improved clinical outcomes. One potential explanation for the observed difference between the PAM and control groups with regard to viral suppression is that the PAM intervention resulted in patients receiving more frequent follow-up and provided more opportunities for education, support, and coaching regarding the importance of adherence, treatment goals, and management of adverse effects. While more frequent monitoring of viral load was observed in the PAM group, our results showed that the effect of PAM on viral suppression remained after accounting for different monitoring patterns, suggesting that the effect of PAM was not entirely explained by monitoring frequency. With regard to the longer durability of initial regimens among PAM patients in the adjusted analysis, we speculate that several factors may have contributed to this finding: initial selection of more potent or better-tolerated regimens, more effective management of adverse effects and toxicities through more frequent monitoring, and more intensive adherence counseling than was available for the control patients.
Another objective of the study was to describe changes in prescribing patterns in the PAM and non-PAM groups. Changes in prescribing patterns over the study period in both the PAM and control groups were consistent with the availability of new antiretroviral agents over time. With the introduction of the first once-daily single-tablet regimen (tenofovir, emtricitabine, and efavirenz in a combined tablet form) in 2006, we observed an increase in the prescribing of NNRTI-based regimens relative to PI-based regimens. The most notable differences in prescribing patterns between the PAM and control groups occurred earlier in the study, during the period 1999–2008, when PAM patients were prescribed PI-based regimens more frequently than control patients.
A potential explanation for that difference in prescribing patterns is that, until 2006, baseline genotype tests were not routinely ordered prior to initiating treatment in treatment-naive patients; in the absence of baseline data on genetic resistance to HIV-1, patients with more advanced disease were typically initiated on PI-based regimens because of the higher HIV-1 resistance thresholds such regimens offer relative to NNRTI-based regimens. When assessment of baseline resistance became the standard of care for treatment-naive patients, a shift toward NNRTI-based regimens and away from PI-based regimens was observed. Another possible explanation for the observed preference for PI-based regimens in the PAM group during the early years of the study period may pertain to clinician estimation of patient medication adherence. In a study of predictors of early ART adherence conducted in the same clinic involved in our study and published in 2002, pharmacists were found to predict ART adherence more accurately than other providers.13 For patients who required ART around that time but were deemed to be at risk for suboptimal adherence, PI-based regimens likely would have been preferred for their ability to foster a higher genetic barrier to resistance than that produced by NNRTI-based regimens and, thus, better accommodate imperfect adherence. Although unboosted PI regimens required more frequent dosing relative to NNRTI regimens, in the early 2000s clinical data indicating that ritonavir-boosted PI regimens could allow less frequent dosing and were associated with a lack of protease resistance at first viral failure were emerging.14 Guidelines published at the time also recognized the difficulty of designing effective regimens after viral failure on the first regimen and mentioned the preference of some experts for ritonavir-boosted PI regimens.15
Lastly, we investigated changes over time in the proportion of patients referred for PAM. Initially during the study period, approximately 60% of treatment-naive patients were referred for PAM for initiation of ART. With the availability of relatively less complex and better-tolerated regimens approved for treatment-naive patients, there may have been a sense among some providers that initiating ART did not require the extensive counseling or education required with traditional, more complex regimens. Although regimen simplification and a reduced pill burden help improve adherence, other predictors of adherence, such as health beliefs, also have a significant impact on adherence and the ultimate success of viral suppression. Even after controlling for the year of entry into treatment, the PAM group had accelerated viral suppression relative to the control group, indicating a benefit of PAM with regard to achieving HIV treatment goals despite the availability of once-daily, one-tablet regimens.
There were several limitations to our study. First, the quality of the data depended on the accuracy and consistency of documentation in the EHR. There may have been some misclassification of PCP types resulting from investigator reliance on documented visit dates closest to ART initiation for provider classification purposes. Also, dates of ART initiation and regimen change were those recorded in the EHR and may not have corresponded to actual initiation and change dates. Second, the results of our study reflect experience in a treatment-naive population. It is likely that in a treatment-experienced population, the PAM group would have performed even better because of the expertise of the PAM clinicians in complex drug interactions, resistance, and adherence counseling. Third, the operational definition of the PAM group (i.e., it included any patient who was referred to the pharmacists and had a documented visit up to 365 days prior to ART initiation) was somewhat arbitrary, and a sensitivity analysis of the pretreatment PAM initiation window was not conducted. Because variations in the components and intensity of the PAM intervention were not explored, the transferability (external validity) of the intervention may be difficult to assess. Fourth, our definition of PAM did not account for the possibility of undocumented consultations between clinic pharmacists and providers prior to treatment initiation (this “curbside consulting” often happens in practice and may have an impact on treatment outcomes); the occurrence of informal pharmacist consultations would have tended to attenuate differences in evaluated outcomes between the two study groups. Fifth, although we attempted to use inverse probability weighting to control for confounding due to nonrandomized PAM assignment and selection bias due to differential viral load ascertainment frequency and differential data censoring, our inferences were subject to residual confounding and selection bias from unmeasured sources. Lastly, our analysis did not identify the specific elements of the PAM intervention (e.g., patient education, adherence counseling, toxicity recognition and management, regimen selection, monitoring plans) responsible for earlier viral suppression. Further investigation of these variables may provide more insight into mediating and moderating factors contributing to the observed differences in viral suppression between the PAM and control groups.
The study had two notable strengths: There was very minimal subject dropout prior to viral suppression, which decreased the likelihood of selection bias due to differential follow-up, and we accounted for different viral load monitoring frequencies in the PAM and control groups, which allowed us to separate the effect of the PAM program on the time to viral suppression from the effect of earlier detection of viral suppression.
Conclusion
In treatment-naive patients, suppression of HIV viral load occurred earlier when pharmacists assisted with initiating ART than when ART was initiated without that assistance.
Acknowledgments
Supported by the Clinical Investigation and Biostatistics Core of the University of California San Diego Center for AIDS Research (CFAR), through grant AI036214; the University of North Carolina at Chapel Hill CFAR, through grant P30 AI50410; and the CFAR Network of Integrated Clinical Systems, a National Institutes of Health–funded program (through grant R24 AI067039) that was made possible by the National Institute of Allergy and Infectious Diseases and the National Heart, Lung and Blood Institute.
Footnotes
The authors have declared no potential conflicts of interest.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. [2014 May 7];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 2.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Powderly WG, Saag MS, Chapman S, et al. Predictors of optimal virological response to potent antiretroviral therapy. AIDS. 1999;13:1873–80. doi: 10.1097/00002030-199910010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Townsend D, Troya J, Maida I, et al. First HAART in HIV-infected patients with high viral load: value of HIV RNA levels at 12 weeks to predict virologic outcome. J Int Assoc Physicians AIDS Care. 2009;8:314–7. doi: 10.1177/1545109709343966. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita TE, Phair JP, Munoz A, et al. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. AIDS. 2001;15:735–46. doi: 10.1097/00002030-200104130-00009. [DOI] [PubMed] [Google Scholar]
- 6.Saberi P, Dong BJ, Johnson MO, et al. The impact of HIV clinical pharmacists on HIV treatment outcomes: a systematic review. Patient Prefer Adherence. 2012;6:297–322. doi: 10.2147/PPA.S30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Howe CJ, Cole SR, Westreich DJ, et al. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22:874–5. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–88. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 11.Hernan MA, McAdams M, McGrath N, et al. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009;18:27–52. doi: 10.1177/0962280208092345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramowitz PW, Shane R, Daigle LA, et al. Pharmacist interdependent prescribing: a new model for optimizing patient outcomes. Am J Health-Syst Pharm. 2012;69:1976–81. doi: 10.2146/ajhp120546. [DOI] [PubMed] [Google Scholar]
- 13.Mathews WC, Mar-Tang M, Ballard C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Pat Care STDS. 2002;16:157–72. doi: 10.1089/10872910252930867. [DOI] [PubMed] [Google Scholar]
- 14.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–46. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 15.Dybul M, Fauci AS, Bartlett JG, et al. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm Rep. 2002;51(RR-7):1–55. [PubMed] [Google Scholar]