Abstract
Maintenance of CTL-, Th1-, and NK cell-mediated type-1 immunity is essential for effective antitumor responses. Unexpectedly, we observed that the critical soluble mediators of type-1 immune effector cells, IFNγ and TNFα, synergize in the induction of cyclooxygenase 2 (COX2), the key enzyme in prostaglandin (PG)E2 synthesis, and the subsequent hyperactivation of myeloid-derived suppressor cells (MDSCs) within the tumor microenvironment (TME) of ovarian cancer patients. MDSC hyperactivation by type-1 immunity and the resultant overexpression of indoleamine 2,3-dioxygenase (IDO), inducible nitric oxide synthase (iNOS/NOS2), IL10, and additional COX2 result in strong feedback suppression of type-1 immune responses. This paradoxical immune-suppression driven by type-1 immune cell activation was found to depend on the synergistic action of IFNγ and TNFα, and could not be reproduced by either of these factors alone. Importantly from a therapeutic standpoint, this negative feedback limiting type-1 responses could be eliminated by COX2 blockade, allowing amplification of type-1 immunity in the ovarian cancer TME. Our data demonstrate a new mechanism underlying the self-limiting nature of type-1 immunity in the human tumor microenvironment, driven by the synergistic induction of COX2 by IFNγ and TNFα, and provide rationale for targeting the COX2-PGE2 axis to enhance the effectiveness of cancer immunotherapies.
Keywords: COX2, prostaglandins, myeloid-derived suppressor cells, ovarian cancer, tumor microenvironment
Introduction
Type-1 immunity, characterized by the development of cytotoxic CD8+ T cell (CTL), type-1 helper CD4+ T cell (Th1), and natural killer (NK) cell responses producing IFNγ and TNFα, have been shown to be essential for effective antitumor immunity (1–3). Driven by the strong positive prognostic relevance of intratumoral type-1 lymphocytes to clinical outcome, current cancer immunotherapies often focus on enhancing the accumulation, activation, and function of such immune effector cells within the tumor microenvironment (TME) (4). Unfortunately, the clinical success of these approaches is often limited to only a proportion of patients (5, 6).
The immune-suppressive nature of the TME has emerged as a critical regulator of antitumor immune responses, and is increasingly recognized as a major barrier to the effectiveness of cancer immunotherapies (7). Prostaglandin (PG)E2 and its key synthesizing enzyme cyclooxygenase 2 (COX2) have been implicated in this regard given the ability of COX2 blockade to enhance the effectiveness of cancer vaccination (8, 9). While the COX2-PGE2 axis has been described to have negative regulatory effects on type-1 effector cell priming, activity, and trafficking in the setting of chronic inflammation (10), the mechanism by which COX2-interference promotes type-1-directed immunotherapy within the human TME remains unclear.
The recruitment and induction of suppressive cell populations within the TME, particularly myeloid-derived suppressor cells (MDSCs) (11), has also emerged as a major determinant of the outcome of antitumor immunotherapy. Characterized in human cancer by a lineage negative CD11b+CD33+HLA-DRlow/− phenotype, MDSCs have been demonstrated to potently inhibit both innate and adaptive immune responses through such mechanisms as inducible nitric oxide synthase (iNOS/NOS2), indoleamine 2,3-dioxygenase (IDO), and IL10 (12–15). While the suppressive effect of MDSCs on immune effector cells has been extensively reported, understanding of the reciprocal interactions between effector cells and MDSCs remains limited.
Here, we demonstrate that suppressive counter-regulation is a direct consequence of type-1 immune activation within the human TME. This counter-regulation results from the IFNγ/TNFα-dependent hyperactivation of MDSCs, driven by amplification of COX2-PGE2 feedback. Blockade of the COX2-PGE2 axis eliminated this IFNγ/TNFα-driven negative feedback within the human TME, providing a rationale for designing interventions that block COX2-PGE2 in cancer immunotherapy.
Materials and Methods
Patients
Human ovarian cancer (OvCa) ascites cells were obtained intraoperatively from previously untreated patients with primarily advanced (stage III or IV) epithelial OvCa undergoing primary surgical debulking for clinical staging (see Supplemental Table S1 for patient/tumor characteristics). All specimens were provided under protocols approved by the University of Pittsburgh or Roswell Park Cancer Institute Institutional Review Boards (UPCI07-058 and CIC02-15) in accordance with the World Medical Association’s Declaration of Helsinki, and written informed consent was obtained prior to any specimen collection. Human OvCa ascites obtained from the University of Pittsburgh Cancer Institute (UPCI07-058) were used in the isolation of cancer-associated CD11b+ cells (MDSCs), NK cells, and T cells. Human OvCa ascites obtained from the Roswell Park Cancer Institute (CIC02-15) were used in the isolation of bulk OvCa primary cells for mRNA analysis.
Isolation of OvCa ascites cells
Primary OvCa ascites cells were harvested from bulk ascites by centrifugation. When indicated, bulk OvCa ascites cells were stimulated with combinations of IL18 (200 ng/ml; MBL International), IFNα (1000 IU/ml; Intron A, IFN-α-2b; Schering-Plough), IL12 (5 ng/ml; PeproTech), IL2 (250 IU/ml; Chiron), monoclonal antibody (mAb) to CD3 (clone OKT3; 1 μg/ml; eBioscience), and CD3/CD28 Human T cell-Activator Dynabeads (5 μl/ml; Invitrogen). NK cells were depleted from bulk OvCa ascites cells by CD56 positive magnetic selection (StemCell Technologies). MDSCs were depleted or isolated using CD11b magnetic selection (Miltenyi Biotech). This procedure has been previously shown (16, 17) to be highly effective in isolating > 95% pure CD11b+ cells uniformly expressing the CD11b+CD33+CD14+HLA-DRlow/− monocytic MDSC phenotype from human OvCa ascites cells (16, 17) (also CD34+CD80−CD83−DC-SIGN−ILT2-4+ expressing ARG1, NOS2, IDO1, IL10, COX2, and IL4Rα; please see Results and Supplemental Material). Control CD11b+ cells were isolated from healthy donor peripheral blood using the same method.
Isolation of NK cells and CD8+ T cells
Peripheral blood from healthy donors was harvested by venipuncture under IRB-approved protocols. NK cells (CD56+CD3−) and naïve CD8+ T cells (CD8+CD45RA+CCR7highCD45RO−CD56−CD57−) were isolated by negative magnetic selection (> 95% pure in both cases) using the EasySep system (StemCell Technologies), according to the manufacturer’s protocol.
Cell culture
Bulk OvCa ascites cells, MDSCs, NK cells, and T cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) containing 10% fetal bovine serum and 1% L-glutamine and penicillin/streptomycin (all from Gibco, Invitrogen).
MDSC activation
For NK cell activation of MDSCs, NK cells (0.5 × 105 cells/well) were cocultured with MDSCs (1 × 105 cells/well) in 96-well plates in the presence of IL18 (200 ng/ml) and IFNα (1000 IU/ml). When indicated, soluble decoy receptors to IFNγ (sIFNγR1; 10 μg/ml; R&D Systems) and TNFα (sTNFR1; 1 μg/ml; R&D Systems) were added to cultures at coculture initiation. For CD8+ T cell activation of MDSCs, T cells (0.5 × 105 cells/well) were cocultured with MDSCs (1×105 cells/well) in 96-well plates in the presence of CD3 mAb (1 μg/ml) and IL12 (5 ng/ml). As an alternative method of MDSC activation, MDSCs were cultured with IFNγ (1000 IU/ml, unless otherwise noted; Miltenyi Biotech) and TNFα (50 ng/ml, unless otherwise noted; Miltenyi Biotech). After MDSC activation by coculture with lymphocytes (24 h activation for assessment of mRNA expression and 36 h activation for assessment of intracellular protein staining/ELISA), NK cells or CD8+ T cells were removed by CD56 or CD8 positive magnetic selection, respectively, and MDSCs were subsequently assessed for mRNA expression, intracellular protein staining, and/or ELISA analysis of supernatants.
CD8+ T cell suppression
Naïve CD8+ T cells (1 × 105 cells/well) labeled with CFSE (Invitrogen; labeled according to the manufacturer’s protocol) were stimulated with CD3/CD28 Human T cell-Activator Dynabeads (5 μl/ml; Invitrogen) in the presence or absence of MDSCs (0.25 × 105 cells/well) and/or IL18/IFNα-activated NK cells (0.25 × 105 cells/well) in 96-well plates. As an alternative method for CD8+ T cell expansion, CFSE-labeled naïve CD8+ T cells (1 × 105 cells/well) were stimulated with staphylococcal enterotoxin B-pulsed mature monocyte-derived DCs (1 × 104 cells/well; matured for 48 h with 50 ng/ml TNFα), as previously described (18). When indicated, cells were co-cultured in the additional presence of small-molecule inhibitors or blocking antibodies against suppressive factors. On day 4–6, expanded CD8+ T cells were analyzed for proliferation via CFSE dilution and intracellular granzyme B expression. The following inhibitors/blocking antibodies were used in this study: celecoxib (20 μM; BioVision), 1-methyl-DL-tryptophan (1 mM; Sigma-Aldrich), L-NMMA (200 μM; Cayman Chemical), IL10 mAb (clone 25209; 1 μg/ml; R&D Systems), and nor-NOHA (200 μM; Cayman Chemical). The concentrations used did not affect viability in cell cultures, as confirmed by live cell counts.
Flow cytometry
Cell surface and intracellular immunostaining analyses were performed using an Accuri C6 Flow Cytometer. NK cells and T cells were stained with the dye-conjugated mouse mAbs to human CD56-PE-Cy5 (Beckman Coulter), CD3-PE (eBioscience), CCR7-FITC (R&D Systems), granzyme B-PE (Invitrogen), and CD16-FITC, CD8-PE-Cy5, CD45RA-FITC, CD45RO-PE, and CD57-FITC (BD Biosciences). MDSCs were stained for CD11b-FITC, CD14-PE, CD33-APC, CD34-PE-Cy5, CD11c-PE, HLA-DR-PE, DC-SIGN-FITC, CD80-FITC, CD86-FITC, and CD83-PE (BD Biosciences and eBioscience), as well as IDO-A488 (R&D Systems), NOS2-PE (Santa Cruz Biotechnology), and COX1-FITC/COX2-PE (BD Biosciences). The corresponding mouse antibody isotype controls IgG1-FITC, IgG2b-FITC, IgG1-PE, IgG2a-PE, IgG1-PE-Cy5, IgG1-APC, and IgG1-A488 (BD Biosciences) were used, as appropriate. Before staining, the cells were treated for 20 min at 4°C in PBS buffer containing 2% human serum, 0.5% BSA, 0.1% NaN3, and 1 μg/ml of mouse IgG (Sigma-Aldrich) to block non-specific binding. Cell permeabilization for intracellular staining was performed using the Foxp3 Fix/Perm Buffer Set (eBioscience), according to the manufacturer’s protocol. Cells were stained for 40 min at 4°C followed by washing with PBS buffer containing 0.5% BSA and 0.1% NaN3, then fixed and stored in 4% paraformaldehyde until analysis.
ELISA
Supernatants from 36 h cocultures of NK cells and MDSCs or cultures of MDSCs activated by IFNγ/TNFα were analyzed for IL10 by indirect sandwich ELISA using specific matched primary and biotinylated-secondary antibody pairs (R&D Systems), as previously described (16).
Quantitative real-time PCR
Analysis of mRNA expression was performed using the StepOne Plus System (Applied Biosystems), as previously described (17), using inventoried primer/probe sets. Expression of CD8α, NKG2D, IFNγ, and COX2 were assessed in bulk OvCa ascites cells immediately after harvesting. Expression of IFNγ, TNFα, IDO1, NOS2, IL10, and/or COX2 was assessed 24 h following bulk OvCa ascites cell activation, or following MDSC activation with type-1-activated lymphocytes or IFNγ/TNFα. The expression of each gene was normalized to HPRT1 and expressed as fold increase (2−ΔCT), where ΔCT = CT (target gene) − CT (HPRT1).
Statistics
Pearson correlations between type-1 immune markers and MDSC markers were calculated on logarithmically transformed data. Comparisons of continuous variables between groups were conducted using unpaired t tests (two-tailed) and one-way and two-way ANOVA, where appropriate. Significance was judged at an α of 0.05. Where indicated, data from multiple different patients and control donors are recorded as means (± SD) from n different donors, described in the figure legends. Data from representative experiments are presented as means (± SD) from triplicate cultures, and were confirmed in multiple independent experiments, described in the figure legends.
Results
Activated type-1 immune cells promote suppression
Given the known pleiotropic suppressive effects of the COX2-PGE2 axis on type-1 immunity (10) and the documented ability of tumor-associated COX2 blockade to skew toward a type-1 cytokine response (19), we anticipated a negative correlation between COX2 and the local expression of type-1 immune markers within the human TME. Unexpectedly, however, our analysis of bulk ascites cell samples from 15 late-stage ovarian cancer (OvCa) patients, used as a model for the human TME, instead demonstrated a positive correlation between COX2 and the local expression of type-1 immune markers, including CD8α, NKG2D, a marker of activated CTLs and NK cells, and IFNγ, the prototypical cytokine of type-1 immunity (Fig. 1A). These findings suggest a surprising positive correlation between type-1 immunity and local immune-suppressive events within the TME.
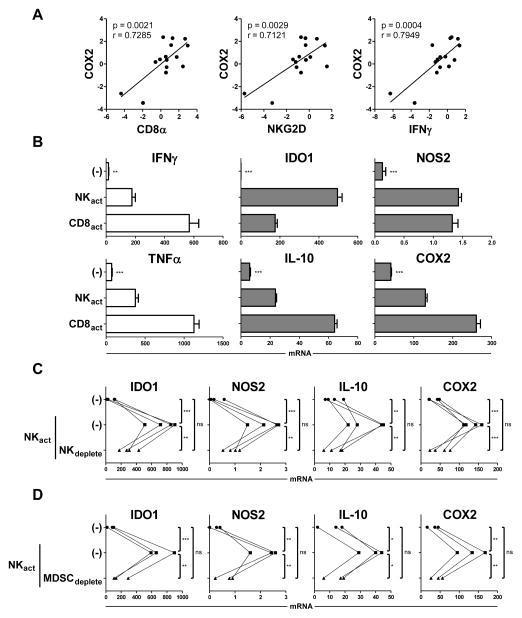
Figure 1.
Type-1 activation of immune cells within the bulk ovarian cancer tumor microenvironment enhances local MDSC-mediated expression of suppressive factors. (A) Bulk ascites cells freshly-isolated from ovarian cancer (OvCa) patients were lysed and analyzed for expression of type-1 immune markers (CD8α, NKG2D, and IFNγ) and COX2. Data are expressed as ratios between the expression of individual genes and HPRT1, and represent 15 independent patients. (B) Bulk OvCa ascites cells were cultured for 24 h in the absence or presence of the NK cell-activating (NKact) stimuli IL18/IFNα or the CD8+ T cell–activating (CD8act) stimuli anti-CD3/IL12, and analyzed for expression of IFNγ, TNFα, IDO1, NOS2, IL10, and COX2. Data are expressed as ratios between the expression of individual genes and HPRT1, and shown as the mean expression (± SD) of triplicate cultures. Data represent one of three independent experiments, all yielding similar results. (C) Bulk OvCa ascites cells or ascites cells depleted of CD56+ NK cells (NKdeplete) were cultured for 24 h in the absence or presence of the NK cell-activating (NKact) stimuli IL18/IFNα, and analyzed for expression of IDO1, NOS2, IL10, and COX2. Data are expressed as ratios between the expression of individual genes and HPRT1, and represent 4 independent patients. (D) Bulk OvCa ascites cells or ascites cells depleted of MDSCs (MDSCdeplete) were cultured for 24 h in the absence or presence of the NK cell–activating (NKact) stimuli IL18/IFNα, and analyzed for expression of IDO1, NOS2, IL10, and COX2. Data are expressed as ratios between the expression of individual genes and HPRT1, and represent 3 independent patients. ***P < 0.001, ** P < 0.01, * P < 0.05, ns: P > 0.05 compared to indicated group or compared to all groups when not specifically indicated.
In order to directly evaluate the effect of type-1 immune activation on the larger TME, we stimulated bulk OvCa ascites cells with factors known to induce type-1 immune activation (20–22), including IL18/IFNα and anti-CD3/IL12 to activate NK cells and CD8+ T cells, respectively. While, as expected, these stimuli induced high expression of the signature type-1 cytokines IFNγ and TNFα (Fig. 1B, left) implicated heavily in the promotion of antitumor responses (23, 24), the same type-1–driving factors also induced significant expression of the known suppressive factors IDO1, NOS2, IL10, and COX2 (Fig. 1B, middle and right). This effect was a general consequence of type-1 activation within the bulk TME, as a similar enhancement in the expression of these suppressive factors was observed upon ascites cell treatment with several other known type-1–driving NK cell and T cell stimuli (Supplemental Fig. S1).
Using IL18/IFNα-driven NK cell activation as a model of type-1 immune cell activation, we further found that this enhancement in suppressive factors induced by type-1-driving stimuli was indeed due to lymphocyte activation within the TME, as prior NK cell depletion abrogated this effect (Fig. 1C). No influence on suppressive factor expression was observed after NK cell depletion in the absence of activation stimuli (data not shown). Given that MDSCs are known to be potent producers of these suppressive factors within the human ovarian cancer TME (16, 17), we hypothesized that these cells may be involved in this phenomenon. Depleting MDSCs from the bulk ascites cell population (CD11b+CD33+CD14+HLA-DRlow/− MDSCs previously shown to be highly enriched in human ovarian cancer ascites; see Methods and Supplemental Fig. S2 for MDSC isolation approach and characterization) prior to treatment with NK cell–activating stimuli resulted in a strong reduction in the expression of suppressive factors induced by NK cell activation (Fig. 1D). Collectively, these results suggest that type-1–activated lymphocytes may promote immune-suppressive molecules within the TME through the augmentation of MDSC activity.
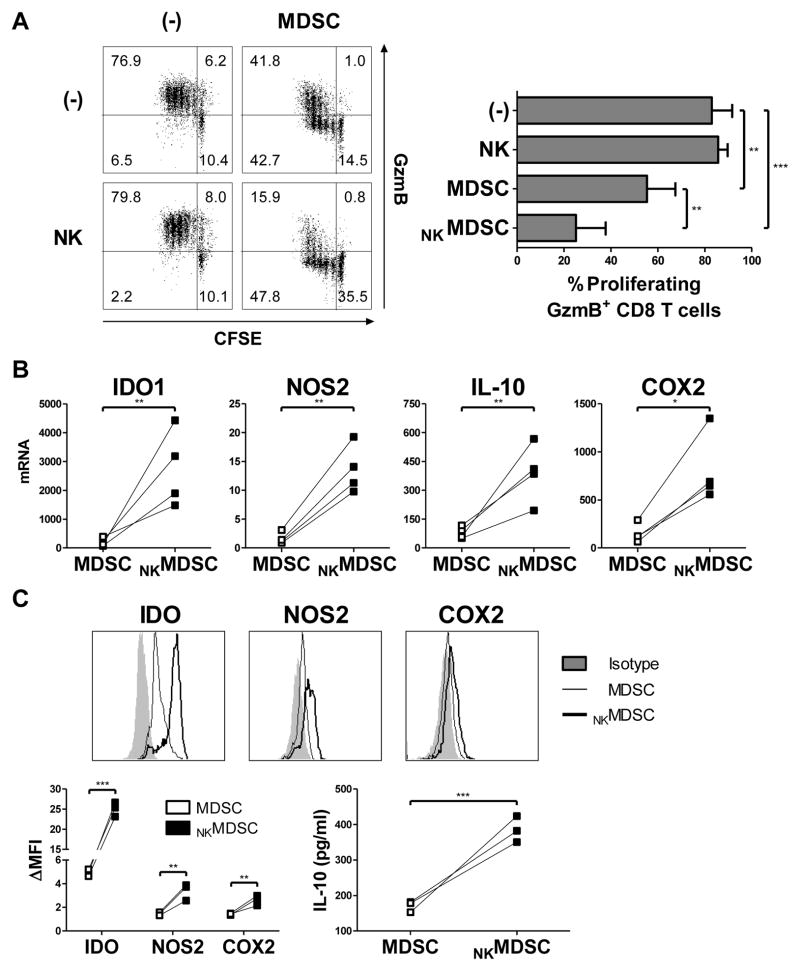
Indeed, direct coculture of activated NK cells and isolated MDSCs significantly enhanced the ability of these MDSCs to suppress the proliferation and granzyme B acquisition of naïve CD8+ T cells driven by CD3/CD28 activation (Fig. 2A, Supplemental Fig. S3), an effect that was not observed with NK cell–activated control CD11b+ myeloid cells isolated from the peripheral blood of healthy donors (Supplemental Fig. S4A). This heightened MDSC suppressive activity was accompanied by enhanced MDSC expression of IDO1, NOS2, IL10, and COX2 mRNA (Fig. 2B) and protein (Fig. 2C). A similar augmentation of MDSC suppressive factors was likewise observed following MDSC coculture with type-1–activated CD8+ T cells (Supplemental Fig. S4B). No suppression was observed by activated NK cells alone (Fig. 2A), which did not express IDO1 and COX2 and showed only marginal expressionof NOS2 and IL10 (data not shown). These data indicate that MDSC suppressive activity is enhanced by direct interaction with type-1–activated lymphocytes.
Figure 2.
Activated type-1 immune effector cells enhance MDSC suppressive activity. (A) Percentage of proliferating, granzyme B (GzmB) positive naïve CD8+ T cells following 4 d activation with anti-CD3/CD28 antibodies in the absence or presence of OvCa ascites-isolated MDSCs and/or IL18/IFNα-activated NK cells (NKMDSC), measured by CFSE dilution and intracellular GzmB staining presented in representative cultures (left) or as the mean (± SD) of 5 independent patients (right). (B–C) Expression of IDO1, NOS2, IL10, and COX2 assessed by mRNA (B) and protein (C) levels in OvCa ascites-isolated MDSCs cultured (24 h for mRNA; 36 h for protein) with or without IL18/IFNα-activated NK cells (NKMDSC). Data of mRNA levels are expressed as ratios between the expression of individual genes and HPRT1, and represent 4 independent patients. Data of protein levels are shown in representative histograms (top) or represented as the fold change of the mean fluorescence intensity (ΔMFI) over the isotype control or as levels detected by specific ELISA in 36 h supernatants across 3 independent patients. *** P < 0.001, ** P < 0.01, * P < 0.05 compared to indicated group.
IFNγ and TNFα key to MDSC suppressive activity
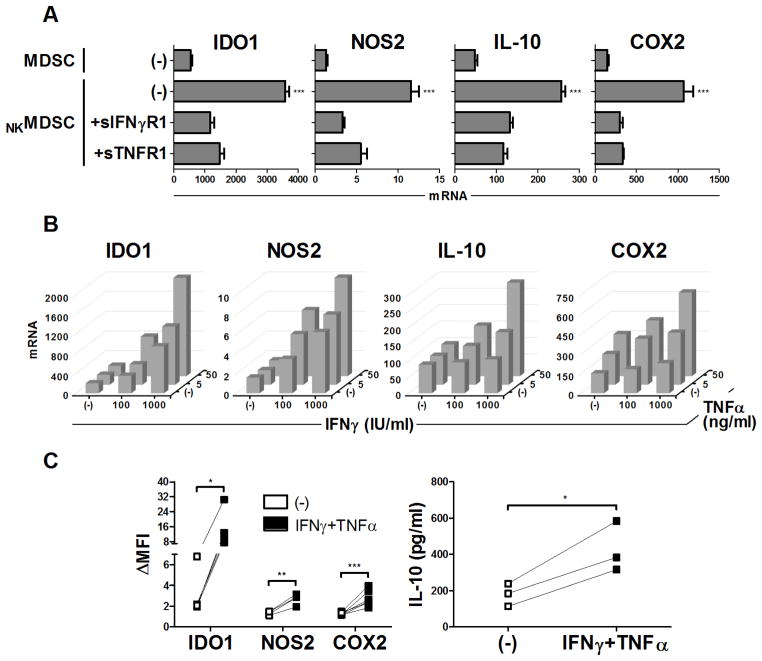
Blockade of IFNγ and TNFα (using soluble decoy receptors) in MDSC cocultures with activated NK cells counteracted the MDSC upregulation of multiple suppressive factors (Fig. 3A), revealing the key synergistic role of these cytokines in driving the enhanced MDSC suppressive activity. Indeed, treatment of MDSCs with exogenous IFNγ and TNFα mirrored the enhanced expression of these suppressive factors seen after coculture with type-1–activated lymphocytes, again at both the mRNA (Fig. 3B) and protein (Fig. 3C) levels.
Figure 3.
IFNγ and TNFα are critical mediators of MDSC hyper-activation induced by type-1 immune effector cells. (A) Expression of IDO1, NOS2, IL10, and COX2 in OvCa ascites-isolated MDSCs cultured for 24 h with or without IL18/IFNα-activated NK cells (NKMDSC), in the additional presence or absence of soluble IFNγ (sIFNγR1) or TNF (sTNFR1) decoy receptors. Data are expressed as ratios between the expression of individual genes and HPRT1, and shown as the mean expression (± SD) of triplicate cultures. Data represent one of three independent experiments, all yielding similar results. (B–C) Expression of IDO1, NOS2, IL10, and COX2 assessed by mRNA (B) and protein (C) levels in OvCa ascites-isolated MDSCs cultured (24 h for mRNA; 36 h for protein) with or without varying concentrations of IFNγ and TNFα. Data of mRNA levels are expressed as ratios between the expression of individual genes and HPRT1, and represent one of three independent experiments, all yielding similar results. Data of protein levels are represented as the fold change of the mean fluorescence intensity (ΔMFI) over the isotype control, and represent n independent patients (n = 5 patients for IDO1, n = 4 for NOS2, n = 6 for COX2), or as levels detected by specific ELISA in 36 h supernatants, representing 3 independent patients. *** P < 0.001, ** P < 0.01, * P < 0.05 compared to indicated group or compared to all groups when not specifically indicated.
Enhanced MDSC activity requires COX2-PGE2
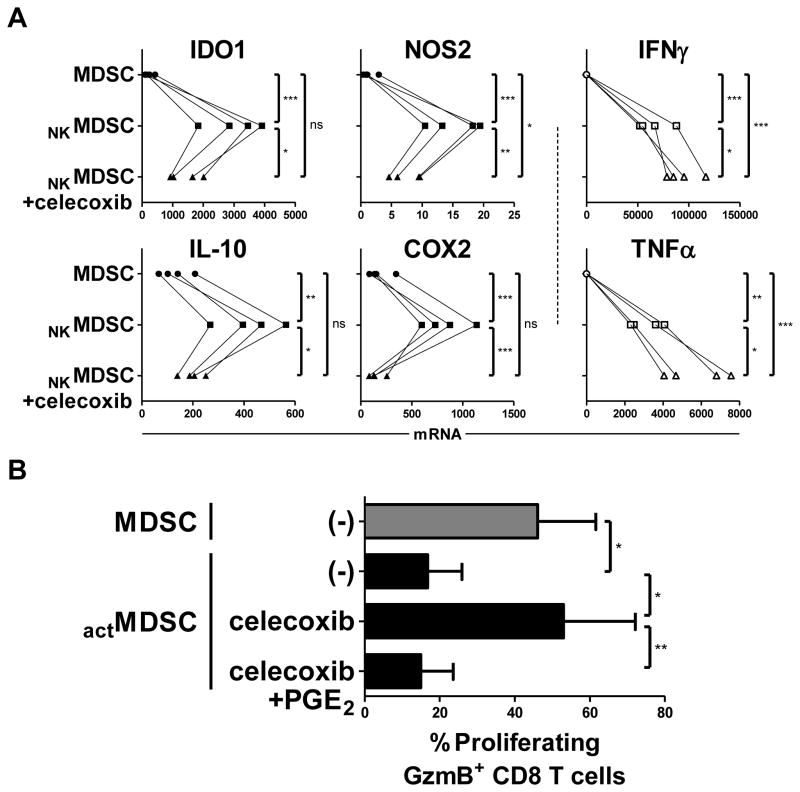
Although individual inhibition of IDO, NOS, IL10, and arginase partially reversed the activation-induced MDSC suppression of CD8+ T cell proliferation and granzyme B acquisition (Supplemental Fig. S5), sole inhibition of COX2 was significantly better than inhibition of any of the other pathways tested, even when blockade of all of these other pathways were combined. Closer investigation of NK cell–activated MDSCs revealed that COX2 blockade coordinately antagonized the expression of multiple other suppressive factors, as well as its own expression (Fig. 4A, left and middle). Whereas PGE2, the product of COX2, has been described to have direct suppressive effects (10), these results suggest that the superior ability of COX2 inhibition in reversing MDSC-mediated immune suppression is also likely to act through the regulation of other suppressive factors. Across cells derived from multiple patients, celecoxib-mediated COX2 blockade was capable of completely reversing the enhanced suppressive ability of hyperactivated MDSCs, which was restored upon exogenous PGE2 supplementation (Fig. 4B, Supplemental Fig. S6). Notably, COX2 blockade was also capable of increasing the expression of IFNγ and TNFα in lymphocyte-MDSC cocultures (Fig. 4A, right), indicating the differential COX2/PGE2-mediated modulation of stimulatory and suppressive factors.
Figure 4.
Type-1 effector cell-driven hyperactivation of MDSCs requires the intact COX2-PGE2 axis. (A) Expression of IDO1, NOS2, IL10, COX2, IFNγ, and TNFα in OvCa ascites-isolated MDSCs cultured for 24 h with or without IL18/IFNα-activated NK cells (NKMDSC), in the additional presence or absence of celecoxib (COX2 inhibitor). Data are expressed as ratios between the expression of individual genes and HPRT1, and represent 4 independent patients. (B) Percentage of proliferating GzmB+ naïve CD8+ T cells following 4 d activation with anti-CD3/CD28 antibodies in the presence of resting or IFNγ/TNFα-activated OvCa ascites-isolated MDSCs (actMDSC), in the additional presence of celecoxib (COX2 inhibitor) and/or exogenous PGE2. Data represent the mean (± SD) of 4 independent patients. *** P < 0.001, ** P < 0.01, * P < 0.05, ns: P > 0.05 compared to indicated group.
Discussion
The current data demonstrate that type-1–activated immune cells and the key cytokines mediating their antitumor activities, IFNγ and TNFα (23, 24), directly promote counter-regulatory suppressive events within the human TME through the COX2/PGE2-driven amplification of MDSC activity and its coordinated enhancement of multiple suppressive pathways. Our data help to reconcile reports demonstrating both antitumor and protumor activities of type-1 cytokines (24–26), and identify the COX2-PGE2 pathway as a key target for the therapeutic separation of opposing stimulatory and suppressive outcomes induced by type-1 immunity within the human TME.
Although many suppressive pathways, including the IDO, NOS, and IL10 mechanisms described here, have been implicated in tumor-associated immune dysfunction, these data indicate that upregulation of these suppressive pathways can be a direct consequence of type-1 immune responses within the human TME. The upregulation of many of these suppressive factors have long been associated with inflammatory mediators such as IFNγ in physiologic settings, including for homeostatic T cell contraction following infection (27), control of autoimmune responses (28–30), and immunologic tolerance during pregnancy (31). These findings suggest that counter-regulatory suppression induced by type-1 immunity may be a mechanism of normal endogenous immune control to prevent overactive responses. However, in the setting of cancer, this mechanism may be co-opted in the TME to support tumor progression. Indeed, recent clinical evidence in melanoma demonstrated that an enhanced intratumoral type-1 immune signature correlated with clinical response to ipilimumab, but was also associated with expression of IDO1 (32), potentially limiting the magnitude of these responses. We identify here that the type-1 immune-mediated hyperactivation of MDSCs, suppressive cells which are profoundly enriched within the human TME (33), is likely to play an important role in this process.
Activation of MDSCs by pro-inflammatory factors has been described (34–36), but here we find that type-1 inflammatory mediators potentiate multiple MDSC suppressive pathways, using the COX2-PGE2 axis as the critical intermediate step. COX2/PGE2 has also been shown to be involved in the de novo induction of MDSCs (16, 37, 38) as well as their recruitment to the tumor environment (17, 39), and has been further implicated in numerous other tumor cell-intrinsic and microenvironmental cancer-promoting activities (reviewed in (10, 40)). Thus, the type-1 immune-mediated enhancement of the COX2-PGE2 axis described here may result in an even-larger expansion of tumor environment-associated suppression and the reinforcement of a suppressive feedback loop, severely limiting spontaneous or therapy-induced type-1 responses..
Our data demonstrate that IFNγ and TNFα produced by type-1 lymphocytes are the primary synergistic enhancers of the observed immune suppression. Nevertheless, these molecules have also been extensively demonstrated to be critical for the effectiveness of antitumor immunity (23, 24), limiting the possibility of the therapeutic blockade of these factors as a part of cancer treatment. Identification of COX2/PGE2 as a central regulator of multiple suppressive pathways downstream of IFNγ and TNFα secretion provides a key therapeutic target to maintain the antitumor features of these type-1 cytokines while preventing suppressive consequences. Indeed, our present data show that COX2 blockade strongly reverses hyperactivated MDSC suppression of CD8+ T cells in coculture and is more effective even than the combined treatment with IDO, NOS, arginase, and IL10 inhibitors. The degree to which such COX2-mediated suppressive feedback driven by type-1 immunity affects the mouse TME is still unclear. It is intriguing to consider whether intrinsic species differences in such type-1 feedback regulation between mouse and humans may help explain the historically poor translation of type-1–promoting immune-modulators (demonstrating robust responses in pre-clinical mouse models) to clinical efficacy (41).
Despite the recent FDA approval of several new forms of immunotherapy, including sipuleucel-T (Provenge) for prostate cancer (42) and ipilimumab (CTLA4 antagonist) (43, 44), pembrolizumab (PD1 antagonist) (45), and pegylated IFNα for melanoma (46), often only a proportion of patients benefit from these immune therapies, and many of the responding patients eventually progress. In addition, recent data demonstrating cancer progression even in the presence of 10–40% tumor-specific T cells in the blood of vaccinated patients (47, 48) highlight the need to promote entry and local effector functions of these T cells within tumor tissues, which may be further enhanced by the antagonism of suppressive feedback mechanisms. Since both CTLA4 and PD1/PDL1 expression in the TME are believed to be enhanced in the course of local immune responses, and CTLA4 and PD1/PDL1 blockade are thought to promote the duration of anti-cancer immunity (49), it remains to be tested whether their effectiveness and the duration of their activity can be enhanced by simultaneous blockade of PGE2 synthesis or responsiveness to PGE2 using available inhibitors of PGE2 synthesis and signaling (reviewed in (10)). Indeed, codelivery of celecoxib and anti–PD-1 can synergistically improve antitumor immunity in preclinical models, in which effects were associated with a reduction in the numbers of MDSCs (50, 51).
In summary, these findings demonstrate the existence of a new mechanism contributing to the self-limiting nature of type-1 immunity in human cancer, and provide rationale for targeting the COX2-PGE2 axis as a key part of therapeutic approaches seeking to enhance the magnitude and duration of type-1 immune responses in the human TME.
Supplementary Material
Acknowledgments
The authors thank Dr. Ravikumar Muthuswamy for technical support and critical discussion of the manuscript, and Dr. Julie Urban, Dr. Erik Berk, Dr. Eva Wieckowski, Dr. Trang Nguyen, and Dr. Morten Hansen for critical discussion of the manuscript.
Financial support: The authors acknowledge support from the following NIH grants: P01 CA132714 (P.K.), P50 CA159981 (K.O.), F30 CA165410 (J.L.W), T32 CA082084 (J.L.W), and TL1 RR024155 (J.L.W). All UPCI authors were supported by P30 CA047904.
Footnotes
Conflicts of interest: The authors declare that no conflicts of interest exist.
References
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 2.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209:201–9. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy--revisited. Nature reviews Drug discovery. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Science translational medicine. 2012;4:127ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12:214–22. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- 9.Hahn T, Alvarez I, Kobie JJ, Ramanathapuram L, Dial S, Fulton A, et al. Short-term dietary administration of celecoxib enhances the efficacy of tumor lysate-pulsed dendritic cell vaccines in treating murine breast cancer. International journal of cancer Journal international du cancer. 2006;118:2220–31. doi: 10.1002/ijc.21616. [DOI] [PubMed] [Google Scholar]
- 10.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 13.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends in immunology. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–97. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 15.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 16.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–83. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S, Zhu L, Yang SC, Zhang L, Lin J, Hillinger S, et al. Cyclooxygenase 2 inhibition promotes IFN-gamma-dependent enhancement of antitumor responses. J Immunol. 2005;175:813–9. doi: 10.4049/jimmunol.175.2.813. [DOI] [PubMed] [Google Scholar]
- 20.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 21.Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, et al. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–53. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong JL, Mailliard RB, Moschos SJ, Edington H, Lotze MT, Kirkwood JM, et al. Helper activity of natural killer cells during the dendritic cell-mediated induction of melanoma-specific cytotoxic T cells. J Immunother. 2011;34:270–8. doi: 10.1097/CJI.0b013e31820b370b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. The lancet oncology. 2013;14:e218–28. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 24.Balkwill F. Tumour necrosis factor and cancer. Nature reviews Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 25.Zaidi MR, Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17:6118–24. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–22. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, et al. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon gamma, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–30. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn DA, Archer DC, Gold DP, Kelly CJ. Adjuvant immunotherapy is dependent on inducible nitric oxide synthase. J Exp Med. 2001;193:1261–8. doi: 10.1084/jem.193.11.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–94. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 31.Kudo Y, Boyd CA, Sargent IL, Redman CW. Modulation of indoleamine 2,3-dioxygenase by interferon-gamma in human placental chorionic villi. Molecular human reproduction. 2000;6:369–74. doi: 10.1093/molehr/6.4.369. [DOI] [PubMed] [Google Scholar]
- 32.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–31. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 34.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 37.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 38.Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–87. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 39.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Seminars in immunopathology. 2013;35:123–37. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 43.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 45.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouwhuis MG, Suciu S, Testori A, Kruit WH, Sales F, Patel P, et al. Phase III trial comparing adjuvant treatment with pegylated interferon Alfa-2b versus observation: prognostic significance of autoantibodies--EORTC 18991. J Clin Oncol. 2010;28:2460–6. doi: 10.1200/JCO.2009.24.6264. [DOI] [PubMed] [Google Scholar]
- 47.Astsaturov I, Petrella T, Bagriacik EU, de Benedette M, Uger R, Lumber G, et al. Amplification of virus-induced antimelanoma T-cell reactivity by high-dose interferon-alpha2b: implications for cancer vaccines. Clin Cancer Res. 2003;9:4347–55. [PubMed] [Google Scholar]
- 48.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 49.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology. 2015 doi: 10.1080/2162402X.2015.1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, Reis e Sousa C. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.