Abstract
Objectives
The capacity of a human cell line to secrete recombinant factor VIII with a F309S point mutation was investigated, as was the effect of the addition of chemical chaperones (betaine and sodium-4-phenylbutyrate) on the secretion of factor VIII.
Methods
This work used a vector with a F309S mutation in the A1 domain to investigate FVIII production in the HEK 293 human cell line. Factor VIII activity was measured by chromogenic assay. Furthermore, the effects of chemical drugs on the culture were evaluated.
Results
The addition of the F309S mutation to a previously described FVIII variant increased FVIII secretion by 4.5 fold. Moreover, the addition of betaine or sodium-4-phenylbutyrate increased the secretion rate of FVIIIΔB proteins in HEK 293 cells, but the same effect was not seen for FVIIIΔB-F309S indicating that all the recombinant protein produced had been efficiently secreted.
Conclusion
Bioengineering factor VIII expressed in human cells may lead to an efficient production of recombinant factor VIII and contribute toward low-cost coagulation factor replacement therapy for hemophilia A. FVIII-F309S produced in human cells can be effective in vivo.
Keywords: HEK 293 cells, Recombinant factor VIII, F309S mutation, Betaine, Sodium-4-phenylbutyrate
Introduction
Hemophilia A is an inherited disorder linked to the X chromosome which results in deficiency or abnormality of blood coagulation factor VIII (FVIII). The current therapy for hemophilia A patients is the intravenous infusion of plasma-derived or recombinant factor VIII (rFVIII). The major problem of replacement therapy with rFVIII is the low productivity which increases the cost of therapy. The high costs are mostly attributable to several biochemical characteristics of the recombinant protein such as the retention of FVIII within the endoplasmic reticulum (ER) due to its interaction with different ER chaperones.1
Several studies have provided important information that helped in the design and development of techniques to bioengineer rFVIII with better secretion efficiency.2, 3 Swaroop4 developed an rFVIII with a point mutation in the A1 domain (Phe309Ser) to increase secretion of the protein.4 However despite many significant advances in the heterologous expression of FVIII, a low production rate persists.
Recently, it was suggested that recombinant concentrates might be associated with a higher incidence of inhibitor development.5 This may be associated with the different glycosylation pattern found in hamster cell lines [available products are produced in Chinese hamster ovary (CHO) and Baby hamster kidney (BHK) cells] compared to human cells: Gal·1-3Galβ1-(3) 4GlcNAc (α-Gal) epitopes and N-glycolylneuraminic acid (Neu5Gc) are not present in humans.6
As an alternative, human cell lines can be used to produce recombinant coagulation factors. This heterologous expression system is capable of producing proteins with post-translational modifications similar to their original counterpart which might reduce the possibility of immunogenic reactions.6
This study investigated the production of FVIII containing the F309S point mutation in the HEK 293 human cell line and the efficacy of the molecule in vivo in a hemophilia A mouse model.
Methods
Lentiviral vectors
A human immunodeficiency virus-1 (HIV-1)-based lentiviral vector containing the human FVIII cDNA with a deletion of a large portion of the B domain (FVIIIΔB) and a second vector with the Phe309Ser mutation (A1 domain) were used to increase the secretion of the protein. The transgene expression of both vectors was driven by the internal myeloproliferative sarcoma virus (MSV) promoter with selection using the neomycin gene. The vectors were kindly provided by Daniel Gibson from the Craig Venter Institute.
Transient expression
FVIIIΔB and FVIIIΔB-F309S constructs were transfected (10 μg of DNA) into HEK 293 cells using lipofectamine® 2000 reagent (Life Technologies) following the manufacturer's instructions. Conditioned medium was harvested at 48 h and 72 h after transfection and analyzed using Asserachrom® VIII:Ag (Diagnostica Stago).
Production of lentiviral particles
To generate lentiviral particles, the construct DNAs were transiently introduced into 293FT cells by triple co-transfection with the packaging construct pCMVΔR8.91 encoding gag, pol, and rev and the pseudotyping construct pMD2.VSVG coding for the vesicular stomatitis virus glycoprotein (VSV-G). Transfection of plasmid DNAs was performed using lipofectamine® (Life Technologies) following the manufacturer's instructions. Viral particles were harvested at 48 h and 72 h post-transfection and filtered through a 0.22 μm filter (Millex®-GV). The viral particles were concentrated by ultracentrifugation (1.40 h at 31,000 × g). The concentrated virus was stored at −80 °C.
Lentiviral titers were determined by quantitative polymerase chain reaction (PCR) of genomic DNA from transduced 293FT cells. Briefly, 1 × 105 293FT cells were seeded in a six-well plate and transduced with serial dilutions of vector supplemented with 8 μg/mL polybrene (Sigma-Aldrich). Genomic DNA was extracted from transduced cells 72 h after transduction using the DNeasy Blood and Tissue Kit (Qiagen) as per the manufacturer's instructions, and quantitative PCR was carried out in duplicate on samples to determine both total viral DNA and human β-actin levels (ACTB). The integrating copy number per transduced cell was normalized assuming two ACTB alleles per cell and infectious units per milliliter were calculated as total viral DNA/(ACTB/2) × (1 × 105) × dilution factor. Primers and probe used have been described previously.7
Expression of stable HEK 293 cells
HEK 293 cells were maintained in Dulbecco's modified eagle medium (DMEM – Life Technology) supplemented with 10% (v/v) fetal bovine serum (Thermo Scientific), 1% (v/v) penicillin/streptomycin solution (Gibco) in 5% CO2 at 37 °C. Cells were plated onto 24-well plates (approximately 100,000 cells) and transduced at a multiplicity of infection (MOI) of 40 for transduction 1–4, MOI of 80 for transduction 5, and MOI of 160 for transduction 6–10 with viral particles in a final volume of 500 μL of DMEM supplemented with 8 μg/mL of polybrene (Sigma). At 24 h post-transduction, virus-containing media was replaced with fresh DMEM and cells were allowed to recover by culturing overnight at 37 °C in 5% CO2. Subsequently, transduced cells were replaced for another round of transduction until the tenth cycle and analyzed for FVIII activity, copy number integration, transcript expression and protein analysis.
Factor VIII quantification and antigen analysis
FVIII activity was determined by a two-stage assay using the COAMATIC FVIII (Chromogenix) according to the manufacturer's instructions. Normal human reference plasma was used to generate the standard curve. The FVIII antigen was quantified by an enzyme-linked immunosorbent assay (ELISA) method using the Asserachrom VIII:Ag (Stago) commercial Kit according to the manufacturer's instructions.
Reverse transcription polymerase chain reaction analysis
RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed using the High Capacity cDNA reverse transcription Kit (Life Technology). This was followed by PCR with primers BiPfor (5′-CCA ACG CCA AGC AAC CAA AG-3′), BiPrev(5′-CTT CTC CCC CTC CCT CTT AT-3′), GAPDHfor (5′-GCC TCA AGA TCA TCA GCA ATG C-3′), GAPDHrev (5′-CAT GGA CTG TGG TCA TGA GTC CT-3′). SYBR Green real-time PCR was carried out using ABI Prism 7500 Sequence Detection System (Applied Biosystems) in a total volume of 10 μL containing 2.5 pM of each specific primer and 2 μL of cDNA. Gliceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control to standardize the amount of applied RNA. Relative immunoglobulin-binding protein (BiP) transcription levels were measured by applying the 2−Δ(ΔCt) equation.
Western blot analysis
Conditioned medium was collected from transduced and nontransduced HEK 293 cells after 72 h and total protein was quantified by the BCA Protein Assay Kit (Pierce). The cultured cells were lysed in ice-cold lysis buffer containing protease inhibitors (Complete mini-protease inhibitor cocktail, Roche) and centrifuged at 16,000 × g for 15 min. Total protein was quantified by the BCA Protein Assay Kit (Pierce). Total protein (35 μg and 40 μg extracted from conditioned medium and lysed cells, respectively) was separated by SDS-PAGE (4–20% Mini-PROTEAN, BIO-RAD), transferred to nitrocellulose membranes (40 μm, Hybond-C Extra, Amersham Bioscience) and probed with anti-FVIII light chain mouse antibody (Santa Cruz Biotechnology) or anti-BiP/GRP78 mouse antibody (BD Bioscience). Internal control was achieved using anti β-actin mouse antibody (Sigma-Aldrich).
Chemical chaperone treatment of transduced cells
Transduced cells were seeded at a cell density of about 3.5 × 106 cells per plate and were incubated in the presence of chemical chaperones: betaine (Sigma-Aldrich) and sodium 4-phenylbutyrate (Sigma-Aldrich) at different concentrations. After 72 h of incubation with chemical chaperones, levels of active FVIII were determined in the supernatants. Experiments were performed in duplicate.
In vivo assay
Hemophilia A mice (B6;129S4-F8tm1kaz/J) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Adult mice, aged 8–12 weeks, were used for tail clipping. All protocols were conducted in accordance with the Ethical Code for Animal Experimentation of the Council for International Organizations of Medical Sciences (CIOMS) and the Colégio Brasileiro de Experimentação Animal (COBEA). This study was approved by the Ethics Committee for Animal Research of the Universidade de São Paulo (USP), Ribeirão Preto (#14.1.784.53.8).
Severe tail-bleeding model
The tail-clip challenge was designed to analyze hemophilic A mice after receiving one unit of culture supernatant containing FVIIIΔB-F309S (n = 3 mice), FVIIIΔB (n = 3 mice) or phosphate-buffered saline (PBS) (n = 3 mice). Mice were anesthetized with 4.5% isoflurane and received culture supernatant via retro orbital injection. After 10 min, bleeding was induced by cutting 1 cm of the tail and animal survival was monitored over 50 h.
Statistical analysis
Results are expressed as means ± standard error of the mean (SEM) or standard deviation (SD) as appropriate. Student's unpaired t-test and 95% confidence interval were used for comparisons between the groups. Two-way analysis of variance (ANOVA) was used to compare differences between the indicated groups and the log-rank test was used to compare the survival curves after the tail-clipping challenge. p-Values <0.05 were considered statistically significant.
Results
Transient assay
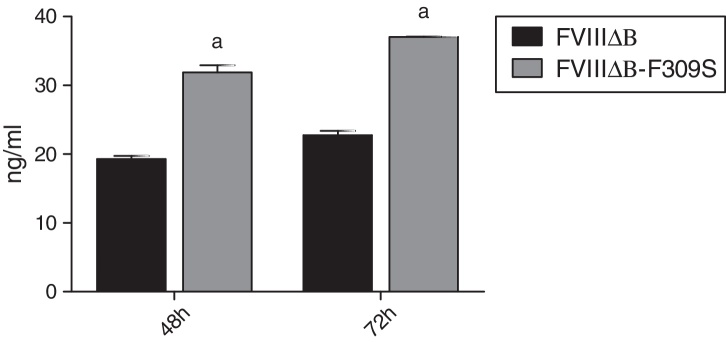
In order to study the role of the F309S mutation on the secretion and function of FVIII in human cell lines, a molecule with this mutation was synthesized and cloned into a lentiviral vector. After, its secretion was analyzed by transient transfection in HEK 293 cells. FVIII two-stage activity assays performed on conditioned media harvested 48 h and 72 h after transfection revealed that there was a better secretion of FVIIIΔB-F309S over the FVIIIΔB levels (Figure 1).
Figure 1.
Transient production of FVIIIΔB and FVIIIΔB-F309S in HEK 293 cells. The factor FVIII production was measured by the enzyme-linked immunosorbent assay (ELISA) technique. aStatistically significant differences between groups using Student's unpaired t-test (FVIIIΔB: p-value = 0.0042; FVIIIΔB-F309S: p-value = 0.0009).
The FVIIIΔB-F309S presented on average, 1.5 and 2 fold higher secretion than FVIIIΔB in HEK 293 cells, after 48 h and 72 h of transfection. These results were reproducible and consistent in three separate transfection experiments.
Generation of the HEK 293 cell line stably expressing FVIIIΔB and FVIIIΔB-F309S
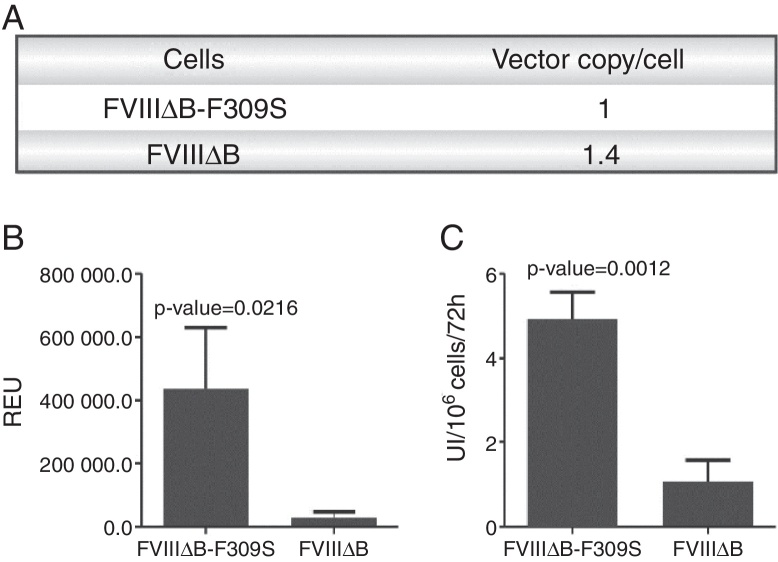
To compare whether the mutation could also result in increased secretion of FVIII in stable expression, HEK 293 cell lines stably expressing FVIIIΔB and FVIIIΔB-F309S were generated. These cell lines were generated by lentiviral infection followed by selection using geneticin. The number of lentiviral vectors integrated into the genome were quantified for the two generated strains (Figure 2A). The cell line FVIIIΔB-F309S has one copy/cell of the lentiviral vector whereas the FVIIIΔB has 1.4 copies/cell integrated in its genome.
Figure 2.
(A) Number of lentiviral vectors integrated into genome; (B) relative expression units (REU) of both recombinant FVIII expressions and (C) Factor VIII second-stage activity by a chromogenic assay (COAMATIC). Data presented are the mean of three independent experiments and the error bars represent the standard deviation. Asterisks designate statically significant differences between groups using two-way ANOVA
The cell line containing the FVIII mutation expresses 9-fold more FVIII mRNA than the cell with FVIIIΔB (Figure 2B). The level of protein secreted in the cell with FVIIIΔB-F309S is greater than the cell line with FVIIIΔB. The cell line containing the F309S mutation secreted an average of five IU/106 cells/72 h of FVIII, that is, 4.5-fold more efficiently than FVIIIΔB as determined by two-stage clotting activity assay (Figure 2C).
Characterization of secreted FVIIIΔB-F309S
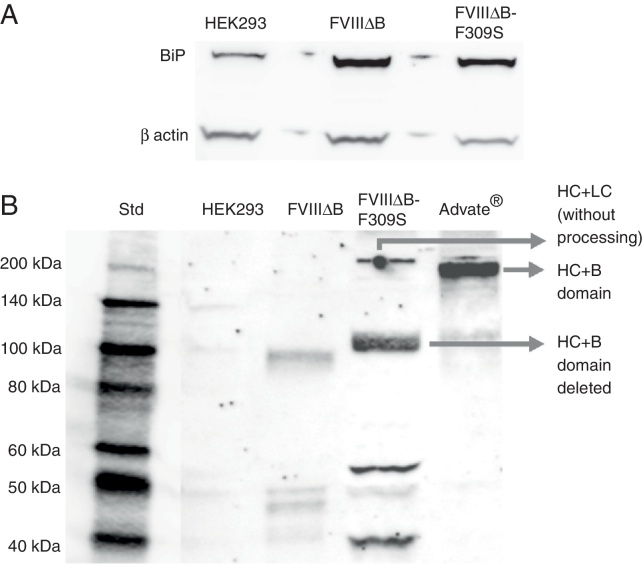
FVIII protein has a 110-amino acid region within the A1-domain that inhibits its secretion and contains multiple short peptide sequences that have potential to bind to BiP. The low level of FVIII secretion correlates with binding to the BiP, within the lumen of the ER. To assess whether the high expression of FVIII induces the production of BiP, the BIP level was evaluated by Western blot. Figure 3 shows that FVIII expression leads to an increased amount of BiP. However, there are no significant differences in BiP amounts between HEK 293 cells expressing FVIIIΔB and those expressing FVIIIΔB-F309S (Figure 3A).
Figure 3.
Characterization of FVIII producing cell lines by Western blot. (A) Cell lysates were fractionated by SDS–PAGE, and immunoblotted with BiP (78 kDa) and b-actin (42 kDa) antibodies. (B) Supernatants of non-transduced HEK 293 cells, 293 cells transduced with FVIIIΔB and FVIIIΔB-F309S, and the control Advate® immunoblotted with anti-heavy chain FVIII.
In order to characterize the secreted FVIIIr, Western blot was performed with the anti-heavy chain monoclonal antibody. As expected, the HEK 293 cells expressing FVIIIΔB-F309S secrete more FVIII than HEK 293 cells expressing FVIIIΔB. It was possible to detect a 90 kDa band referring to the heavy chain (Figure 3B).
However, it was not possible to identify clearly the band referring to the heavy chain in the FVIIIΔB sample, probably because this cell produces a low amount of FVIII thereby hindering antibody binding. In the band related to the heavy chain, another band of ∼200 kDa was detected in commercial FVIII corresponding to the heavy chain with B domain (Advate®, Baxter). FVIIIΔB-F309S, which has the partial deletion of B domain, also shows a band of ∼200 kDa, suggesting that part of the FVIIIr released is composed of heavy and light chains without intracellular cleavage (Figure 3B).
The FVIIIΔB-F309S sample also showed fragments of 53 kDa and 43 kDa, which are probably related to the degradation of FVIII. The 53 kDa band may correspond to part of the A2 domain with the A1 domain (∼55 kDa) and the 43 kDa is related to the A2 domain (Figure 3B).
Chemical chaperone supplementation increases the secretion of FVIIIΔB but not of FVIIIΔB-F309S
In addition, two different compounds termed chemical chaperones (CC) were tested. These compounds are responsible for non-specifically stabilizing native protein conformations and supporting escape from the endoplasmic quality control system. Members of different compound classes of CC (Betaine and sodium-4-phenylbutyrate) were tested for their effect on FVIIIΔB and FVIIIΔB-F309S expression in HEK 293 cells. Betaine and sodium-4-phenylbutyrate were used at concentrations of 1 mM and 100 mM. The drugs were tested individually and in combination in the modified cell lines expressing FVIIIΔB and FVIIIΔB-F309S.
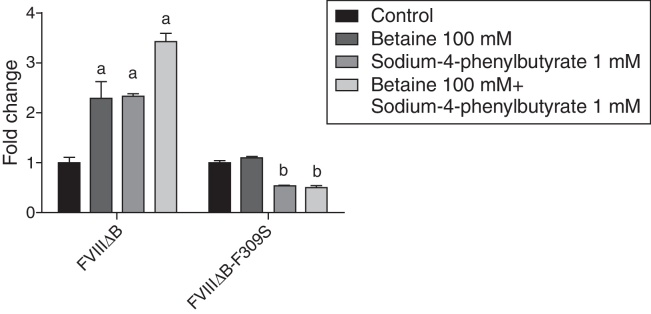
The addition of betaine or sodium-4-phenylbutyrate increased the secretion rate of FVIII-ΔB proteins in HEK 293 cells (Figure 4). The combination of both drugs led to a further increase in the secretion of FVIIIΔB. However, no increase in the secretion of FVIIIΔB-F309S was observed with the addition of betaine or sodium-4-phenylbutyrate or with the combination of these two compounds (Figure 4).
Figure 4.
Effect of the betaine and sodium-4-phenylbutyrate on FVIII secretion. HEK 293 cells expressing FVIIIΔB or FVIIIΔB-F309S were incubated with betaine and sodium-4-phenylbutyrate alone or in combination. FVIII activity was determined in cell supernatants after 72 h by chromogenic assay (n = 3). aStatistically significant differences between groups using two-way ANOVA. p-Value = 0.0045. bStatistically significant differences between groups using two-way ANOVA. p-Value = 0.0005.
FVIIIΔB and FVIIIΔB-F309S are functionally active in hemophilia A mice.
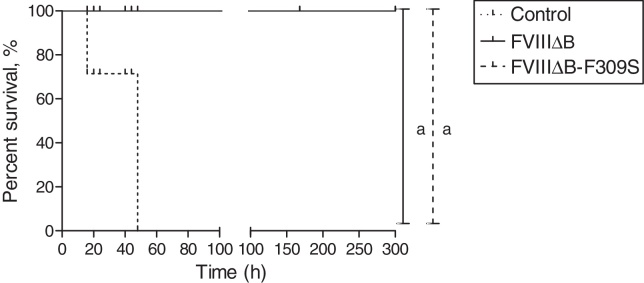
In order to investigate the FVIIIΔB and FVIIIΔB-F309S activity profile in vivo and whether they are able to stop bleeding in hemophilia A mice, a dosage of 1 IU/mL of FVIIIΔB, FVIIIΔB-F309S or PBS (as a negative control) was applied and then tail bleeding was induced. Both FVIIIΔB and FVIIIΔB-F309S groups were successful in stopping the bleeding. Furthermore, all mice in the PBS group died within 50 h (Figure 5).
Figure 5.
Survival curve of hemophilia A mice submitted to treatment with FVIII-ΔB (n = 4), FVIIIΔB-F309S (n = 4) or phosphate-buffered saline (n = 4) and challenged by tail clipping. aStatistically significant values (p-value = 0.0136).
Discussion
Recombinant FVIII protein is one of the most complex proteins for industrial production due to the low efficiency of gene transcription, protein interactions with retention in the ER, inappropriate transport from the ER to the Golgi apparatus and the instability of the secreted protein.8, 9, 10 Over the years, other research groups have studied ways to improve the expression, secretion and to increase the half-life of coagulation factors, especially FVIII.2, 11
In this study, the HEK 293 cell line was chosen due to the various advantages offered by this strain, for example, robust pattern of growth, easy maintenance, and high transfection efficiency and production of proteins.12 The transient production of rFVIII using the serum-free HEK 293 cell line in suspension has been demonstrated yielding approximately 0.64 IU/mL FVIIIr.13 More recently, the pharmaceutical industry has developed the first rFVIII produced in human cells (using the HEK293F strain) which showed efficiency in terms of safety and production.14
The current study showed that the F309S mutation was able to increase FVIII secretion by 3-fold compared to FVIIIΔB in a stable HEK 293 cell line. This result is in accordance with previous works.2, 4 It is known that the inefficient secretion of FVIII is correlated with binding to the protein identified as BiP, also known as the glucose-regulated protein 78 (GRP78) within the lumen of the ER.15 Marquette et al. located a 110 amino acid region within the A1 domain that inhibits FVIII secretion. This region is clustered with multiple short peptide sequences that have potential to bind to BiP.16 The results of Western Blot in this study revealed that both FVIIIΔB-F309S and FVIIIΔB increased BiP expression, although FVIII production was not affected.
Another strategy used in this work to improve FVIII secretion was to add chemical chaperones to culture cells. Betaine is a chemical chaperone that can inhibit the aggregation of FVIII and restore the intracellular trafficking of loosely coiled proteins. In addition to betaine, sodium butyrate, an organic compound that has various effects on cell cultures, was also tested; one effect is the induction of gene expression by histone hyperacetylation promoting the activation of genes.17 This study shows that HEK 293 cells expressing FVIIIΔB that had been treated with betaine and sodium butyrate increased FVIII production. However, no effect was observed in HEK 293 cells expressing FVIIIΔB-F309S treated with betaine. Roth18 tested several chemical chaperones, including betaine and reported that this compound increased the secretion of FVIII because of the increased solubility of intracellular FVIII aggregates and improved transport from the ER to the Golgi apparatus.18 By contrast, the data of this study suggest that all the FVIII produced by HEK 293 cells expressing FVIIIΔB-F309S was secreted out of the cell and did not form aggregates.
On the other hand, sodium butyrate increased FVIII production in HEK 293 cells expressing FVIIIΔB either alone or in combination with betaine. Previous studies have showed that sodium butyrate produces a wide variety of effects on cells in culture: arrest of cell growth, reversion of the transformation characteristics of cells, and induction of proteins, including enzymes, peptide hormones and hemoglobin.17 However, the same effect was not noticed in HEK 293 cells expressing FVIIIΔB-F309S with the reason for this difference not being clear and thus further investigations are needed.
Conclusion
The main contribution of this work is the production of a FVIII molecule with a high secretion rate (with the F309S mutation in the A1 domain), in order to increase the productivity and decrease the production cost. Furthermore, this study utilized a human cell line, HEK 293, to produce an rFVIII more similar to the existing FVIII in human plasma, and less immunogenic than the rFVIII commercially produced in hamster cells.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Cleide Araújo Silva and Sandra Navarro Bresciani who helped with the animal experiments and drew the figures, respectively. This work was supported by CAPES, FAPESP and CNPq.
References
- 1.Dorner A.J., Wasley L.C., Kaufman R.J. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989;264(34):20602–20607. [PubMed] [Google Scholar]
- 2.Miao H.Z., Sirachainan N., Palmer L., Kucab P., Cunningham M.A., Kaufman R.J. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103(9):3412–3419. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- 3.Dooriss K.L., Denning G., Gangadharan B., Javazon E.H., McCarty D.A., Spencer H.T. Comparison of factor VIII transgenes bioengineered for improved expression in gene therapy of hemophilia A. Hum Gene Ther. 2009;20(5):465–478. doi: 10.1089/hum.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaroop M., Moussalli M., Pipe S.W., Kaufman R.J. Mutagenesis of a potential immunoglobulin-binding protein-binding site enhances secretion of coagulation factor VIII. J Biol Chem. 1997;272(39):24121–24124. doi: 10.1074/jbc.272.39.24121. [DOI] [PubMed] [Google Scholar]
- 5.Calvez T., Chambost H., Claeyssens-Donadel S., d’Oiron R., Goulet V., Guillet B. Recombinant factor VIII products and inhibitor development in previously untreated boys with severe hemophilia A. Blood. 2014;124(23):3398–3408. doi: 10.1182/blood-2014-07-586347. [DOI] [PubMed] [Google Scholar]
- 6.Picanco-Castro V., Biaggio R.T., Cova D.T., Swiech K. Production of recombinant therapeutic proteins in human cells: current achievements and future perspectives. Protein Pept Lett. 2013;20(12):1373–1378. doi: 10.2174/092986652012131112130322. [DOI] [PubMed] [Google Scholar]
- 7.Matsui H., Shibata M., Brown B., Labelle A., Hegadorn C., Andrews C. Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo factor VIII expression from lentivirally engineered endothelial progenitors. Stem Cells. 2007;25(10):2660–2669. doi: 10.1634/stemcells.2006-0699. [DOI] [PubMed] [Google Scholar]
- 8.Soukharev S., Hammond D., Ananyeva N.M., Anderson J.A., Hauser C.A., Pipe S. Expression of factor VIII in recombinant and transgenic systems. Blood Cells Mol Dis. 2002;28(2):234–248. doi: 10.1006/bcmd.2002.0508. [DOI] [PubMed] [Google Scholar]
- 9.Chuah M.K., Vandendriessche T., Morgan R.A. Development and analysis of retroviral vectors expressing human factor VIII as a potential gene therapy for hemophilia A. Hum Gene Ther. 1995;6(11):1363–1377. doi: 10.1089/hum.1995.6.11-1363. [DOI] [PubMed] [Google Scholar]
- 10.Becker S., Simpson J.C., Pepperkok R., Heinz S., Herder C., Grez M. Confocal microscopy analysis of native, full length and B-domain deleted coagulation factor VIII trafficking in mammalian cells. Thromb Haemost. 2004;92(1):23–35. doi: 10.1160/TH03-06-0360. [DOI] [PubMed] [Google Scholar]
- 11.Dumont J.A., Liu T., Low S.C., Zhang X., Kamphaus G., Sakorafas P. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119(13):3024–3030. doi: 10.1182/blood-2011-08-367813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas P., Smart T.G. HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51(3):187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Swiech K., Kamen A., Ansorge S., Durocher Y., Picanço-Castro V., Russo-Carbolante E.M. Transient transfection of serum-free suspension HEK 293 cell culture for efficient production of human rFVIII. BMC Biotechnol. 2011;11:114. doi: 10.1186/1472-6750-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentino L.A., Negrier C., Kohla G., Tiede A., Liesner R., Hart D. The first recombinant FVIII produced in human cells – an update on its clinical development programme. Haemophilia. 2014;20(Suppl. 1):1–9. doi: 10.1111/hae.12322. [DOI] [PubMed] [Google Scholar]
- 15.Munro S., Pelham H.R. An Hsp70-like protein in the ER: identity with the 78 kDa glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 16.Marquette K.A., Pittman D.D., Kaufman R.J. A 110-amino acid region within the A1-domain of coagulation factor VIII inhibits secretion from mammalian cells. J Biol Chem. 1995;270:10297–10303. doi: 10.1074/jbc.270.17.10297. [DOI] [PubMed] [Google Scholar]
- 17.Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- 18.Roth S.D., Schüttrumpf J., Milanov P., Abriss D., Ungerer C., Quade-Lyssy P. Chemical chaperones improve protein secretion and rescue mutant factor VIII in mice with hemophilia A. PLoS ONE. 2012;7(9):e44505. doi: 10.1371/journal.pone.0044505. [DOI] [PMC free article] [PubMed] [Google Scholar]