Abstract
BACKGROUND:
In developing countries, Helicobacter pylori (H. pylori) infection is mainly acquired during childhood and may be a predisposing factor for peptic ulcer or gastric cancer later in life. Noninvasive diagnostic tools are particularly useful in children for screening tests and epidemiological studies. Data on serologic testing of children are lacking. Accurate noninvasive tests for diagnosing Helicobacter pylori infection in children are strongly required.
AIM:
The aim of this study was to evaluate the performance of a serological test (serum IgG antibody for H. pylori) in Egyptian children with recurrent abdominal pain necessitating endoscopy.
SUBJECTS AND METHODS:
One hundred children, referred to the endoscopy unit at Mansoura University. Upper endoscopy was done for each with rapid urease test (RUT) and histological examination as the gold standard test for detection of H. pylori infection. Serum samples were collected for detecting IgG for H. pylori infection.
RESULTS:
The mean age of the subjects included in the study was 7.23 ± 1.94 year. Serological test (IgG to H. pylori) was positive in 60% of all cases. A highly significant association between the standard test and the serological test at a cutoff > 10 U/ml at p = 0.001 were detected for the diagnosis of H. pylori infection. The sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio for the IgG antibody a cutoff > 10 U/ml, were 96.5%, 93%, 13.83, 0.038 respectively.
CONCLUSION:
Serum IgG antibody to H. pylori infection has a high diagnostic value and can be considered as a suitable and reliable noninvasive test for detection of H. pylori infection.
Keywords: Helicobacter pylori, serologic test, children, Egypt
Introduction
At least half of the world’s population is estimated to be infected with H. pylori. However, the prevalence of this infection varies widely across both geographic regions and ethnic groups. Overall, rates of H. pylori infection are markedly higher in developing countries compared to developed countries. For example, prevalence rates that approach or even exceed 90% have been reported in multiple studies conducted in Bangladesh, Egypt, Russia, Siberia, and Africa. In contrast, prevalence rates are much lower in developed regions, including the United States (6.8–79%), Europe (7.3–70%), and Australia (15.5–23%) [1-3]. Most infections are probably acquired in childhood, mainly via oral-oral or fecal-oral routes [4].
Helicobacter pylorus (H. pylori) causes gastrointestinal diseases such as gastritis and peptic ulcer in adults and children. In addition, previous reports have linked H. pylori infection with gastric cancer, mucosa-associated lymphoid tissue (MALT) lymphoma, iron deficiency anemia and thrombocytopenic purpura in children [5-7].
Diagnostic methods for H. pylori infection are usually classified as invasive and noninvasive. The invasive tests including histology, urease tests and culture, require upper gastrointestinal endoscopy for obtaining the diagnostic sample. On the other hand, non-invasive methods include the urea breath test, serology and stool antigen test. Bacterial culture from the gastric biopsy is the gold standard technique, and is recommended for antibiotic susceptibility test. Serology is used for initial screening and the stool antigen test is particularly used when the urea breath test is not available [8]. To define the value or usefulness of a diagnostic test, each test has to be compared to a gold standard [9]. There are few data on serologic tests for children, and thus it remains unclear whether the serology cutoffs used for adults are applicable to children.
The aim of this study was to determine the accuracy of the noninvasive serologic test in comparison with the invasive gold standard (endoscopy with biopsy analyses) for the diagnosis of H. pylori in Egyptian children with different upper gastrointestinal disorder.
Material and Methods
One hundred children (age range 4-10 years), referred to the endoscopy unit at Mansoura University Children Hospital for upper gastrointestinal disorder, were recruited in the present study. Informed consent was obtained from the parents of the children. The study was approved by the Ethical Committee of National Research Centre.
Patients were excluded from the study if they had received treatment with antibiotics, proton pump inhibitors, and H2 receptor antagonists within the last four weeks. Patients with previous gastric surgery, long-term use of corticosteroid and immunosuppressant, and history of bleeding or active gastrointestinal bleeding were also excluded from the study.
During upper endoscopy (Olympus GIF P 230; Olympus Optical Co., Tokyo, Japan), three gastric biopsies (two taken within 3 cm from the pylorus and one from the corpus) were taken. One biopsy was used for rapid urease test (RUT) (Dio-Helico, Diomed), and the remaining two biopsies were used for histological examination (Hematoxiline and Eosin staining) for H. pylori infection. A rapid urease test result was obtained by adding a biopsy specimen to a urea broth (NaCl, KH2PO4, and NaOH); the result of the test was considered positive if there was a change of urea broth color from yellow-gold to pink-red due to an increase in pH induced by H. pylori [10].
Serum samples were stored at −20°C until the laboratory assay was performed. Serum antibodies (IgG) to H. pylori were examined using a microplate enzyme immunoassay (EIA) and an antibody determination kit (E-Plate Eiken H. pylori antibody, Eiken Chemical Co., Ltd., Tokyo, Japan). All samples were analyzed according to the manufacturer’s instructions, and the cutoff point was set at 10 U/ml. All assays were performed by experimenters blinded to the clinical status of the patients.
The gold standard for the presence of H. pylori infection was defined as both the histological examination and rapid urease test being positive. The absence of H. pylori infection required both tests to be negative.
Statistical Analysis
Statistical analysis was carried out using the statistical package for social sciences, version 16 for windows (SPSS Inc., USA). Continuous data were expressed as mean± SD, while Categorical data were expressed as frequencies and percentages, and were analyzed with the two-tailed chi-square test. The chi-square(χ2) test, odds ratio (OR) and 95% confidence interval (CI) were used to evaluate the association between serum IgG at a cutoff 10 U/ml and the gold standard (RUT and histological examination) for detection of H. pylori infection. To assess the criterion validity of the serologic test, sensitivities, specificities, positive likelihood ratios, and negative likelihood ratios were estimated relative to the gold standard, across all possible cutoff values for the serologic test. Receiver operating characteristics (ROC) analysis was also conducted using the gold standard to assess the performance of serum IgG in detection of H. pylori infection. The 95% CI of the area under the ROC curve (AUC) was calculated. P value < 0.05 was considered statistically significant.
Results
Of the one hundred subjects included in the study 57 were male and 43 were female. Their age ranged from 4-10 years with a mean age 7.23 ± 1.94 year. Standard test were positive in 57% of all cases and negative in 43%. Serological test (IgG to H. pylori) was positive in 60% of all cases and negative in 40%.
Table 1 showed a highly significant association between the standard test and the serological test at a cutoff > 10 U/ml (Chi-square = 80.6, Odds ratio = 5.904, 95% Confidence interval = 4.069-7.739, and p < 0.001).
Table 1.
Association between serological test and the standard test
| Standard test | Total | χ2 | OR | 95% CI | p | |||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
| Serology test (IgG> 10 U/ml) | Positive | 55 | 3 | 58 | 80.6 | 5.904 | 4.069-7.739 | 0.000* |
| Negative | 2 | 40 | 42 | |||||
| Total | 57 | 43 | 100 | |||||
P < 0.005 is significant; χ2 = Chi-square; OR = Odds ratio; CI = Confidence interval.
As shown in Table 2, when the cutoff point of IgG antibody to H. pylori recommended by the manufacturer was used, the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio were 96.5%, 93%, 13.83, 0.038, respectively. Sensitivity and specificity at different cutoff values of IgG antibodies to H. pylori were shown in the Table 2.
Table 2.
Sensitivity and specificity of anti-H. pylori IgG antibody test for Egyptian children, by cutoff point
| Cutoff (U/ml) | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR- (95% CI) |
|---|---|---|---|---|
| 3 | 99.1 [0.922-0.999] | 98.9 [0.899-0.999] | 86.243 [5.481-135.11] | 0.009 [0.001-0.139] |
| 4 | 99.1 [0.922-0.999] | 85.3 [0.622-0.719] | 80,136 [0.969-1.115] | 0.187 [0.009-4.042] |
| 5 | 99.1 [0.922-0.999] | 87.9 [0.722-0.819] | 54.345 [1.14-1.659] | 0.031 [0.002-0.513] |
| 6 | 98.2 [0.907-0.997] | 86.3 [0.625-0.711] | 1.837 [1.387-2.432] | 0.038 [0.005-0.27] |
| 7-9 | 96.5 [0.881-0.99] | 93 [0.814-0.976] | 13.83 [4.638-41.239] | 0.038 [0.01-0.148] |
| 10 | 96.5 [0.881-0.99] | 93 [0.814-0.976] | 13.83 [4.638-41.239] | 0.038 [0.01-0.148] |
| 11 | 82.5 [0.706-0.902] | 95.3 [0.845-0.987] | 17.728 [4.557-68.974] | 0.184 [0.104-0.324] |
LR = likelihood ratio.
Figure 1 showed receiver operating characteristics (ROC) curve for anti-H. pylori IgG antibody test, with the histological examination and RUT as the gold standard. The area under the curve (AUC) for the anti-H. pylori IgG antibody test was 0.953 and 95% Confidence interval 0.907-0.999.
Figure 1.
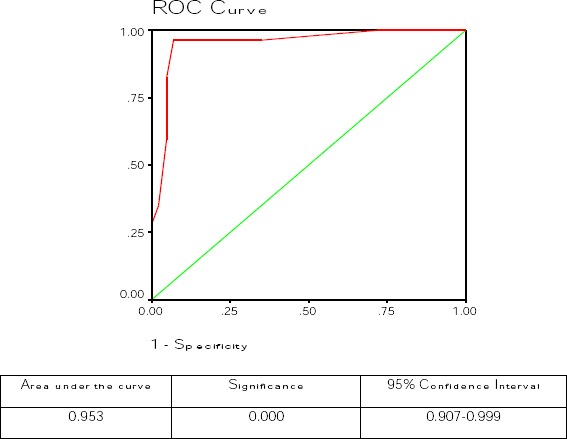
Receiver operating characteristics (ROC) curve for anti- H. pylori IgG antibody test, with the histological examination and RUT as the gold standard.
Discussion
H. pylori is acquired in childhood and survives in the human stomach. Noninvasive testing for H. pylori has been strongly recommended as it is less expensive and more patient-friendly than invasive testing that requires endoscopy [11]. Serology was one of the first methods used for diagnosis of H. pylori infection. Detection of antibodies is useful for detecting past or present exposure [12]. In fact, a limitation of serology tests is the failure to distinguish between past and current H. pylori infection [13].
Serological tests have several advantages, namely they are non-invasive and they do not produce false negative results in patients receiving treatment (proton pump inhibitors and antibiotics) or presenting acute bleeding [14]. The success of a serology test depends on the use of antigens that are present in H. pylori strains from a given population. Moreover, kits developed using H. pylori strains from the west are not suitable for detecting H. pylori infection in the East [15]. The use of high-molecular-weight cell associated antigens that are conserved in H. pylori strains overcomes this limitation [16].
In the present study, a highly significant association was detected between the standard test for H. pylori (histological examination and RUT) and serological test (IgG antibody for H. pylori) for detection of H. pylori infection. A sensitivity and specificity of 96.5% and 93% were detected using a cutoff level of IgG of 10 U/ml with AUC of 0.953 with significance of <0.001 at the ROC curve.
Previous studies reported debate regarding the use of serologic tests for detection of H. pylori infection in children [18, 19]. Okuda et al., [18] studied 157 children for comparing antibodies to H. pylori (IgG and IgA enzyme linked immunosorbent assay (ELISA)) with H. pylori stool antigen (HpSA) enzyme immunoassay. They concluded that an immature immune response or tolerance to H. pylori exists in childhood and sero diagnosis of H. pylori infection is less useful in children aged below 10. Frenck et al., [19] studied children between 2 and 17 years of age, evaluated at the Cairo University School of Medicine Pediatric Gastroenterology Clinic who were already scheduled for upper endoscopy. Rapid urease, histology, and culture were done as invasive tests. Urea breath test performed. Stool and serum samples were tested for the presence of H. pylori by using commercially available enzyme-linked immunosorbent assay-based technology. They concluded that urea breath test and stool enzyme-linked immunosorbent assay kit had the highest sensitivity and specificity (sensitivity and specificity: 98 and 89 [urea breath test] and 94 and 81 [HpSA], respectively). In contrast to the present study, the serologic kit in their results had an unacceptably low sensitivity (50%).
In agreement with the results of the current study, a 2008 meta-analysis of 42 studies of children showed a sensitivity of 79.2% (95% CI, 77.3–81.0) and a specificity of 92.4% (95% CI, 91.6–93.3) for a serologic IgG antibody test [19]. In 2014, Ueda et al., [20] studied the performance of the E-plate anti-H. pylori IgG antibody test and it was found to be comparable to that of the stool antigen test. In concordance with the result of the present study, they found sensitivity, specificity, AUC of IgG (cutoff value 10 U/ml) of 91.18%, 97.44%, o.96 respectively. They concluded that IgG antibody to H. pylori might be useful in epidemiologic studies involving large numbers of participants. In agreement with the present study, Alam El-Din et al., [21] concluded that diagnosis of H. pylori infection by noninvasive methods, including the serum antibody test, revealed a sensitivity and positive predictive value of 88.9% and 94.2%, respectively.
A previous study released by (HO and Marshall,) [22] recommended that, in studies using serologic tests, researchers should reexamine the test results and determine if it is necessary to adopt specialized cutoff points in children. Accordingly, the performance of the serologic test was evaluated in the present study by using various cutoff points and it was found that cutoff points in the range of 7 to9 U/ml yielded optimal sensitivity, specificity, and positive likelihood ratio.
The present study has several limitations. First, because only a subset of children for whom both blood and endoscopy was available were included in this study. The results need to be replicated in other, larger samples of consecutive patients. Second, IgG antibodies can be detected approximately 3 weeks after H. pylori infection. Therefore, the latent period between H. pylori infection and antibody production may be a source of misclassification. Finally, information, such as number of siblings and birth order, have not been collected that may be related to transmission of H. pylori infection among children.
In conclusion, IgG antibody for the detection of H. pylori infection seems to be a good alternative for invasive diagnostic tests such as urea breath test. IgG antibody to H. pylori has a high diagnostic value and can be considered as a suitable and reliable noninvasive test for detection of H. pylori infection. The choice of test kit depends on the sensitivity and specificity in each region and the circumstances of each patient. Further studies are needed to explore genetic differences among populations and their effects on immune responses.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Elfant AB. Helicobacter pylori:History, Prevalence, and Association with disease. Gastroenterology & Hepatology. 2012;8(Supplement 7)(11):3–6. [Google Scholar]
- 2.Mansour MMHK, Al Hadidi KH M, Omar MA. Helicobacter pylori and recurrent abdominal pain in children:Is there any relation? Tropical Gastroenterology. 2012;33(1):55–61. doi: 10.7869/tg.2012.9. [DOI] [PubMed] [Google Scholar]
- 3.Hamed ME, Hussein HM, El Sadany HF, Elgobashy AA, Atta AH. Seroprevalence of Helicobacter pylori infection among family members of infected and non-infected symptomatic children. J Egypt Soc Parasitol. 2013;43(3):755–66. doi: 10.12816/0006432. [DOI] [PubMed] [Google Scholar]
- 4.Prasanthi CH, Prasanthi NL, Manikiran SS, Rama Rao NN. Focus on current trends in the treatment of Helicobacter pylori infection:An update. Inter J Pharm Sci Rev Res. 2011;1:42–51. [Google Scholar]
- 5.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 6.Tan HJ, Goh KL. Extra gastrointestinal manifestations of Helicobacter pylori infection:Facts or myth?A critical review. J Dig Dis. 2012;13:342–9. doi: 10.1111/j.1751-2980.2012.00599.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Wang J, Tanaka T, Hosono A, Ando R, Soeripto S, Ediati Triningsih FX, Triono T, Sumoharjo S, Astuti EY, Gunawan S, Tokudome S. Association between HLA-DQ genotypes and haplotypes vs. Helicobacter pylori infection in an Indonesian population. Asian Pac J Cancer Prev. 2012;13:1247–51. doi: 10.7314/apjcp.2012.13.4.1247. [DOI] [PubMed] [Google Scholar]
- 8.Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection -recent developments in diagnosis. World J Gastroenterol. 2014;20(28):9299–9313. doi: 10.3748/wjg.v20.i28.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarner J, Kalach N, Elitsur Y, Koletzko S. Helicobacter pylori diagnostic tests in children:Review of the literature from 1999 to 2009. Eur J Pediatr. 2010;169:15–25. doi: 10.1007/s00431-009-1033-x. [DOI] [PubMed] [Google Scholar]
- 10.Sabbi T, De Angelis P, Colistro F, Dall’Oglio L, di Abriola GF, Castro M. Efficacy of noninvasive tests in the diagnosis of Helicobacter pylori infection in pediatric patients. Arch Pediatr Adolesc Med. 2005;159:238. doi: 10.1001/archpedi.159.3.238. [DOI] [PubMed] [Google Scholar]
- 11.Valliani A, Khan F, Chagani B, Khuwaja AK, Majid S, Hashmi S, Nanji K, Valliani S. Factors associated with Helicobacter pylori infection, results from a developing country -Pakistan. Asian Pac J Cancer Prev. 2013;14:53–6. doi: 10.7314/apjcp.2013.14.1.53. [DOI] [PubMed] [Google Scholar]
- 12.Burucoa C, Delchier JC, Courillon-Mallet A, de Korwin JD, Mégraud F, Zerbib F, Raymond J, Fauchère JL. Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter. 2013;18:169–179. doi: 10.1111/hel.12030. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, Kim CH, Kim D, Chung SJ, Song JH, Kang JM, Yang JI, Park MJ, Kim YS, Yim JY, Lim SH, Kim JS, Jung HC, Song IS. Prospective evaluation of a new stool antigen test for the detection of Helicobacter pylori, in comparison with histology, rapid urease test, (13)C-urea breath test, and serology. J Gastroenterol Hepatol. 2011;26:1053–1059. doi: 10.1111/j.1440-1746.2011.06705.x. [DOI] [PubMed] [Google Scholar]
- 14.Braden B. Diagnosis of Helicobacter pylori infection. BMJ. 2012;344:e828. doi: 10.1136/bmj.e828. [DOI] [PubMed] [Google Scholar]
- 15.McNulty CA, Lehours P, Mégraud F. Diagnosis of Helicobacter pylori Infection. Helicobacter. 2011;16(Suppl 1):10–18. doi: 10.1111/j.1523-5378.2011.00875.x. [DOI] [PubMed] [Google Scholar]
- 16.Marchildon PA, Sugiyama T, Fukuda Y, Peacock JS, Asaka M, Shimoyama T, Graham DY. Evaluation of the effects of strain-specific antigen variation on the accuracy of serologic diagnosis of Helicobacter pylori infection. J Clin Microbiol. 2003;41:1480–1485. doi: 10.1128/JCM.41.4.1480-1485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuda M, Miyashiro E, Koike M, Tanaka T, Bouoka M, Okuda S, Yoshikawa N. Serodiagnosis of Helicobacter pylori infection is not accurate for children aged below 10. Pediatr Int. 2002;44:387–90. doi: 10.1046/j.1442-200x.2002.01585.x. [DOI] [PubMed] [Google Scholar]
- 18.Frenck RW, Jr, Fathy HM, Sherif M, Mohran Z, El Mohammedy H, Francis W, Rockabrand D, Mounir BI, Rozmajzl P, Frierson HF. Sensitivity and specificity of various tests for the diagnosis of Helicobacter pylori in Egyptian children. Pediatrics. 2006;118:e1195–202. doi: 10.1542/peds.2005-2925. [DOI] [PubMed] [Google Scholar]
- 19.Leal YA, Flores LL, García-Cortés LB, Cedillo-Rivera R, Torres J. Antibody-based detection tests for the diagnosis of Helicobacter pylori infection in children:A meta-analysis. PLoS One. 2008;3:e3751. doi: 10.1371/journal.pone.0003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda J, Okuda M, Nishiyama T, Lin Y, Fukuda Y, Kikuchi S. Diagnostic accuracy of the E-plate serum antibody test kit in detecting Helicobacter pylori infection among Japanese children. J Epidemiol. 2014;24(1):47–51. doi: 10.2188/jea.JE20130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam El-Din HM, Hashem AG, Ragab YM, Hussein IL, Mohamed DB, Mohamed el-CB. Evaluation of noninvasive versus invasive techniques for the diagnosis of Helicobacter pylori infection. Appl Immunohistochem Mol Morphol. 2013;21(4):326–33. doi: 10.1097/PAI.0b013e31826e4e61. [DOI] [PubMed] [Google Scholar]
- 22.Ho B, Marshall BJ. Accurate diagnosis of Helicobacter Pylori. Serologic testing. Gastroenterol Clin North Am. 2000;29:853–62. doi: 10.1016/s0889-8553(05)70152-7. [DOI] [PubMed] [Google Scholar]


