Abstract
Vitamin D deficiency is associated with increased risk of schizophrenia. However, it is not known whether this association is causal or what the direction of causality is. We performed two sample bidirectional Mendelian randomization analysis using single nucleotide polymorphisms (SNPs) robustly associated with serum 25(OH)D to investigate the causal effect of 25(OH)D on risk of schizophrenia, and SNPs robustly associated with schizophrenia to investigate the causal effect of schizophrenia on 25(OH)D. We used summary data from genome-wide association studies and meta-analyses of schizophrenia and 25(OH)D to obtain betas and standard errors for the SNP-exposure and SNP-outcome associations. These were combined using inverse variance weighted fixed effects meta-analyses. In 34,241 schizophrenia cases and 45,604 controls, there was no clear evidence for a causal effect of 25(OH)D on schizophrenia risk. The odds ratio for schizophrenia per 10% increase in 25(OH)D conferred by the four 25(OH)D increasing SNPs was 0.992 (95% CI: 0.969 to 1.015). In up to 16,125 individuals with measured serum 25(OH)D, there was no clear evidence that genetic risk for schizophrenia causally lowers serum 25(OH)D. These findings suggest that associations between schizophrenia and serum 25(OH)D may not be causal. Therefore, vitamin D supplementation may not prevent schizophrenia.
Vitamin D deficiency has been linked with increased risk of a number of neuropsychiatric disorders, including schizophrenia1. Recent meta-analyses have shown that individuals with schizophrenia or psychotic disorders have lower levels of serum vitamin D and a higher prevalence of vitamin D deficiency relative to healthy controls, although not relative to individuals with other psychiatric disorders2,3. The association between low vitamin D and schizophrenia is supported by data on the prevalence of schizophrenia by latitude and by season of birth, which suggest that lower exposure to sunlight, which is required for the production of vitamin D, is associated with higher schizophrenia risk1,4. Vitamin D deficiency has also been proposed as a potential explanation for higher prevalence of schizophrenia amongst dark-skinned second generation migrants living in temperate climates4. It has been suggested that this observational association between lower vitamin D levels and increased schizophrenia risk may be due to a pathophysiological role of vitamin D in the aetiology of schizophrenia3. Furthermore, data from longitudinal studies provide some evidence that vitamin D supplementation or higher dietary vitamin D intake may reduce risk of psychosis or schizophrenia in some groups5,6. This has led to suggestions that randomised clinical trials (RCTs) are appropriate to explore the potential benefits of vitamin D supplementation for the prevention of schizophrenia4.
However, RCTs of vitamin D supplementation for schizophrenia prevention would be challenging and expensive, given that schizophrenia occurs at a relatively low frequency in the population. It is therefore important to establish with a high degree of certainty that vitamin D plays a causal role in the aetiology of schizophrenia, since if it does not an RCT will almost certainly fail. Unfortunately, establishing causality from observational data is notoriously problematic, due to well-described difficulties associated with confounding and reverse causality. Lower vitamin D levels in patients with schizophrenia may arise due to a pathophysiological causal effect of vitamin D levels on schizophrenia risk, or could be due to reverse causality, for example if individuals with schizophrenia are more likely to stay indoors and have reduced exposure to sunlight. Alternatively, the association between the two may not be causal at all but may arise due to confounding by independent risk factors. Possible confounders of this relationship include ethnicity, dietary factors, sedentary lifestyle, physical activity, body mass index, urban living and socioeconomic position, which have all been shown to be associated with both vitamin D levels and schizophrenia4,7,8,9,10,11,12,13.
Mendelian randomization (MR) is instrumental variable analysis that uses genetic variants as unconfounded proxies (i.e., instruments) for the exposure of interest. Due to the random nature of inheritance of genetic information, it can be assumed that we inherit each variant (for the most part) independently from other genetic variants, and independently from environmental factors, meaning that such variants are unlikely to be associated with potential confounding factors14. Moreover, because our genome is determined at conception, associations between genetic variants and outcomes cannot arise from reverse causation. To be a suitable instrument for a MR analysis, a genetic variant must be: 1) robustly associated with an exposure of interest, 2) not associated with potential confounding factors of the exposure-outcome association, 3) only associated with the outcome through the exposure of interest (i.e., not pleiotropic). If these assumptions are met, a genetic variant can be used in a MR experiment to test whether an observed association between and exposure and an outcome is likely to be causal14,15. Genomewide association studies (GWAS) have identified genetic variants (single nucleotide polymorphisms (SNPs)) which are robustly associated with circulating 25 hydroxyvitamin D (25(OH)D) (the most commonly measured biomarker of vitamin D) levels16,17. When combined in an allele score, these variants do not appear to associate with other lifestyle factors (with the exception of geographical region) which could confound the observed relationship between vitamin D and health outcomes18. Previous MR studies, using the serum 25(OH)D related genetic variants have provided evidence that serum 25(OH)D is unlikely to cause changes in body mass index7 or influence risk of diabetes, coronary artery disease or stroke19,20, but may causally influence multiple sclerosis21, all cause mortality22 and blood pressure23. More recently, variants robustly associated with schizophrenia have been identified in a GWAS conducted by the Psychiatric Genomics Consortium24. The associations of polygenic risk scores combining these variants with schizophrenia risk have been replicated in a number of independent populations25,26 and are now being used to investigate associations between schizophrenia and other phenotypes26,27.
We therefore took advantage of these findings and conducted a bidirectional Mendelian randomization analysis of the association between serum 25(OH)D levels and schizophrenia risk, to establish whether the observational association is likely to be causal. We conducted this analysis to examine two possibilities: 1) that serum 25(OH)D levels influence schizophrenia risk, and 2) that biological risk for schizophrenia influences serum 25(OH)D levels. In a conventional Mendelian randomization analysis, data on genotype, exposure and outcome from the same data set are analysed. However, obtaining complete exposure data may be difficult in some settings, due to high measurement costs or lack of appropriate biospecimens28. Two-sample Mendelian randomization refers to the use of data from separate samples, where data on genotype and the exposure of interest are available in one sample, and data on genotype and the outcome of interest available in the other28.
Methods
Study Design
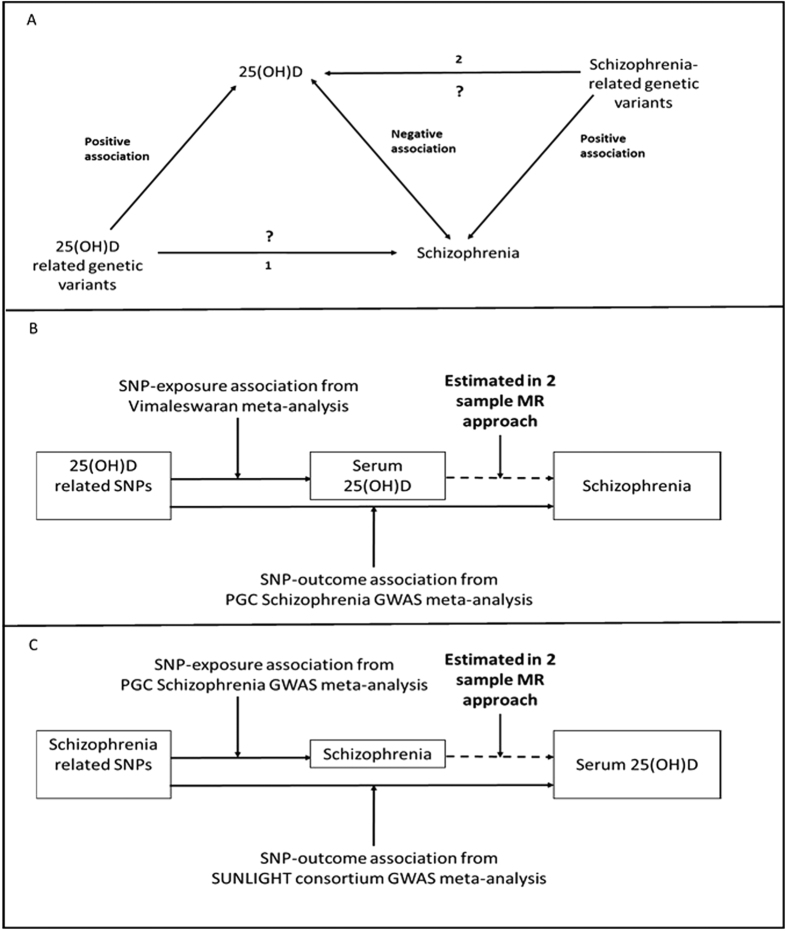
We performed a two-sample bidirectional Mendelian randomization analysis. To investigate whether vitamin D causes schizophrenia we used single nucleotide polymorphisms (SNPs) associated with serum 25(OH)D levels as proxies for measured serum 25(OH)D in an analysis with schizophrenia as the outcome. To investigate whether schizophrenia may affect vitamin D levels, we used SNPs associated with schizophrenia as proxies for biological risk of schizophrenia in an analysis with serum 25(OH)D levels as the outcome. To obtain causal estimates from a Mendelian randomization analysis, the association (beta coefficients from linear regression or log odds ratios from logistic regression) between the SNPs and the outcome of interest (SNP-outcome association) are divided by the association of the SNP and the exposure of interest (SNP-exposure association). This is known as the Wald estimator28. For each analysis, estimates of the SNP-exposure and SNP-outcome associations were obtained from different sources. A schematic of the analysis is shown in Fig. 1.
Figure 1. Bidirectional Mendelian randomization study of the association of 25(OH)D and schizophrenia.
(A) Indicates direction of published genetic and observational associations. Analyses will provide estimates of (1) the causal effect of serum 25(OH)D on schizophrenia and (2) the causal effect of schizophrenia risk on serum 25(OH)D levels. (B) Shows data sources for investigating whether serum 25(OH)D causes schizophrenia. (C) Shows data sources for investigating whether biological risk for schizophrenia causally affects serum 25(OH)D.
Ethical approval
This analysis is based on summary statistics obtained from previously published analyses and therefore we have not sought additional ethical approval. Each of these studies obtained appropriate ethical approval7,16,24.
Vitamin D to Schizophrenia
To construct a genetic instrument for vitamin D, we used four genetic variants which demonstrated genome-wide significant associations with serum 25(OH)D levels in the SUNLIGHT GWAS meta-analysis (N = 16,125 in the discovery sample, N = 17,871 in the replication sample) conducted in individuals of European ancestry16. These variants were located in or close to the following genes: GC (rs2282679), CYP2R1 (rs10741657), DHCR7 (rs12785878) and CYP24A1 (rs6013897). These genetic variants affect 25(OH)D levels through two distinct pathways (synthesis: CYP2R1 and DHCR7 and metabolism: GC and CYP24A1)18. Variants in or near three of these genes (GC, CYP2R1, DHCR7) have also reached genome-wide significance with serum 25(OH)D levels in a smaller GWAS17. None of these variants is in linkage disequilibrium (LD).
For the SNP-exposure association we used beta coefficients and standard errors from a meta-analysis (of up to 42,024 participants) conducted by Vimaleswaran and colleagues in individuals of European ancestry7 (see Table S1 in Supplementary material). These were reported as percentage change in serum 25(OH)D per effect allele. Effect sizes for associations with serum 25(OH)D were not available from the SUNLIGHT GWAS. This GWAS was conducted using a weighted z-score based approach16, meaning that contributing studies only shared z-scores (and not beta coefficients or standard errors) with the consortium.
For the SNP-outcome association, we used odds ratios and standard errors for the association of the vitamin D related variants with schizophrenia from the Schizophrenia GWAS meta-analysis conducted by the Psychiatric Genetics Consortium (PGC) (see Table S2 in Supplementary material)24. This is a case control study of 36,989 individuals with diagnosed schizophrenia and 113,075 controls (primarily of European ancestry). We used statistics from the GWAS discovery sample which comprised 34,241 cases and 45,604 controls and 3 family-based samples (1,235 parent affected-offspring trios). The summary statistics from the PCG GWAS meta-analysis are publicly available and can be downloaded at: http://www.med.unc.edu/pgc/downloads.
Schizophrenia to Vitamin D
To construct a genetic instrument for schizophrenia, we used variants which reached genome-wide significance in the PGC schizophrenia GWAS, described above24. A total of 128 independent variants were identified (explaining around 3.4% of the variance in risk of schizophrenia), in 108 physically distinct genetic loci.
For the SNP-exposure association, we used odds ratios and standard errors for the association with schizophrenia from the discovery sample of the PGC24. Where SNPs from the PGC were not available in SUNLIGHT, we extracted proxy SNPs from SUNLIGHT that were in high LD with these variants (R2 > 0.9). A list of the proxies used is provided in Supplementary material (see Table S3 in Supplementary material). Of the 128 independent PGC GWAS significant variants, 81 SNPs were matched in SUNLIGHT (54 SNPs were matched directly, 27 were matched to proxy SNPs). Twelve (6 pairs) of the 81 SNPs included in the analysis were in low linkage disequilibrium with each other, with R2 values ranging from 0.03–0.20.
For the SNP-outcome association, we used the z-scores for the association of the schizophrenia-related SNPs with serum 25(OH)D from the SUNLIGHT consortium meta-analysis.
Sample Overlap
We were unable to precisely determine the degree of sample overlap between studies contributing the gene-outcome and gene-exposure effect sizes. From the list of included studies, it is possible that up to 13% of the controls were in the Vimaleswaran meta-analysis and 12% of individuals in the Vimaleswaran meta-analysis were also PGC controls. Up to 6% of individuals included in the SUNLIGHT GWAS may have been PGC controls and 19% of PGC controls may have been in the SUNLIGHT GWAS. It is unlikely that there was any sample overlap between the vitamin D meta-analyses and the PGC cases.
Statistical Analysis
Vitamin D to schizophrenia
SNP-exposure (serum 25(OH)D) and SNP-outcome (schizophrenia) associations for the four vitamin D related SNPs were combined in two different ways to estimate the association of vitamin D with schizophrenia. First, we used a fixed effects meta-analysis approach, which combines the ratio estimates (SNP-exposure divided by SNP-outcome) from individual variants using inverse variance weighting29. Standard errors for this method were approximated using the delta method30. Second, we used a likelihood based approach31. In both analyses, results are expressed as the odds ratio for schizophrenia per 10% increase in serum 25(OH)D. We combined estimates from all four vitamin D related SNPs in a single analysis. However, we also performed sensitivity analyses combining the estimates from SNPs in the synthesis (rs10741657 and rs12785878) and metabolism (rs2282679 and rs6013897) pathways separately. This has been done in previous Mendelian randomization studies investigating the causal effects of vitamin D7,19. In addition, we performed two further analyses: 1) excluding just the DHCR7 SNP (rs12785878), which is strongly related to ancestry18 and 2) excluding just the GC SNP (rs2282679), as there is evidence that this SNP may have opposing effects on bioavailable vitamin D to its effects on 25(OH)D32 and that it could have potential pleiotropic effects, as the protein itself has been identified in cerebrospinal fluid33,34.
Schizophrenia to vitamin D
As associations of SNPs with serum 25(OH)D in SUNLIGHT were only available as test statistics (z-scores), we constructed beta coefficients and standard errors for the association of the schizophrenia-related SNPs with serum 25(OH)D from the z-scores, effect allele frequencies and sample size (see Table S4 in Supplementary material for formula). We then combined the SNP-exposure (schizophrenia) and SNP-outcome (serum 25(OH)D) associations for the 81 schizophrenia-related SNPs using the inverse variance weighted fixed effects meta-analysis approach and the likelihood based approach29. As the SNP-outcome associations in this analysis are constructed from z-scores, neither the beta coefficients for associations with serum 25(OH)D nor the effect sizes from the Mendelian randomization analysis have interpretable units. However, this analysis provides a direction of association and a P-value indicating the strength of the evidence for an association. To investigate the potential impact of correlated SNPs in this analysis, we performed a sensitivity analysis, excluding one SNP at random from the 6 pairs of SNPs in LD.
Detection of violations of the Mendelian randomization assumptions
A high degree of heterogeneity between estimates of the exposure-outcome association from SNPs included in the meta-analysis could indicate violation of the Mendelian randomization assumption that there is no pleiotropy (i.e., that all SNPs are only impacting on the outcome through their effect on the exposure)35. We therefore calculated Cochran’s Q and the I-squared statistic to estimate the degree of heterogeneity in the fixed effects meta-analysis. To further investigate potential bias due to pleiotropy, we performed Egger regression, as described by Bowden and colleagues35. The intercept from Egger regression is an estimate of the average pleiotropic effect of a SNP and so provides a test of directional pleiotropy, and the coefficient for the slope from Egger regression can provide a valid test of the causal effect estimate in Mendelian randomization analysis, even when one or more SNPs are pleiotropic.
In all analyses, confidence intervals were calculated as beta coefficients ± 1.96 * standard error. Analyses were performed in R (version 3.0.1) and Stata (version 11).
Results
Vitamin D to Schizophrenia
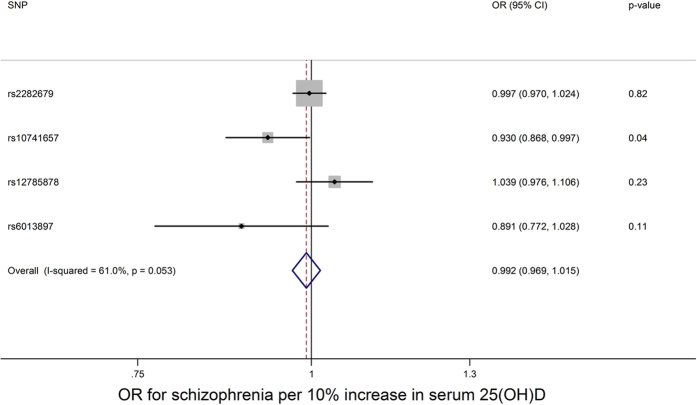
Using genetic variants related to serum 25(OH)D levels as instruments for measured serum 25(OH)D levels, there was no clear evidence from the inverse variance weighted fixed effects meta-analysis for a causal effect of serum 25(OH)D levels on schizophrenia (OR for schizophrenia per 10 percent increase in serum 25(OH)D: 0.992, 95% CI: 0.969 to 1.015) (see Fig. 2). The likelihood method produced a similar result (OR: 0.991, 95% CI: 0.969 to 1.015).
Figure 2. Mendelian randomization analysis of the effect of 25(OH)D on schizophrenia.
OR = odds ratio. Results from fixed effects inverse variance weighted meta-analysis. Weights for each SNP in the meta-analysis are as follows: rs2282679: 72.6%, rs10741657: 11.2%, rs12785878: 13.6%, rs6013897: 2.6%.
There was some evidence for heterogeneity in the analysis (I2 = 61%, 95% CI: 0, 87), indicating that the SNPs did not demonstrate associations with schizophrenia consistent with the magnitudes of their effect on serum 25(OH)D. However, in Egger regression, there was no clear evidence for directional pleiotropy (Intercept: 0.982 (95% CI: 0.959, 1.005). There was no clear evidence that the causal effect estimate from Egger regression differed from the estimate we obtained from the inverse variance weighted and likelihood approaches (OR: 1.023, 95% CI: 0.977 to 1.072).
There was no clear evidence for causal effects of serum 25(OH)D on schizophrenia in the analyses using just the SNPs involved in 25(OH)D synthesis (OR from fixed effects meta-analysis: 0.988, 95% CI: 0.944 to 1.035) or in 25(OH)D metabolism (OR from fixed effects meta-analysis: 0.993, 95% CI: 0.967 to 1.020) (see Figs S1 and S2 in Supplementary material).
Schizophrenia to Vitamin D
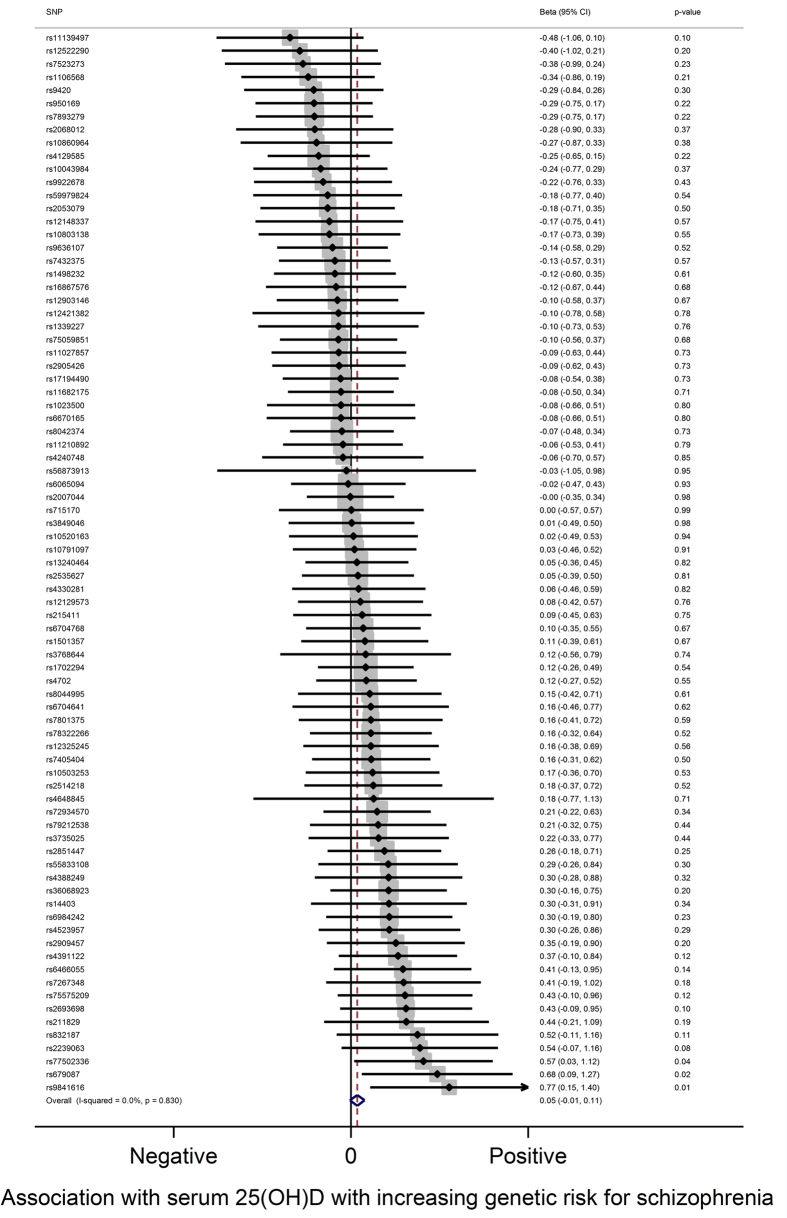
The analysis using genetic variants related to schizophrenia as instruments for risk for schizophrenia indicated only suggestive evidence for a causal effect of schizophrenia on vitamin D levels (P-value = 0.08 in the fixed effects meta-analysis). The effect was in the direction of higher genetic risk for schizophrenia increasing serum 25(OH)D levels (see Fig. 3). The likelihood approach produced similar results (positive direction of effect, P-value = 0.05). Excluding one of each SNP pair in LD slightly weakened the evidence for an effect (P-value from fixed effects meta-analysis = 0.14), but the direction of effect remained the same.
Figure 3. Mendelian randomization analysis of the effect of genetic risk for schizophrenia on serum 25(OH)D levels.
Results from fixed effects inverse variance weighted meta-analysis. Weights for individuals SNPs ranged from 0.4 to 2.8%. Overall effect is in the positive direction (higher genetic risk for schizophrenia associated with higher 25(OH)D). Magnitude of effects from this analysis are not interpretable.
There was no evidence for heterogeneity in this analysis (I2 = 0%) and no clear evidence for directional pleiotropy (P-value = 0.79) or a causal effect (P-value = 0.54) from Egger regression. The full output from the fixed effect meta-analysis, the likelihood approach and Egger regression are presented in Supplementary material (Table S5).
Discussion
Although there are strong observational associations between vitamin D deficiency and schizophrenia, our Mendelian randomization results do not provide any clear evidence that serum 25(OH)D levels play a causal role in schizophrenia, or that genetic risk for schizophrenia (as indexed by a schizophrenia polygenic risk score) causally lowers serum 25(OH)D levels. These findings would suggest that observational associations between serum 25(OH)D levels and schizophrenia risk could be explained by confounding due to lifestyle factors and health behaviours. Lack of evidence for a causal role of serum 25(OH)D in schizophrenia indicates that vitamin D supplementation may not be effective for prevention of schizophrenia.
We investigated the potential causal relationship between serum 25(OH)D and schizophrenia in the largest genetic study of schizophrenia published to date24. Using genetic markers related to serum 25(OH)D levels in a Mendelian randomization approach removes the possibility of reverse causality in this analysis and is likely to minimise confounding due to other lifestyle factors. As data on serum 25(OH)D levels are not available in the PGC, we have assumed that the vitamin D related genetic variants are associated with serum 25(OH)D levels in the schizophrenia cases and controls of the PGC consortium and that the relationship between serum 25(OH)D levels and schizophrenia risk is linear. Given the relatively well described function of the vitamin D genetic variants, which are located in or close to genes coding for proteins in vitamin D synthesis and metabolism pathways18, and the replication of these variants with serum 25(OH)D in additional populations7,19,20, we think that this first assumption is likely to be valid. With regards to the second assumption, it is possible that schizophrenia risk is only increased amongst individuals with 25(OH)D deficiency; if this is the case, the test for a causal effect of 25(OH)D from the MR analysis would still be a valid test of a causal link between 25(OH)D deficiency and schizophrenia. However, our power to detect threshold effects would be lower, particularly if prevalence of 25(OH)D deficiency is very low in the schizophrenia GWAS sample. There is evidence that the variants we have used are associated with likelihood of being 25(OH)D deficient16 and prevalence of 25(OH)D deficiency in European populations is reported to be reasonably high. For example, the average year-round prevalence of serum 25(OH)D below 25 nmol/L in adults in the UK (2008–2012) was reported to be 24% in males and 22% in females36. However, we cannot completely rule out the possibility of a threshold effect from the results of these analyses. For similar reasons, we must be cautious in generalising these results to populations of non-European ancestry, which have been shown to have higher prevalence of vitamin D deficiency and schizophrenia (as well as other mental health conditions)4,37,38.
Mendelian randomization is potentially an efficient method to establish or refute causality with respect to known observational associations. Indeed, it has been argued that the primary value of this approach will be to identify which observational associations are not likely to be causal, and thereby prevent investment in clinical trials of interventions which are likely to be unsuccessful39. Our results would appear to align with this objective, and suggest that clinical trials aimed at increasing serum 25(OH)D to prevent schizophrenia are not warranted. We assume that the 25(OH)D related genetic variants affect concentrations of serum 25(OH)D throughout the life-course. Whilst these results are therefore consistent with no causal effect of life-long lower serum 25(OH)D levels on schizophrenia risk, we may not be able to rule out there being a specific critical exposure period where low vitamin D increases risk of schizophrenia. For example, maternal 25(OH)D status during pregnancy, has been identified as a possible risk factor for schizophrenia40,41. The 25(OH)D SNPs should be associated with maternal serum 25(OH)D levels of the individuals included in this analysis, but only half as strongly. Furthermore, as our analysis was conducted in a case control sample of schizophrenia, we cannot use these data to make inferences about the potential impact of vitamin D supplementation in individuals already diagnosed with schizophrenia as an intervention for reducing symptoms3.
Well powered studies are required to have confidence that lack of evidence for causal effects in Mendelian randomization studies reflect true null associations39. Along with sample size, the strength of the association between the genetic variant and the exposure is a key determinant of power in Mendelian randomization analysis. Whilst the genetic variants used in this analysis only explain a small proportion of the variation in serum 25(OH)D levels (3.6% in the study by Ye and colleagues19), the increase in serum 25(OH)D conferred by each copy of the major allele of the GC SNP (rs2282579) has been shown to be roughly equivalent to taking a daily vitamin D supplement in some populations16. Therefore, failure of the combined 25(OH)D related genetic variants to demonstrate clear evidence of an association with increased risk of schizophrenia in the largest genetic case control sample of schizophrenia available to date enables us to be reasonably confident that 25(OH)D levels are not a major contributing factor for schizophrenia in European populations.
The analysis presented relates to serum 25(OH)D levels, the major circulating metabolite of vitamin D, which is a precursor to the active form (1, 25- dihydroxyvitamin D3)18. Therefore, whilst serum 25(OH)D is observationally very strongly associated with schizophrenia risk, we must exercise some caution in drawing conclusions regarding the role of biologically active vitamin D19. There is some evidence that one of the metabolism variants associated with increased serum 25(OH)D levels (rs2282679) may in fact reduce the bioavailability of vitamin D19,20,32. However, when we excluded this variant from the meta-analysis, there was no clear evidence for any association of serum 25(OH)D with schizophrenia (see Fig. S3 in Supplementary material). Furthermore, the SNP scores based on genetic variants involved in synthesis and metabolism also failed to provide convincing evidence for an effect of serum 25(OH)D on schizophrenia.
We also investigated the potential causal impact of genetic risk for schizophrenia on serum 25(OH)D, using genetic variants which have been identified to increase risk of schizophrenia24. Whilst there was some weak evidence for an association of the risk score with serum 25(OH)D levels, it was in the direction of schizophrenia increasing serum 25(OH)D, which is opposite to what is seen observationally. Therefore, this analysis does not provide convincing evidence that genetic risk for schizophrenia decreases serum 25(OH)D levels. In this analysis, the gene-exposure associations for the schizophrenia related genetic variants were derived from a case control sample of individuals with and without schizophrenia, but here we have applied them the studies to contributing to the SUNLIGHT consortium (largely comprising general population samples), where schizophrenia prevalence is likely to be low. There is evidence that schizophrenia symptoms exist on a continuum42,43,44, although this is debated44,45. If this is indeed the case, genetic risk for schizophrenia will likely influence schizophrenia-like symptoms in individuals without diagnosed schizophrenia. However, we are assuming in our analysis that this genetic risk on schizophrenic traits would be sufficiently strong to alter lifestyle in a way that could impact upon serum vitamin D in individuals without severe disease. Furthermore, we are assuming that this genetic risk would impact upon lifestyle in the same direction in the general population as in those with severe disease (i.e., that if individuals with severe disease have lower exposure to sunlight, higher genetic risk in individuals without clinical disease is also associated with lower exposure to sunlight). We should therefore treat the results of our analysis of schizophrenia risk on vitamin D with some caution.
There are some general limitations to Mendelian randomization that should also be considered when interpreting these results. First, we were not able to exclude the possibility of sample overlap in our analysis. With a small overlap, as it most likely the case here, it is likely that any bias would be conservative, and would not lead to a false positive finding28. Second, we cannot completely rule out population stratification and pleiotropy as sources of bias in these analyses. However, restriction of the data included to almost exclusively European individuals as well as adjustment for principal components within the contributing studies7,16,24 is likely to protect against spurious findings due to population stratification. Furthermore, removal of the DHCR7 SNP (which is strongly associated with ancestry) from the analysis did not appear to influence the results (see Fig. S4 in Supplementary material). In addition, the results of Egger regression (which should be less subject to bias than inverse variance weighted meta-analysis) did not provide clear support for the existence of directional pleiotropy or for a non-null causal effect. However, Egger regression has much lower power to detect causal effects than inverse variance weighted meta-analysis, and the power of Egger regression to detect pleiotropy decreases with decreasing numbers of genetic variants35.
In conclusion, our findings, which should be subject to lower bias from potential confounding by lifestyle factors than conventional epidemiological studies, do not provide evidence that vitamin D levels causally affect schizophrenia. This finding is valuable as it suggests that vitamin D supplementation in the general population is unlikely to have large effects on schizophrenia risk, although we cannot completely rule out the possibility that deficient individuals could be at higher risk. Two-sample Mendelian randomization analysis using summary data is an efficient strategy for investigating potential causal links in the absence of large studies with genetic data, vitamin D and schizophrenia. Follow up of these findings in such studies would be beneficial in the future for better characterising the nature of the vitamin D and schizophrenia associations. Furthermore, similar analyses in populations of different ethnicities, who are more at risk of vitamin D deficiency and schizophrenia would help to clarify whether there may be subgroups who could benefit from supplementation.
Additional Information
How to cite this article: Taylor, A. E. et al. Investigating causality in the association between 25(OH)D and schizophrenia. Sci. Rep. 6, 26496; doi: 10.1038/srep26496 (2016).
Supplementary Material
Acknowledgments
We would like to thank the SUNLIGHT Consortium for providing the summary estimates from their GWAS of 25(OH)D16. AET, JJW, SHG and MRM are members of the UK Centre for Tobacco and Alcohol Studies, a UK Clinical Research Council Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. Support from the Medical Research Council (MC_UU_12013/1, MC_UU_12013/6) is also gratefully acknowledged. SB is supported by the Wellcome Trust (grant number 100114). JJW is supported by a Post-Doctoral Research Fellowship from the Oak Foundation.
Footnotes
Author Contributions M.R.M. came up with the concept for this study. A.E.T. performed the statistical analysis and wrote the initial draft of the manuscript. S.B. advised on the statistical analysis. J.J.W. and S.H.G checked the statistical analyses. G.D.S and J.B.R advised on the methods and interpretation of the results. All authors commented on a draft of the manuscript and approved the final version.
References
- Eyles D. W., Burne T. H. & McGrath J. J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 34, 47–64 (2013). [DOI] [PubMed] [Google Scholar]
- Valipour G., Saneei P. & Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J Clin Endocrinol Metab 99, 3863–72 (2014). [DOI] [PubMed] [Google Scholar]
- Belvederi Murri M. et al. Vitamin D and psychosis: mini meta-analysis. Schizophr Res 150, 235–9 (2013). [DOI] [PubMed] [Google Scholar]
- McGrath J. Is it time to trial vitamin D supplements for the prevention of schizophrenia? Acta Psychiatr Scand 121, 321–4 (2010). [DOI] [PubMed] [Google Scholar]
- McGrath J. et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res 67, 237–45 (2004). [DOI] [PubMed] [Google Scholar]
- Hedelin M. et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry 10, 38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimaleswaran K. S. et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. Plos Med 10, e1001383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voortman T. et al. Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J Nutr 145, 791–8 (2015). [DOI] [PubMed] [Google Scholar]
- Saraceno B., Levav I. & Kohn R. The public mental health significance of research on socio-economic factors in schizophrenia and major depression. World Psychiatry 4, 181–5 (2005). [PMC free article] [PubMed] [Google Scholar]
- McNamee L., Mead G., MacGillivray S. & Lawrie S. M. Schizophrenia, poor physical health and physical activity: evidence-based interventions are required to reduce major health inequalities. Br J Psychiatry 203, 239–41 (2013). [DOI] [PubMed] [Google Scholar]
- Peet M. Diet, diabetes and schizophrenia: review and hypothesis. Br J Psychiatry Suppl 47, S102–5 (2004). [DOI] [PubMed] [Google Scholar]
- McGrath J. J. & Lawlor D. A. The search for modifiable risk factors for schizophrenia. Am J Psychiatry 168, 1235–8 (2011). [DOI] [PubMed] [Google Scholar]
- Pedersen C. B. & Mortensen P. B. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry 58, 1039–46 (2001). [DOI] [PubMed] [Google Scholar]
- Davey Smith G. & Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32, 1–22 (2003). [DOI] [PubMed] [Google Scholar]
- Davey Smith G. Mendelian Randomization for Strengthening Causal Inference in Observational Studies: Application to Gene x Environment Interactions. Perspectives on Psychological Science 5, 527–545 (2010). [DOI] [PubMed] [Google Scholar]
- Wang T. J. et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376, 180–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J. et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 19, 2739–45 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. J., Vimaleswaran K. S., Whittaker J. C., Hingorani A. D. & Hypponen E. Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. Plos One 7, e37465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. et al. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol 3, 35–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong A. et al. The causal effect of vitamin D binding protein (DBP) levels on calcemic and cardiometabolic diseases: a Mendelian randomization study. Plos Med 11, e1001751 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokry L. E. et al. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. Plos Med 10.1371/journal.pmed.1001866 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal S., Brondum-Jacobsen P., Bojesen S. E. & Nordestgaard B. G. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 349, g6330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimaleswaran K. S. et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol 2, 719–29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R. A. et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat Neurosci 18, 953–5 (2015). [DOI] [PubMed] [Google Scholar]
- Agerbo E. et al. Polygenic Risk Score, Parental Socioeconomic Status, Family History of Psychiatric Disorders, and the Risk for Schizophrenia: A Danish Population-Based Study and Meta-analysis. JAMA Psychiatry 72, 635–41 (2015). [DOI] [PubMed] [Google Scholar]
- Power R. A. et al. Genetic predisposition to schizophrenia associated with increased use of cannabis. Mol Psychiatry 19, 1201–4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce B. L. & Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 178, 1177–84 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S. et al. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 30, 543–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Lawlor D. A. & Thompson J. R. Re: Estimation of bias in nongenetic observational studies using “Mendelian triangulation” by Bautista et al. Ann Epidemiol 17, 511–3 (2007). [DOI] [PubMed] [Google Scholar]
- Burgess S., Butterworth A. & Thompson S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37, 658–65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe C. E. et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 369, 1991–2000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon M. et al. Vitamin D-binding protein interacts with A beta and suppresses A beta-mediated pathology. Cell Death and Differentiation 20, 630–638 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi A. O. et al. Increased Circulating Levels of Vitamin D Binding Protein in MS Patients. Toxins 7, 129–137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J., Davey Smith G. & Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44, 512–25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro A. & Buttriss J. L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr Bull 39, 322–350 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker M. & Holick M. F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol 5, 51–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerhus M. et al. Vitamin D status in psychotic disorder patients and healthy controls - The influence of ethnic background. Psychiatry Res 230, 616–21 (2015). [DOI] [PubMed] [Google Scholar]
- VanderWeele T. J., Tchetgen Tchetgen E. J., Cornelis M. & Kraft P. Methodological challenges in mendelian randomization. Epidemiology 25, 427–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. J. et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 67, 889–94 (2010). [DOI] [PubMed] [Google Scholar]
- McGrath J. J., Burne T. H., Feron F., Mackay-Sim A. & Eyles D. W. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophr Bull 36, 1073–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R. et al. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry 57, 1053–8 (2000). [DOI] [PubMed] [Google Scholar]
- Kendler K. S., McGuire M., Gruenberg A. M. & Walsh D. Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am J Psychiatry 152, 755–64 (1995). [DOI] [PubMed] [Google Scholar]
- van Os J., Linscott R. J., Myin-Germeys I., Delespaul P. & Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med 39, 179–95 (2009). [DOI] [PubMed] [Google Scholar]
- Zammit S. et al. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am J Psychiatry 170, 742–50 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.