Abstract
Purpose
Early detection of hearing loss in all newborns and timely intervention are critical to children's cognitive, verbal, behavioral, and social development. The initiation of appropriate early intervention services before 6 months of age can prevent or reduce negative developmental consequences. The purpose of this study was to assess, using large, population-based registries, the effect of co-occurring birth defects (CBDs) on the timing and overall rate of hearing screening and diagnosis.
Method
The authors linked statewide data from newborn hearing screenings, a birth defects registry, and birth certificates to assess the timeliness of newborn hearing screening and diagnosis of hearing loss (HL) for infants with and without CBDs in 485 children with confirmed HL.
Results
Nearly one third (31.5%) of children with HL had 1 or more CBDs. The presence of CBDs prolonged the time of the initial infant hearing screening, which contributed to further delays in the subsequent diagnosis of HL.
Conclusions
Better coordination of HL assessment into treatment plans for children with CBDs may enable earlier diagnosis of HL and provide opportunities for intervention that will affect long-term developmental outcomes for these children.
Keywords: hearing loss, congenital abnormalities/anomalies, hearing screening, coordination of care
Congenital hearing loss (HL) constitutes one of the most common groups of birth defects in the United States, with a birth prevalence of 1.2 to 1.9 per 1,000 newborns (Centers for Disease Control and Prevention [CDC], 2010; Morton & Nance, 2006). The total lifetime societal cost of HL for children born in 2000 was estimated to be $1.9 billion, including $640 million in special education costs (CDC, 2004). Timely screening for and diagnosis of HL are crucial, because delays in intervention may affect the cognitive, verbal, behavioral, and social development of infants with HL (Yoshinaga-Itano, 2003). Some of these developmental problems can be prevented if appropriate intervention for children with HL is initiated by 6 months of age (Kennedy et al., 2006; Robinshaw, 1995; Sininger et al., 2009; Yoshinaga-Itano, Sedey, Coulter, & Mehl, 1998). Before the advent of universal newborn hearing screening, age at diagnosis of HL consistently exceeded 24 months; it now typically occurs between 2 and 10 months of age (Harrison & Roush, 1996; Harrison, Roush, & Wallace, 2003; Sininger et al., 2009). The beneficial impact of universal newborn hearing screening on developmental outcomes has been clearly demonstrated: Compared with infants with HL born in hospitals that offered universal newborn hearing screening, infants with HL born at hospitals that did not offer screening had lower scores on tests of receptive and expressive language, poorer speech intelligibility, and smaller expressive vocabularies (Yoshinaga-Itano, Coulter, & Thomson, 2000, 2001).
Although nearly 95% of all infants born are screened for HL at birth, with referral rates for diagnostic testing being less than 6% (low false-positive rates; www.infanthearing.org; White, Forsman, Eichwald, & Muñoz, 2010), successful follow-up and tracking of these infants remains a challenge because of many barriers (Hayes, 1999; Shulman et al., 2010). Nearly half of all infants who do not pass the initial hospital hearing screening do not receive timely, appropriate follow-up care (CDC, 2003, 2010; Smith et al., 2007; White, 2004). Indeed, the follow-up of these infants remains one of the biggest challenges to state early hearing detection and intervention programs; even in research studies, in which diagnostic evaluation is a priority, as many as half of children referred are lost to follow-up (White, 2003). Some evidence suggests that the presence of comorbid conditions may affect both the timing and overall rate of screening, diagnosis, and intervention for HL. Studies of children and youth with special health care needs have shown that children with more than one condition (Bitsko et al., 2009), and more severe conditions (Rosenberg et al., 2005; Tippy, Meyer, Aronson, & Wall, 2005), had an increased risk for having unmet needs, including obtaining hearing aids or other devices. In a 3-year cohort study of 39,000 infants born in Rhode Island, Vohr, Moore, and Tucker (2002) found that infants in the neonatal intensive care unit (NICU) were 16.4 times more likely to miss the initial hearing screening and nearly six times more likely to miss their rescreen compared with infants in the well-baby nursery. They suggested that these missed hearing screenings were due to transfers before screening occurred, increased risk of social and environmental barriers, and illness severity among the NICU infants.
The purpose of the current study was to quantify the impact of co-occurring birth defects (CBDs) on the timing of hearing screening and diagnosis for infants with HL, using statewide birth defect and hearing screening registries. We use the term co-occurring because the genesis or pathophysiological connections among various birth defects in this study are not known, and we lacked information on other conditions that may truly be comorbid. We hypothesized that CBDs would be associated with delays in hearing screening and diagnosis and that children with multiple CBDs would experience longer delays than those with one CBD.
Method
Data Sources
We extracted information regarding newborn hearing screening and confirmatory diagnoses from the Virginia Early Hearing Detection and Intervention (VEHDI) program database for children born between January 1, 2002, and December 31, 2006. The Code of Virginia (§32.1-64.1) and Virginia Regulation 12 VAC 5-80, promulgated in 1999, require that all hospitals with infant nurseries and all hospitals with neonatal intensive care services screen the hearing of all infants before they are discharged from the facility. If the infant does not pass the initial screening, the hospital must refer the infant for diagnostic evaluation.
For the time period covered by this study, hospitals were required to provide the results of initial hearing screening tests to the VEHDI program for infants who failed their initial screen and for infants who passed the initial screen but were at risk for developing early childhood HL. All persons who provide audiologic services must also report the status and/or results of diagnostic evaluations to the VEHDI program for infants and children up to 2 years of age (see Virginia Department of Health, 2004, for more details). HL in this study was defined as having one of the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM; World Health Organization, 1977) codes reported in at least one ear at a follow-up assessment by a licensed audiologist: 389.0 (conductive hearing loss), 389.1 (sensorineural hearing loss), 389.2 (mixed hearing loss), and 389.9 (undetermined hearing loss).
Birth defect diagnoses were extracted from the Virginia Congenital Anomalies Reporting System (VaCARES) for children born within the same time period as the VEHDI program data. VaCARES is a passive compliant birth multi-source defects registry (Kirby, 2000) that receives diagnosis codes from hospitals regarding children from birth to 2 years of age covering 86 categories of structural, functional, or biochemical abnormalities as well as information from Virginia's newborn dried-blood spot screening program (Virginia Department of Health, 2003).
Because of concerns regarding the specificity of ICD-9-CM diagnosis codes and the fact that VaCARES is a passive registry, we excluded some birth defects from analyses. We also excluded all “unspecified” diagnoses, with the exception of unspecified laterality, site, or limb. HL diagnosis codes (e.g., 389.x) reported to VaCARES were excluded, but other ear anomalies were considered to be CBDs. Patent ductus arteriosus, undescended testicle, and scaphocephaly were excluded for infants born preterm. Because multiple ICD-9-CM codes related to the same birth defect are commonly reported, we grouped the codes into one of 13 general categories of birth defects based on organ system: (a) blood/immune, (b) cardiovascular, (c) chromosomal, (d) central nervous system, (e) ocular, (f) gastrointestinal, (g) genitourinary, (h) metabolic/endocrine, (i) musculoskeletal, (j) orofacial, (k) other ear, (l) prenatal exposures, and (m) respiratory. We then computed for each child the number of CBD categories present. See Virginia Department of Health (2003) for a detailed list of ICD-9-CM codes reported within each of the 13 categories.
Maternal sociodemographic variables (sex, race/ethnicity, age, education, and method of payment) and infant birth status (infant transfer status and gestational age) were extracted from 2002–2006 Virginia resident live birth data, which include data on births to Virginia residents that occurred both in and out of state.
We prepared a de-identified linked VEHDI program, VaCARES, and birth certificate analysis data set on the basis of linkages previously conducted by the first and second authors as part of their regular public health surveillance activities within the Virginia Department of Health. This linkage was created using a combination of deterministic and probabilistic data linkage iterations followed by manual verification and searches for additional records using methods described by Mason and Tu (2008). The final analysis data set consisted of 485 children with confirmed HL who were Virginia residents both at the time of birth and when the diagnosis of HL was made. The children in this study either did not pass their initial hearing screening in one or both ears (96%) or passed with follow-up (4%) and had a confirmed diagnosis of HL reported to VEHDI. Because the data linkage started with cases of confirmed HL, infants who died prior to screening or diagnosis were not included in the study. Because VEHDI mandates reporting up to 2 years of age, only children with a diagnosis date 2 years of age or less were included in analyses involving screening and diagnosis dates.
Screening and Diagnosis Goals
The VEHDI program's mission is to minimize or eliminate communication disorders resulting from HL. This mission is being accomplished in part through the VEHDI program's goal to ensure that all children with HL meet benchmarks, known as the 1–3–6 Plan, recommended by the Joint Committee on Infant Hearing. The 1–3–6 Plan recommends universal hearing screening for newborns before hospital discharge or by 1 month of age, diagnosis by 3 months of age, and enrollment in intervention services by 6 months of age if HL is confirmed (Joint Committee on Infant Hearing, 2007). We were able to directly assess two of the goals related to the 1–3–6 Plan: (a) initial hearing screening occurring by 1 month of age and (b) confirmatory diagnosis occurring by 3 months of age. We also computed the time elapsed between the hearing screening and diagnosis to assess whether there were diagnosis delays independent of the timing of the initial hearing screening.
We were not able to compute the age at hearing screening for 55 children with HL (11.3%) because of invalid or missing screening dates in the VEHDI data. Age at diagnosis was not computed for 59 children (12.2%) who had a missing or invalid confirmatory diagnosis date. Time elapsed between hearing screening and diagnosis could not be computed for the 114 children (23.5%) who were missing either the initial screening date or the diagnosis date. For each target, we examined missing data rates by maternal age, maternal education, insurance status at birth, sex, and race/ethnicity. We found no significant differences between children with missing and valid screening or diagnosis dates for any of these variables, indicating that the dates were missing at random.
Results
The prevalence of HL in this sample was 0.97 per 1,000 infants screened (485/501,408). Nearly one third (31.5%) of these 485 children with HL had at least one other reported birth defect. Among children with both HL and a CBD, more than half (56%) had birth defect codes reported in multiple CBD categories. The most prevalent CBD categories among children with HL were cardiovascular (14.4%), musculoskeletal (8.3%), chromosomal (5.6%), ophthalmologic (5.6%), and other ear anomalies (5.4%). The CBD prevalence and percentage of children who met the hearing screening, diagnosis, and hearing screening-to-diagnosis targets by various sample characteristics are presented in Table 1.
Table 1.
Overall percentage, co-occurring birth defects (CBDs) prevalence, and percentage of infants who met hearing screening (HS), diagnosis (Dx), and HS-to-Dx targets by sample characteristics.
| Overall |
||||||
|---|---|---|---|---|---|---|
| Characteristic | n | % | CBDs prevalence (%) | HS by age 1 month (%) | Dx by age 3 months (%) | Dx within 3 months of HS (%) |
| Sex | ||||||
| Male | 267 | 55.0 | 36.0 | 88.4 | 37.9 | 48.8 |
| Female | 218 | 45.0 | 26.2 | 86.8 | 33.0 | 40.6 |
| Insurance | ||||||
| Self-pay | 34 | 7.1 | 44.1 | 75.0 | 24.1 | 39.1 |
| Medicaid | 106 | 22.2 | 36.8 | 86.0 | 32.6 | 40.5 |
| Private insurance | 338 | 70.7 | 29.0 | 89.2 | 38.3 | 47.2 |
| Race/ethnicity | ||||||
| Black, non-Hispanic | 78 | 16.1 | 32.0 | 79.1 | 20.8* | 27.9* |
| Hispanic | 74 | 15.3 | 35.1 | 92.2 | 43.3 | 48.0 |
| Other, non-Hispanic | 41 | 8.4 | 43.9 | 87.5 | 41.7 | 48.6 |
| White, non-Hispanic | 292 | 60.2 | 28.8 | 88.8 | 37.2 | 48.4 |
| Maternal age (years) | ||||||
| <19 | 26 | 5.4 | 30.8 | 88.0 | 12.5 | 26.1 |
| 19-24 | 103 | 21.2 | 27.2 | 87.8 | 38.9 | 48.0 |
| 25-34 | 251 | 51.8 | 31.9 | 88.7 | 35.6 | 45.5 |
| 35+ | 105 | 21.6 | 35.2 | 85.1 | 38.7 | 46.3 |
| Maternal education (years) | ||||||
| <12 | 85 | 17.7 | 36.5 | 87.0 | 32.4 | 38.1* |
| 12 | 134 | 27.9 | 26.1 | 84.5 | 29.8 | 37.7 |
| 13+ | 261 | 54.4 | 33.0 | 89.7 | 39.8 | 51.0 |
| Infant transferred to another facility | ||||||
| Yes | 26 | 5.4 | 76.9 | 41.7*** | 8.7** | 47.6 |
| No | 459 | 94.6 | 29.0 | 90.4 | 37.2 | 44.9 |
| Preterm birth | ||||||
| Yes | 121 | 25.0 | 41.3 | 56.3*** | 15.7*** | 31.0** |
| No | 364 | 75.0 | 28.3 | 97.6 | 42.0 | 49.1 |
| Hearing loss laterality | ||||||
| Unilateral | 169 | 34.8 | 33.1 | 91.7 | 38.5 | 41.5 |
| Bilateral | 316 | 65.2 | 30.7 | 85.4 | 34.3 | 46.9 |
| CBD category | ||||||
| Isolated hearing loss | 332 | 68.5 | 92.6*** | 40.2** | 46.2 | |
| 1 category | 68 | 14.0 | 88.3 | 35.0 | 46.2 | |
| 2+ categories | 85 | 17.5 | 67.6 | 17.1 | 39.0 | |
Note. This table contains valid percentages, which were computed using only valid and nonmissing data for each combination of variables.
p < .05.
p < .01.
p < .001.
On average, children with isolated HL received their initial hearing screening 25 days earlier than those with multiple CBD categories, t(368) = –7.04, p < .001 (see Table 2). The mean length of time for diagnosis was over 7 months for all groups. The median age that children with isolated HL received their confirmatory diagnosis was over 2.5 months earlier than those with multiple CBD categories. The time elapsed between hearing screening and diagnosis was similar for all groups.
Table 2.
Mean and median age in days at initial HS, confirmatory Dx, and elapsed time between HS and Dx.
| Agea at HS |
Agea at Dx |
HS to Dxb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | M | SD | Mdn | M | SD | Mdn | M | SD | Mdn |
| Isolated hearing loss | 8.4 | 21.3 | 2 | 226.0 | 243.2 | 129.5 | 179.8 | 202.7 | 105 |
| 1 CBD category | 14.0 | 26.8 | 3 | 285.2 | 287.0 | 155.5 | 215.3 | 225.1 | 96 |
| 2+ CBD categories | 33.2 | 43.3*** | 17 | 268.8 | 226.9 | 210.0 | 197.9 | 192.8 | 121 |
Age in days.
Elapsed time in days.
p < .001.
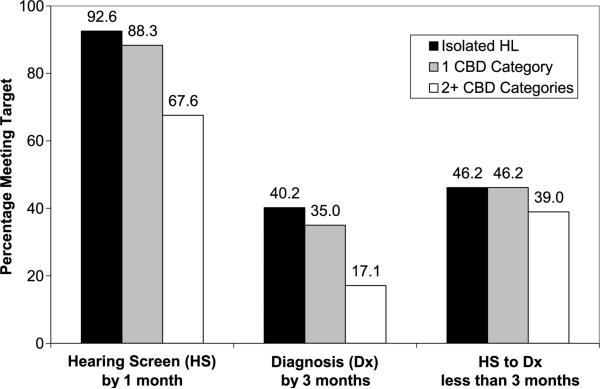
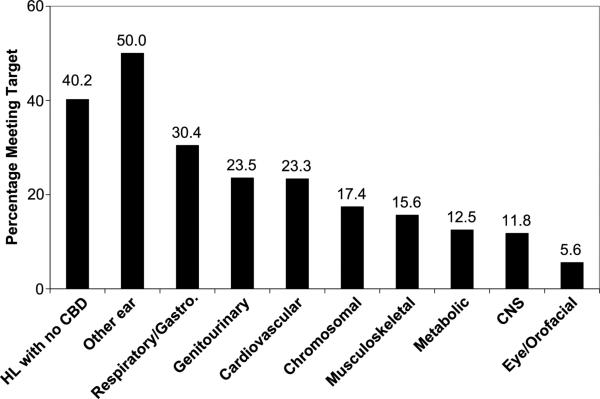
Overall, 87.7% of the 430 children in this study with a valid screening date received their hearing screening by 1 month of age, but only 35.7% were diagnosed by 3 months of age. Less than half of the children (45.0%) received a diagnosis within 3 months of their hearing screening. Children with CBD met all three targets less often than children with isolated HL. The percentages of children who met the hearing screening, diagnosis, and hearing screening-to-diagnosis targets by the number of CBD categories are shown in Figure 1. The percentage of children with HL who were diagnosed by 3 months of age by category of CBD is presented in Figure 2. With the exception of other ear anomalies, the percentage of children in every CBD category had a lower percentage meeting the 3-month diagnosis goal than children with isolated HL.
Figure 1.
Percentage of children with hearing loss (HL) who met various hearing screening (HS) and diagnosis (Dx) targets by the presence and number of co-occurring birth defects (CBDs).
Figure 2.
Percentage of children with HL who received a confirmatory diagnosis by 3 months of age by CBD category. CNS = central nervous system.
We conducted a series of bivariate analyses to identify maternal sociodemographic and birth characteristics that were associated with increased odds of failing to meet hearing screening and diagnosis targets. We then computed adjusted logistic regressions, including potential confounders selected on the basis of at least a 10% change in estimate (Mickey & Greenland, 1989). Bivariate and final adjusted models predicting failure to meet the hearing screening and diagnosis targets are presented in Table 3. For the hearing screening target, preterm birth, infant transfer, and multiple CBD categories remained significant in the adjusted model. Preterm birth and multiple CBD categories were associated with diagnosis delays in the adjusted model. We performed an additional series of regressions using the same variables in Table 3 to predict the odds of an elapsed time between hearing screening and diagnosis greater than 3 months. Four predictors were associated with increased odds of having longer than 3 months elapsed time between hearing screening and diagnosis. Odds ratios for these four predictors (multiple CBD categories, preterm birth, Black non-Hispanic race/ethnicity, and maternal education of 12 years or less) were 5.98, 95% confidence interval (CI) [3.11, 11.48]; 2.15, 95% CI [1.28, 3.61]; 2.43, 95% CI [1.31, 4.51]; and 1.71, 95% CI [1.12, 2.59], respectively. Despite the delays in age at hearing screening and diagnosis associated with having multiple CBD categories, after receiving their hearing screenings, these children did not experience longer delays in elapsed time from hearing screening to diagnosis compared with children with isolated HL after adjusting for preterm birth, race/ethnicity, and maternal education, adjusted OR = 1.20, 95% CI [0.65, 2.18].
Table 3.
Crude and adjusted odds ratios predicting failure to meet initial HS and Dx targets.
| HS >1 month old |
Dx >3 months old |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||
| Variable | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Sex | ||||||||
| Male | 0.86 | 0.49, 1.53 | 0.81 | [0.54, 1.20] | ||||
| Female | 1.00 | 1.00 | ||||||
| Insurance | ||||||||
| Self-pay | 2.77 | [1.09, 7.00] | 1.95 | [0.81, 4.70] | ||||
| Medicaid | 1.35 | [0.68, 2.69] | 1.28 | [0.78, 2.10] | ||||
| Private insurance | 1.00 | 1.00 | ||||||
| Race/ethnicity | ||||||||
| Black, non-Hispanic | 2.10 | [1.04, 4.24] | 2.25 | [1.21, 4.20] | ||||
| Hispanic, any race | 0.67 | [0.25, 1.81] | 0.78 | [0.44, 1.37] | ||||
| Other, non-Hispanic | 1.13 | [0.41, 3.12] | 0.83 | [0.41, 1.69] | ||||
| White, non-Hispanic | 1.00 | 1.00 | ||||||
| Maternal age (years) | ||||||||
| <19 | 0.98 | [0.25, 3.82] | 4.45 | [1.24, 16.04] | ||||
| 19-24 | 1.00 | 1.00 | ||||||
| 25-34 | 0.92 | [0.43, 1.95] | 1.15 | [0.69, 1.91] | ||||
| 35+ | 1.26 | [0.54, 2.94] | 1.01 | [0.56, 1.83] | ||||
| Maternal education (years) | ||||||||
| <12 | 1.30 | [0.59, 2.86] | 1.38 | [0.79, 2.43] | ||||
| 12 | 1.60 | [0.83, 3.08] | 1.56 | [0.98, 2.48] | ||||
| 13+ | 1.00 | 1.00 | ||||||
| Infant transferred to another facility | ||||||||
| Yes | 13.20 | [5.48, 31.63] | 7.14 | [2.18, 23.38] | 6.22 | [1.44, 26.92] | 3.12 | [0.68, 14.17] |
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Preterm birth | ||||||||
| Yes | 30.93 | [13.86, 68.99] | 31.18 | [12.98, 74.88] | 3.89 | [2.18, 6.93] | 3.24 | [1.80, 5.85] |
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| CBD category | ||||||||
| Isolated hearing loss | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 1 category | 1.64 | [0.67, 4.05] | 1.02 | [0.32, 3.23] | 1.25 | [0.70, 2.23] | 1.15 | [0.63, 2.09] |
| 2+ categories | 5.98 | [3.11, 11.65] | 4.29 | [1.81,10.20] | 3.25 | [1.67, 6.31] | 2.43 | [1.22, 4.85] |
Note. Boldface type indicates statistically significant (p < .05). CI = confidence interval.
Discussion
We have presented the results of an analysis of statewide birth defects and newborn hearing screening registries to investigate the effects of CBDs on the timeliness of newborn hearing screening and diagnosis. Although comorbid conditions have been noted as a barrier to receiving a timely diagnosis of HL, to our knowledge this is the first population-based study that quantifies the prevalence of CBDs and the extent to which they influence newborn hearing screening and diagnosis. Nearly one third of children with HL in this sample had a CBD. Having multiple CBD categories was independently associated with delays in initial hearing screening and confirmatory diagnosis, even after adjusting for maternal sociodemographic factors and infant birth status. When delays in the initial screening were taken into account, children with CBDs did not experience diagnosis delays compared with those with isolated HL.
The overall prevalence of HL in this study (0.97 per 1,000) was lower than reported in past studies (1.2–1.9 per 1,000; CDC, 2010; Morton & Nance, 2006), which may reflect incomplete screening of all infants born in the state in the first few years after initiation of universal screening in Virginia in 2001 as well as loss to follow-up of infants who failed their HS. The prevalence of CBDs in this study (31.5%) was higher than previous estimates. A higher prevalence of CBDs was expected given that we used a statewide birth defects registry that included minor anomalies and did not distinguish whether CBDs were directly related to HL (e.g., part of a syndrome or recognizable pattern). Kenna et al. (2007), for example, identified 18% of children from a total of 163 participants, all with bialleleic GJB2 mutations, with structural or developmental abnormalities in addition to the HL, although they presumed that the majority of these findings were not related to the phenotype of HL.
In this sample, 88% of all children with HL received their hearing screening by 1 month of age, but only 36% had a diagnosis by 3 months of age. A number of programmatic elements have been identified as barriers to timely and appropriate diagnosis and treatment following a failed hearing screening, including inadequate data systems to track and manage reported cases (Baroch, 2003; White, 2003); insufficient availability of centers/audiologists for follow-up (Todd, 2006); communication difficulties, including insufficient information and support between parents and providers; and a perceived sense of false positives by many providers, which translates to a less aggressive emphasis on the need for timely follow-up of infants who fail their hearing screening (Russ, Kuo, & Poulakis, 2004; White, 2003).
Smith et al. (2007) conducted a study that involved focus groups with key stakeholders, maternal interviews at the time of discharge in four hospitals, and 190 telephone surveys of mothers whose infants had not passed their hearing screening, and they identified barriers to timely diagnosis of HL in Virginia. Personal (e.g., mothers’ misconceptions about the hearing screening test, physician knowledge of and attitudes about early hearing screening), financial (e.g., lack of or inadequate insurance to cover all the costs of follow-up and intervention), and structural (e.g., a shortage of audiologists primarily due to inadequate insurance reimbursement for diagnostic services) barriers were major challenges to follow-up. In addition, Smith et al. stated that the lack of a “medical home” made it difficult for families to navigate the follow-up process in a timely manner.
The presence of other medical conditions appears to exacerbate problems with obtaining a timely screening and diagnosis in the overall population. Delays in the initial hearing screening and confirmatory diagnosis were strongly associated with infants’ birth status and apparent complexity of medical needs. The three variables that serve as proxy for complexity of medical needs (preterm birth, infant transfer from the birth hospital, and having multiple CBD categories) were independently associated with a four- to 31-fold increase in the odds of missing the hearing screening target even after adjusting for maternal sociodemographic factors. Hospital length of stay and NICU data were not available in the current study, but it is likely that a large number of these high-risk infants may have received their initial screening before discharge but after the 1-month target. Initial screening delays translated into a two- to threefold increase in odds of failing to meet the diagnosis target for children born preterm and those with multiple CBD categories. Given that the median time from initial screen to confirmatory diagnosis was over 3 months in all groups, an initial screening that occurs after 1 month of age will make it extremely difficult for families to obtain a diagnosis by 3 months of age. These findings are consistent with reports of delayed care among children and youth with special health care needs (Bitsko et al., 2009; Dusing, Skinner, & Mayer, 2004; Rosenberg et al., 2005; Tippy et al., 2005; Viner-Brown & Kim, 2005).
Better comprehensive care coordination within the context of a medical home for children with HL and CBDs may result in improvements in the timeliness of diagnosis of HL. One qualitative study explored key themes related to parents’ experiences from the time of newborn hearing screening to diagnosis and intervention; in particular, if their children had other medical or developmental problems, attention to the HL tended to take a back seat, so to speak (Russ et al., 2004). Parents reported challenges such as devastation at first learning of the problem, frustration at delays in diagnosis, and difficulty understanding providers’ communication with them. Although it may be appropriate for hearing loss to take a back seat to life-saving procedures in the NICU, such delays in diagnosis and intervention should occur only as part of a deliberate plan of care.
A strength of our study lies in the use of population-based data on birth defects and hearing loss; however, these data are not without limitations. Because this study involved a secondary analysis of statewide registry data, we were not able to directly identify causes of delays in screening and diagnosis of HL. In addition, information regarding NICU admission, length of stay, and any time periods when an infant's medical state precluded a valid screen was not available. Eleven percent of the sample had a missing/invalid initial screening date, and 12% had a missing/invalid date of diagnosis. However, the concern with missing data was tempered by the fact that cases with missing dates did not differ from those with valid dates on any key predictors. Also, we relied on ICD-9-CM codes reported to a passive birth defects registry that lacked the resources to verify diagnoses through chart review or collection of verbatim data from the medical chart. In hospital discharge data, multiple diagnosis codes are commonly reported for the same birth defect, and components of sequence or syndrome are commonly reported as separate diagnosis codes. Chart reviews have also shown inconsistencies (both over- and underreporting) with passive diagnosis code–based registries (Berman, Stapf, Sciacca, & Young, 2002; Cronk et al., 2003; Frohnert et al., 2005; Hexter et al., 1990). Despite these concerns, we believe that the exclusion of miscoded diagnosis codes, grouping individual codes into 13 general categories, and broad comparisons of one versus multiple categories of CBDs greatly minimize the potential effect on our results. For example, because we used the number of CBD categories as a proxy for the need for medical services, we were interested in the number of organ systems affected regardless of whether they were part of a common sequence. Underreporting of birth defects would result in children with more severe medical conditions being included in our isolated HL group. Similarly, cases of false-positive CBDs would result in children with less severe medical conditions being included in the CBD group. Both situations would, if anything, make our results an underestimate of the effects of CBDs on the timing of hearing screening and diagnosis.
Conclusion
Our results clearly indicate that having multiple CBDs is associated with delays in the screening and diagnosis of HL. After the initial hearing screening, infants with multiple CBDs experienced similar diagnosis delays as infants with isolated HL, which resulted in even longer diagnosis delays. Although in some cases it is possible that medical complications of infants with multiple CBDs prevented a timely initial screen, the high prevalence of HL among these infants underscores the importance of improving follow-up efforts. Even among infants with other ear anomalies, which is obviously associated with increased risk for HL, only half received their HL diagnosis by 3 months of age.
One way to improve time to HL diagnosis is to conduct full diagnostic audiologic assessments before discharge for all infants in the NICU who have failed their initial hearing screening. Although cost and staffing concerns may preclude this, at a minimum, discharge from the NICU should not occur without documentation of the hearing screening and an appointment for audiologic follow-up when indicated. Given recent technological advances, with the availability of better instruments for diagnostic auditory brainstem response in infants, however, it is not inconceivable to consider doing the diagnostic audiologic workup in infants with CBDs before their discharge from the NICU. In addition, with consistent improvement in screening techniques with a simultaneous decrease in false-positive results from audiologic screening, we need to seriously consider a change in terminology from refer to fail, which may influence the number of infants lost to follow-up assessment by conveying a stronger sense of the need for further testing and evaluation. Better coordination of HL assessments into the treatment plan for children with complex medical conditions will help decrease diagnosis delays and provide earlier opportunities for intervention, and it has the potential to improve developmental outcomes for the one third of children with HL who have CBDs.
Acknowledgments
This research was supported in part by grants from the Association of University Centers on Disability Cooperative Agreement with the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (RTOI 2007-01-10), and the Health Resources and Services Administration (H18MC00052-14-01). We acknowledge the work of Andrea Alvarez, who assisted with the identification of cases of hearing loss and extracting data from the newborn hearing screening data system. We thank the Pediatrics Screening and Genetics Services staff at the Virginia Department of Health, who collect and maintain the birth defects and newborn hearing screening registry data used in the research reported in this article.
References
- Baroch KA. Universal newborn hearing screening: Fine-tuning the process. Current Opinion in Otolaryngology & Head and Neck Surgery. 2003;11:424–427. doi: 10.1097/00020840-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Berman MF, Stapf C, Sciacca RR, Young WL. Use of ICD-9 coding for estimating the occurrence of cerebrovascular malformations. American Journal of Neuroradiology. 2002;23:700–705. [PMC free article] [PubMed] [Google Scholar]
- Bitsko RH, Visser SN, Schieve LA, Ross DS, Thurman DJ, Perou R. Unmet health care needs among CSHCN with neurologic conditions. Pediatrics. 2009;124(Suppl. 4):S343–S351. doi: 10.1542/peds.2009-1255D. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Infants tested for hearing loss, United States, 1999–2001. Morbidity and Mortality Weekly Report. 2003;52:981–984. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment: United States, 2003. Morbidity and Mortality Weekly Report. 2004;53:57–59. [Errata in Morbidity and Mortality Weekly Report, 2004, 55, 881.]
- Centers for Disease Control and Prevention Summary of diagnosis and loss to follow-up/loss to documentation (Year 2007) 2010 Retrieved from www.cdc.gov/ncbddd/hearingloss/data.html.
- Cronk CE, Malloy ME, Pelech AN, Miller RE, Meyer SA, Cowell M, McCarver DG. Completeness of state administrative databases for surveillance of congenital heart disease. Birth Defects Research: Part A: Clinical and Molecular Teratology. 2003;67:597–603. doi: 10.1002/bdra.10107. [DOI] [PubMed] [Google Scholar]
- Dusing SC, Skinner AC, Mayer ML. Unmet need for therapy services, assistive devices, and related services: Data from the National Survey of Children With Special Health Care Needs. Ambulatory Pediatrics. 2004;4:448–454. doi: 10.1367/A03-202R1.1. [DOI] [PubMed] [Google Scholar]
- Frohnert BK, Lussky RC, Alms MA, Mendelsohn NJ, Symonik DM, Falken MC. Validity of hospital discharge data for identifying infants with cardiac defects. Journal of Perinatology. 2005;25:737–742. doi: 10.1038/sj.jp.7211382. [DOI] [PubMed] [Google Scholar]
- Harrison M, Roush J. Age of suspicion, identification, and intervention for infants and young children with hearing loss: A national study. Ear and Hearing. 1996;17:55–62. doi: 10.1097/00003446-199602000-00007. [DOI] [PubMed] [Google Scholar]
- Harrison M, Roush J, Wallace J. Trends in age of identification and intervention in infants with hearing loss. Ear and Hearing. 2003;24:89–95. doi: 10.1097/01.AUD.0000051749.40991.1F. [DOI] [PubMed] [Google Scholar]
- Hayes D. State programs for universal newborn hearing screening. Pediatric Clinics of North America. 1999;46:89–94. doi: 10.1016/s0031-3955(05)70083-3. [DOI] [PubMed] [Google Scholar]
- Hexter AC, Harris JA, Roeper P, Coren LA, Krueger P, Gant D. Evaluation of the hospital discharge diagnoses index and the birth certificate as sources of information on birth defects. Public Health Reports. 1990;105:296–307. [PMC free article] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- Kenna MA, Rehm HL, Robson CD, Frangulov A, McCallum J, Yaeger D, Krantz ID. Additional clinical manifestations in children with sensorineural hearing loss and biallelic GJB2 mutations: Who should be offered GJB2 testing? American Journal of Medical Genetics Part A. 2007;143A:1560–1566. doi: 10.1002/ajmg.a.31706. [DOI] [PubMed] [Google Scholar]
- Kennedy CR, McCann DC, Campbell MJ, Law CM, Mullee M, Petrou S, Stevenson J. Language ability after early detection of permanent childhood hearing impairment. New England Journal of Medicine. 2006;354:2131–2141. doi: 10.1056/NEJMoa054915. [DOI] [PubMed] [Google Scholar]
- Kirby RS. Analytical resources for assessment of clinical genetics services in public health: Current status and future prospects. Teratology. 2000;61:9–16. doi: 10.1002/(SICI)1096-9926(200001/02)61:1/2<9::AID-TERA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Mason CA, Tu S. Data linkage using probabilistic decision rules: A primer. Birth Defects Research: Part A: Clinical and Molecular Teratology. 2008;82:812–821. doi: 10.1002/bdra.20510. [DOI] [PubMed] [Google Scholar]
- Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American Journal of Epidemiology. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- Morton CC, Nance WE. Newborn hearing screening—A silent revolution. New England Journal of Medicine. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- Robinshaw HM. Early intervention for hearing impairment: Differences in the timing of communicative and linguistic development. British Journal of Audiology. 1995;29:315–334. doi: 10.3109/03005369509076750. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Onufer C, Clark G, Wilkin T, Rankin K, Gupta K. The need for care coordination among children with special health care needs in Illinois. Maternal and Child Health Journal. 2005;9(Suppl. 2):S41–S47. doi: 10.1007/s10995-005-3857-y. [DOI] [PubMed] [Google Scholar]
- Russ SA, Kuo AA, Poulakis Z. Qualitative analysis of parents’ experience with early detection of hearing loss. Archives of Disease in Childhood. 2004;89:353–358. doi: 10.1136/adc.2002.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S, Besculides M, Saltzman A, Ireys H, White KR, Forsman I. Evaluation of the universal newborn hearing screening and intervention program. Pediatrics. 2010;126(Suppl.):S19–S27. doi: 10.1542/peds.2010-0354F. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Martinez A, Eisenberg L, Christensen E, Grimes A, Hu J. Newborn hearing screening speeds diagnosis and access to intervention by 20–25 months. Journal of the American Academy of Audiology. 2009;20:49–57. doi: 10.3766/jaaa.20.1.5. [DOI] [PubMed] [Google Scholar]
- Smith LR, Layton C, Ramirez C, Hendershot T, Dai L, Hershey J. An evaluation of loss to follow-up in state EHDI Programs: Findings from the Virginia EHDI program. 2007 Retrieved from www.infanthearing.org/states/virginia/
- Tippy K, Meyer K, Aronson R, Wall T. Characteristics of coordinated ongoing comprehensive care within a medical home in Maine. Maternal and Child Health Journal. 2005;9(2 Suppl):S13–S21. doi: 10.1007/s10995-005-4747-z. [DOI] [PubMed] [Google Scholar]
- Todd NW. Universal newborn hearing screening follow-up in two Georgia populations: Newborn, mother and system correlates. International Journal of Pediatric Otorhinolaryngology. 2006;70:807–815. doi: 10.1016/j.ijporl.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Viner-Brown SI, Kim HK. Impact of caring for children with special health care needs on the family: Rhode Island's experience. Maternal and Child Health Journal. 2005;9(2 Suppl):S59–S66. doi: 10.1007/s10995-005-4483-4. [DOI] [PubMed] [Google Scholar]
- Virginia Department of Health Virginia Congenital Anomalies Reporting and Education System: Birth defect surveillance data 1989–1998. 2003 Retrieved from www.vahealth.org/vnsp/documents/docs2006/PDF/VaCARES_Report_1989-1998.pdf.
- Virginia Department of Health Protocols for diagnostic audiological assessment: Follow-up for newborn hearing screening. 2004 Retrieved from www.vahealth.org/hearing/documents/2005/Audiologicprotocolfinal904.pdf.
- Vohr BR, Moore PE, Tucker RJ. Impact of family health insurance and other environmental factors on universal hearing screen program effectiveness. Journal of Perinatology. 2002;22:380–385. doi: 10.1038/sj.jp.7210750. [DOI] [PubMed] [Google Scholar]
- White KR. The current status of EHDI programs in the United States. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:79–88. doi: 10.1002/mrdd.10063. [DOI] [PubMed] [Google Scholar]
- White KR. Early hearing detection and intervention programs: Opportunities for genetic services. American Journal of Medical Genetics: Part A. 2004;130A:29–36. doi: 10.1002/ajmg.a.30048. [DOI] [PubMed] [Google Scholar]
- White KR, Forsman I, Eichwald J, Muñoz K. The evolution of early hearing detection and intervention programs in the United States. Seminars in Perinatology. 2010;34:170–179. doi: 10.1053/j.semperi.2009.12.009. [DOI] [PubMed] [Google Scholar]
- World Health Organization . International Classification of Diseases, Ninth Revision, Clinical Modification. Author; Geneva, Switzerland: 1977. [Google Scholar]
- Yoshinaga-Itano C. Early intervention after universal neonatal hearing screening: Impact on outcomes. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:252–266. doi: 10.1002/mrdd.10088. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Coulter D, Thomson V. The Colorado Newborn Hearing Screening Project: The effects on speech and language development for children with hearing loss. Journal of Perinatology. 2000;20(8, Pt. 2):S132–S137. doi: 10.1038/sj.jp.7200438. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Coulter D, Thomson V. Developmental outcomes of children with hearing loss born in hospitals with and without universal newborn hearing screening programs. Seminars in Neonatology. 2001;6:521–529. doi: 10.1053/siny.2001.0075. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Sedey A, Coulter D, Mehl A. Language of early- and later- identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]