Abstract
BACKGROUND:
The leaves of Ocimum basilicum L. (basil) are used in traditional cuisine as spices; its essential oil has found a wide application in perfumery, dental products as well as antifungal agents.
AIM:
To assess the chemical composition as well as the in vitro antifungal activity of O. basilicum L. essential oil against Aspergillus flavus fungal growth and aflatoxin B1 production.
MATERIAL AND METHODS:
The essential oil of O. basilicum was obtained by hydrodistillation and analysed using gas chromatography (GC) and GC coupled with mass spectrometry (GC/MS). The essential oil was tested for its effects on Aspergillus flavus (A. flavus) mycelial growth and aflatoxin B1 production in Yeast Extract Sucrose (YES) growth media. Aflatoxin B1 production was determined by high performance liquid chromatography (HPLC).
RESULTS:
Nineteen compounds, representing 96.7% of the total oil were identified. The main components were as follows: linalool (48.4%), 1,8-cineol (12.2%), eugenol (6.6%), methyl cinnamate (6.2%), α-cubebene (5.7%), caryophyllene (2.5%), β-ocimene (2.1%) and α-farnesene (2.0%). The tested oil showed significant antifungal activity that was dependent on the used oil concentration. The complete inhibition of A. flavus growth was observed at 1000 ppm oil concentration, while marked inhibition of aflatoxin B1 production was observed at all oil concentrations tested (500, 750 and 1000 ppm).
CONCLUSION:
These results confirm the antifungal activities of O. basilicum L. oil and its potential use to cure mycotic infections and act as pharmaceutical preservative against A. flavus growth and aflatoxin B1 production.
Keywords: Ocimum basilicum L. essential oil, chemical composition, antifungal activity, aflatoxins B1, Aspergillus flavus
Introduction
Basil has been traditionally used as a food spice, in perfumery and medical industries [1]. Ancient Egyptians used it in their medical prescriptions [2]. In folk medicine, the leaves and flowering tops of the plant are prescribed as carminative, galactogogue, stomachic, antispasmodic [3]. However, recently the potential uses of O. basilicum essential oil, particularly as antimicrobial [4], antifungal [5], antioxidant [6] and larvicidal [7] have also been investigated.
The genus Ocimum, Lamiaceae, collectively called basil, has long been acclaimed for its diversity. Ocimum comprises more than 30 species of herbs and shrubs from the tropical and subtropical regions of Asia, Africa, Central and South America, but the main center of diversity appears to be Africa [8].
The O. basilicum essential oils exhibit a wide and varying array of chemical compounds, depending on variations in chemotypes, leaf and flower colours, aroma and origin of the plants [9]. The chief constituents include chavicol methyl ether or estragole, linalool and eugenol [10].
Recently due to the alarming increase in the rate of infection by antibiotic resistant microorganisms, immense clinical problems have been encountered in treatment of infectious diseases. Extensive research work has to be done to eliminate this serious problem. Fortunately, natural products particularly essential oils can participate effectively in this scope. One of these microorganisms is A. favus fungus which produces secondary metabolites known as aflatoxins that are potentially harmful to crops, animals and humans. The aflatoxins B1, B2, G1 and G2 are the major four toxins amongst at least 16 structurally related toxins. Aflatoxin B1 is particularly important since it is the most toxic and potent hepatocarcinogenic natural compound ever characterized [11].
A. flavus causes a broad spectrum of diseases in humans, ranging from hypersensitivity reactions to invasive infections associated with angioinvasion. A. flavus is the second leading cause of invasive and noninvasive aspergillosis [12]. Multiple resistances to known antifungal drugs with high mortality rate have been reported. This increases the need to explore natural drugs of high potency and effectiveness against A. flavus and their aflatoxins.
In this study, the chemical composition and effects of essential oil of Ocimum basilicum L. (family Apiaceae or Umbelliferae) on growth and aflatoxin B1 production of A. flavus were evaluated.
Material and Methods
The protocol of the study was reviewed and approved by Ethical Committee of National Research Center.
Authors declare that there is no actual or potential conflict of interest for this work.
Plant Material
Leaves of Ocimum basilicum L. were purchased from local markets and authenticated in the herbarium of Faculty of Science, Cairo University and National Research Center, Egypt.
Extraction of Essential Oil: 1 kg of Ocimum basilicum L. leaves were subjected to hydrodistillation. The volatile oil then collected and dried in desiccators over anhydrous CaSO4. The volatile oil sample was kept in dark bottle for GC and GC/MS (mass spectrometry) analyses.
Analysis of the Essential Oil GC: The essential oil was analysed on Perkin-Elmer gas chromatograph model 8700, equipped with HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm)programming from 80ºC (3 min) to 220ºC (10 min). Helium as a carrier gas (1.5 ml/min). Injection in split mode (1:100).
GC/MS: The essential oil was also analysed on Agilent-Technologies 6890N network gas chromatographic (GC) system (Little Falls, California, USA), equipped with HP-5 MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm) programming from 80ºC (3 min) to 220ºC (10 min). Helium as a carrier gas (1.5 ml/min). Injection in split mode (1:100) [13, 14].
Identification of Compounds: Compounds were identified by computer search using their mass spectra either with the known components or published spectra and by comparison of their retention indices with those of known compounds [15, 16].
Fungal inoculum preparation
A. flavus strain (ATCC 16872), was used as an aflatoxigenic strain for in vitro experiments. They were kept on potato-dextrose-agar (PDA) media and were incubated at 25ºC for 10 days. From single spore cultures of A. flavus grown on PDA media, the spores were harvested from the surface of the agar plates by washing off the surface of the slant with 10 ml of sterile 0.1% Tween 80 (Merck, Germany) solution to obtain a concentration of 106 spores/ml, and were utilized on the same day.
Antifungal activity of essential oils
Fifteen ml of YES medium in 4 flasks (250 ml) was autoclaved at 120ºC for 15 minutes. 1 ml of a suspension of spores (105 spores) of the toxigenic A. flavus strain was inoculated in each flask with either 50 µl (500 ppm), 100 µl (750 ppm), or 150 µl (1000 ppm), of basil essential oil or without essential oil (control). The flasks were incubated in the dark for 14 days at 25 ºC. After the incubation period, the growth of the aflatoxigenic fungi A. flavus in all flasks was visually examined.
Determination of mycelial weight
Extraction of aflatoxins produced in the YES culture was carried out [17] by harvesting the mycelium of each flask through filtration in Whatman paper (No. 4) then extraction by 100 ml chloroform. The chloroform extract was then dried by adding anhydrous sodium sulfate and evaporated to dryness under nitrogen at temperature below 60ºC. The net dry weight of mycelia was determined. The dry film was then used for the detection of aflatoxins.
Derivatization
Two hundred µl hexane were added to the clean up dry film of control and tested samples followed by 50 µl trifluroacetic acid (TFA) and mixed by vortex vigorously for 30 seconds. The mixture was left to stand for 5 minutes. Then, 450 ml water – acetonitrile (9 +1 v/v) were added and mixed well for 30 seconds. The mixture was left to stand for 10 minutes to form two separate layers. The lower aqueous layer was used for HPLC analysis [18].
HPLC analysis: Using HPLC [Model 1525, Waters 1500 Rheodyne manual injector, 2475 Multi-Wavelength Fluorescence Detector, and a data workstation with software Breeze 2. A phenomenex C18 (250x 4.6 mm i.d), 5 μm from Waters corporation (USA) was used], the mixed solutions of control as well as tested extracts were filtered through a 0.22 mm membrane filter and loaded (20 ml) into a 20 ml injection loop.
The elution order of the four aflatoxins was G2, B2, G2a (G1 derivative), B2a (B1 derivative). AFs contents in samples were calculated from chromatographic peak areas using the standard curve (Fig. 1, 2, 3, 4).
Figure 1.
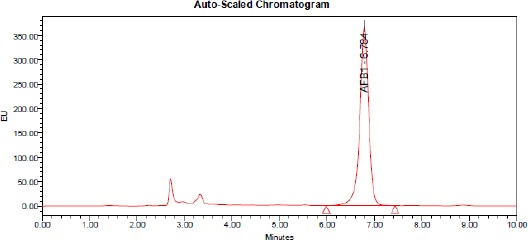
HPLC of aflatoxin B1 produced by A. flavus (control).
Figure 2.
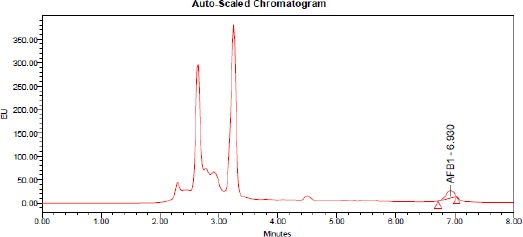
HPLC of aflatoxin B1 produced by A. flavus treated by O. basilicum L. essential oil at 500 ppm concentration.
Figure 3.
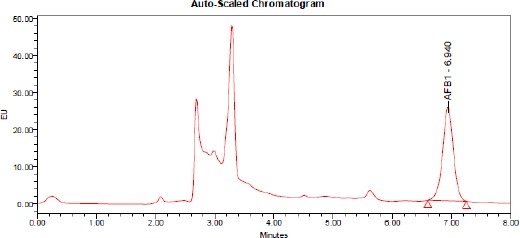
HPLC of aflatoxin B1 produced by A. flavus treated by O. basilicum L. essential oil at 750 ppm concentration.
Figure 4.
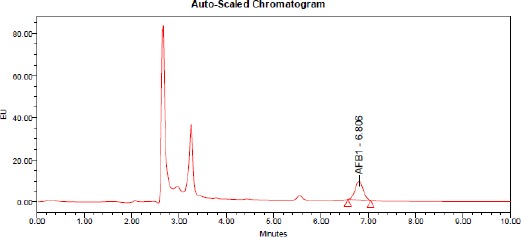
HPLC of aflatoxin B1 produced by A. flavus treated by O. basilicum L. essential oil at 1000 ppm concentration.
The experiments were replicated three times. The inhibitory % of fungal growth and aflatoxin production was calculated according to the equation:
Inhibitory % = (control- treatment /control ×100).
Statistical analysis
All data from three independent replicate trials were subjected to statistical analysis using statistical software (SPSS, 10.0; Chicago, USA). Data are reported as means ± standard deviations. The significant differences between mean values were determined by independent sample t-test (P < 0.05).
Results
Qualitative and quantitative chemical compositional data of Ocimum basilicum oil is given in Table 1.
Table 1.
Ocimum basilicum L. essential oil components
| Component | Composition % | |
|---|---|---|
| 1 | α-Pinene | 0.7 |
| 2 | Camphene | 1 |
| 3 | β-Pinene | 1.5 |
| 4 | β-Myrcene | 1.2 |
| 5 | 1, 8- Cineol | 12.2 |
| 6 | β-Ocimene | 2.1 |
| 7 | γ-Terpin | 1.3 |
| 8 | Linalool | 48.4 |
| 9 | Camphor | 0.8 |
| 10 | Myrtenol | 1.5 |
| 11 | α-Cubebene | 5.7 |
| 12 | Eugenol | 6.6 |
| 13 | Methyl cinnamate | 6.3 |
| 14 | Caryophyllene | 2.5 |
| 15 | Azulene | 1.6 |
| 16 | α-Farnesene | 2 |
| 17 | Germacrene B | 1.9 |
| 18 | Germacrene D | 0.9 |
| 19 | Naphthalene | 0.5 |
| Total % | 96.7 | |
Regarding the antifungal activity of the tested oil, results show that both fungal growth and aflatoxin biosynthesis were significantly suppressed by O. basilicum essential oil compared to control (Table 2).
Table 2.
Effect of different concentrations of Ocimum basilicum L. essential oil on the mycelia dry weight and aflatoxin B1 production
| Treatment | Concentration (ppm) | Mycelia dry weight (g/ml)a | Aflatoxin B1 (lg/ml)a |
|---|---|---|---|
| Ocimum basilicum L. | 500 | 15.35 ± 1.53 a,c | 0.68 ± .053 a,c |
| 750 | 5.333 ± 1.154 b | 0.516 ± .020 | |
| 1000 | .000 ± .000 | 0.496 ± .015 | |
| Positive control | 71.666 ± 1.527 | 25.808 ± .599 |
significant differences between concentration 500 & 750
significant differences between concentration 750 & 1000
significant differences between concentration 500 & 1000 in the same column. a Data are means of triplicates (± standard deviation).
There was no mycelia growth of A. flavus fungus at 1000 ppt concentration of the tested oil (Fig 5).
Figure 5.

Flask A, control flask showing growth of A. flavus. Flask B, containing O.basilicum L. essential oil at concentration of 1000 ppm showing no growth of A. flavus.
The inhibitory % is one of the elements necessary for the evaluation of the effectiveness of an extract. The inhibitory % of the oil increased in proportion to its concentration (Fig. 6, 7).
Figure 6.
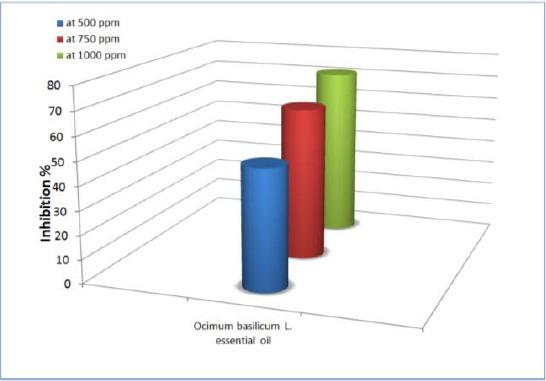
The inhibitory % of O. basilicum L essential oil on mycelia growth of A. flavus.
Figure 7.
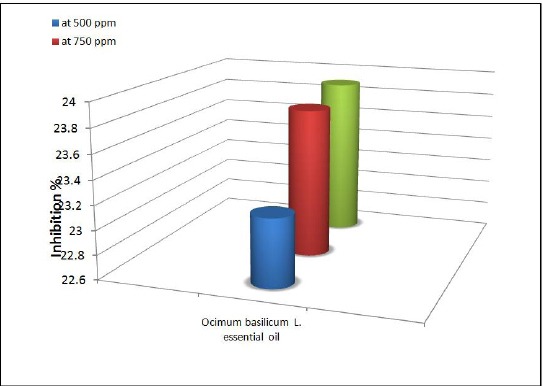
The inhibitory % of O. basilicum L essential oil on aflatoxin B1 of A. flavus.
Discussion
This study confirms the antifungal properties of O. basilicum L. essential oil against A. flavus mycelial growth and aflatoxin B1 production. The tested oil showed significant antifungal activity that was dependent on the used oil concentration. The complete inhibition of A. flavus growth was observed at 1000 ppm oil concentration, while marked inhibition of aflatoxin B1 production was observed at all oil concentrations tested (500, 750 and 1000 ppm).
In agreement with our findings, a number of studies report on strong antifungal action of basil essential oil. Doube et al. [20] using the agar plate method, showed that basil oil, in a concentration of 1.5 ml/l inhibited completely the growth of 22 species of molds, including the aflatoxigenic strains Aspergillus parasiticus and A. flavus.
Another study on Candida albicans and A. flavus [21] reported that basil oil inhibited completely the growth of these organisms at a concentration of 5000 ppm, during 7 days of incubation, using microdilution method. Soliman and Badeaa [22] found that basil oil acts as a fungistatic agent against F. verticillioides in a concentration of 2000 ppm, and as a fungicid agent in concentration of 3000 ppm. While complete growth inhibition of F. verticillioides was noticed in another study at concentrations higher than 2.7 μL/mL [23].
Many researchers reported that essential oil of plants was able to inhibit both growth and/or mycotoxin production [24, 25]. The antiaflatoxigenic actions of essential oil may be related to inhibition of the ternary steps of aflatoxin biosynthesis involving lipid peroxidation and oxygenation [26]. In addition essential oils protect the cells from harmful impact of aflatoxins through two possible mechanisms firstly by reducing DNA binding formation of aflatoxins, secondly by reacting with ROS increased by aflatoxins [27].
Some researchers reported that there is a relationship between the chemical structures of the most abundant compounds in the essential oils and the antimicrobial or antifungal activities [28]. Compositional analysis of O. basilicum essential oil in our study showed that compounds such as linalool, 1,8-cineol, eugenol, methyl cinnamate and α-cubebene were among the main components present. The antimicrobial activity of an essential oil is attributed mainly to its major compounds. However, the synergistic or antagonistic effect of one compound in minor percentage in the mixture has to be considered [29].
Early researches addressed linalol, estragol, eugenol and methyl cinnamate as the major antimicrobial and antifungal components of basil extracts and essential oil [30]. Some pointed to strong antifungal effect of oil which contained estragol as the main component on the growth of Aspergillus niger, A. ochraceus, and Fusarium culmorum (inhibition growth of 71.0 to 94.76%) [31], others found linalol and estragol to be more efficient against R. nigricans (100% of inhibition), compared to eugenol (38.1% of inhibition) [32]. Eugenol was found to exhibit stronger inhibition towards F. oxysporum (100% of inhibition), in contrast to linalool and estragol, where the inhibition values were 26.4 and 30.3%, respectively [4].
Our findings suggest that O. basilicum L. at concentration of 1000 ppt could supress A. flavus fungal growth and inhibit markedly aflatoxin B1 production which could be attributed to its rich content of linalool, 1,8-cineol and eugenol.
In conclusion, these results confirm the antifungal activities of O. basilicum L. oil and its potential use to cure mycotic infections and act as pharmaceutical preservative against A. flavus growth and aflatoxin B1 production.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Telci I, Bayram E, Yilmaz G, Avci B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.) Biochem Syst Ecol. 2006;34:489–497. [Google Scholar]
- 2.Abou El-Soud NH. Herbal medicine in ancient Egypt. Journal of Medicinal Plants Research. 2010;4(2):082–086. [Google Scholar]
- 3.Daneshian A, Shayegh J, Mikaili P, Sharaf J. Antimicrobial activity of essential oil extract of Ocimum basilicum L. leaves on a variety of pathogenic bacteria. Journal of Medicinal Plants Research. 2011;5(15):3453–3456. [Google Scholar]
- 4.Tanackov S, Dimić G, Lević J, Tanackov I, Tuco D. Antifungal activities of basil (Ocimum basilicum L.). extract on Fusarium species. African Journal of Biotechnology. 2011;10(50):10188–10195. [Google Scholar]
- 5.Shirazi M, Gholami H, Kavoosi G, Rowshan V, Tafsiry A. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Science & Nutrition. 2014;2(2):146–155. doi: 10.1002/fsn3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Politeo O, Jukic M, Milos M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007;101:379–385. [Google Scholar]
- 7.Govindarajan M, Sivakumar R, Rajeswary M, Yogalakshmi K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.). against Culex tritaeniorhynchus Aedes albopictus and Anopheles subpictus (Diptera: Culicidae) Experimental Parasitology. 2013;134:7–11. doi: 10.1016/j.exppara.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Paton A. A synopsis of Ocimum L. (Labiatae) in Africa. Kew Bul. 1992;47:403–435. [Google Scholar]
- 9.Da-Silva F, Santos R, Diniz E, Barbosa L, Casali V, De-Lima R. Content and composition of basil essential oil at two different hours in the day and two seasons. Revista Brasileira de Plantas Medicinais. 2000;6(1):33–38. [Google Scholar]
- 10.Rao BR, Kotharia SK, Rajput DK, Patel RP, Darokar MP. Chemical and biological diversity in fourteen selections of four Ocimum species. Nat. Prod. Commun. 2011;6:1705–1710. [PubMed] [Google Scholar]
- 11.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153:1677–1692. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- 12.Morgan J, Wannemuehler K A, Marr K A, Hadley S, Kontoyiannis DP, Walsh TJ, Fridkin SK, Pappas PG, Warnock DW. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43(1):S49–S58. doi: 10.1080/13693780400020113. [DOI] [PubMed] [Google Scholar]
- 13.Minica DN, Bozin B, Sokovic M, Simin N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004;52(7):2485–2489. doi: 10.1021/jf030698a. [DOI] [PubMed] [Google Scholar]
- 14.Vagionas K, Graikou K, Chinou IB, Runyoro D, Ngassapa O. Chemical analysis and antimicrobial activity of essential oils from the aromatic plants Artemisia afra Jacq. and Leonotis ocymifolia (Burm. F.)Iwarsson var raineriana (Vision 1) Iwarsson growing in Tanzania. J Essent Oil Res. 2007;19:396–400. [Google Scholar]
- 15.Masada Y. Analysis of essential oil by gas chromatography and mass spectrometry. New York: John Wiley and Sons Inc. 1976:118–120. [Google Scholar]
- 16.Adams RP. Identification of essential oils components by gas chromatography/quadrupole mass spectroscopy. Carol Stream, IL., USA: Allured Publishing Corporation; 2001. p. 456. [Google Scholar]
- 17.Munimbazi C, Bullerman LB. Isolation and partial characterization of antifungal metabolites of Bacillus pumilus. J Appl Microbiol. 1998;84:959–968. doi: 10.1046/j.1365-2672.1998.00431.x. [DOI] [PubMed] [Google Scholar]
- 18.A.O.A.C Nature Toxins. Official Methods of Analysis of AOAC International. 19th ed. Arlington, USA: AOAC International; 2012. p. 49. [Google Scholar]
- 19.Deabes MM, Darwish HR, Abdel-Aziz KB, Farag IM, Nada SA, Tawfek N S. Protective effects of Lactobacillus rhamnosus GG on Aflatoxins-induced toxicities in male Albino Mice. Journal of environment & Analytical Toxicology. 2012;2(3):2–9. [Google Scholar]
- 20.Doube S, Upadhyay PD, Tripathi SC. Antifungal, physicochemical, and insect repelling activity of the essential oil of Ocimum basilicum. Can J Bot. 1989;67:2085–2087. [Google Scholar]
- 21.Zollo PHA, Biyiti L, Tchoumbougnang F, Menut C, Lamaty G, Bouchet P. Aromatic plants of tropical Central Africa. Part XXXII. Chemical composition and antifungal activity of thirteen essential oils from aromatic plants of Cameroon. Flavour Fragr J. 1998;13:107–114. [Google Scholar]
- 22.Soliman KM, Badeaa RI. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002;40:1669–1675. doi: 10.1016/s0278-6915(02)00120-5. [DOI] [PubMed] [Google Scholar]
- 23.Fandohan P, Gbenou JD, Gnonlonfin B, Hell K, Marasas WFO, Wingfield M J. Effect of essential oils on the growth of Fusarium verticillioides and fumonisin contamination in corn. J Agr Food Chem. 2004;52:6824–6829. doi: 10.1021/jf040043p. [DOI] [PubMed] [Google Scholar]
- 24.Deabes MM, Abou El-Soud NH, Abou El-Kassem L. In Vitro Inhibition of Growth and Aflatoxin B1 Production of Aspergillus Flavus Strain (ATCC 16872) by Various Medicinal Plant Essential Oils. Maced J Med Sci. 2011;4(4):345–350. [Google Scholar]
- 25.Abou El-Soud NH, Deabes MM, Abou El-Kassem L, Khalil MY. Antifungal Activity of Family Apiaceae Essential Oils. Journal of Applied Sciences Research. 2012;8(10):4964–4973. [Google Scholar]
- 26.Bluma VP, Etcheverry MG. Application of essential oils in maize grain: Impact on Aspergillus section Flavi growth parameters and aflatoxin accumulation. Food Microbiolo. 2008;25:324–334. doi: 10.1016/j.fm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Hashim S, Aboobaker VS, Madhubala R, Bhattacharya RK, Rao AR. Modulatory effects of essential oils from spices on the formation of DNA adducts by aflatoxin B1 in vitro. Nutr Cancer. 1994;21:169–175. doi: 10.1080/01635589409514314. [DOI] [PubMed] [Google Scholar]
- 28.Lis-Balchin M, Deans SG, Eaglesham E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr J. 1998;13:104. [Google Scholar]
- 29.Dambolena SJ, Zunino PM, López GA, Rubinstein RH, Zygadlo AJ, Mwangi WJ, Thoithi NG, Kibwage OI, Mwalukumbi MJ, Kariuki TS. Essential oils composition of Ocimum basilicum L. and Ocimum gratissimum L. from Kenya and their inhibitory effect on growth and fumonisin production by Fusarium verticillioides. Innovat Food Sci Emerg Tech. 2010;11(2):239–422. [Google Scholar]
- 30.Reuveni R, Fleisher A, Putievsky E. Fungistatic activity of essential oils from Ocimum basilicum chemotypes. Phytopath. 1984;110:20–22. [Google Scholar]
- 31.Özkan M, Erkmen O. Antimicrobial activity of the essential oils of Turkish plant spices. Eur Food Res Technol. 2001;212:658–660. [Google Scholar]
- 32.Alpsoy L. Inhibitory effect of essential oil on aflatoxin activities. Afr J Biotechnol. 2010;9(17):2474–2481. [Google Scholar]


