Abstract
Introduction
Cerebrospinal fluid (CSF) neurodegenerative markers are measured clinically to support a diagnosis of Alzheimer's disease. Several preanalytical factors may alter the CSF concentrations of amyloid β 1–42 (Aβ1–42) in particular with the potential to influence diagnosis. We aimed to determine whether routine handling of samples alters measured biomarker concentration compared with that of prompt delivery to the laboratory.
Methods
Forty individuals with suspected neurodegenerative diseases underwent diagnostic lumbar punctures using a standardized technique. A sample of each patient's CSF was sent to the laboratory by four different delivery methods: (1) by courier at room temperature; (2) by courier, on ice; (3) using standard hospital portering; and (4) after quarantining for >24 hours. Aβ1–42, total tau (t-tau), and phosphorylated tau (p-tau) levels measured using standard enzyme-linked immunosorbent assay techniques were compared between transfer methods.
Results
There were no significant differences in Aβ1–42, t-tau, or p-tau concentrations measured in samples transported via the different delivery methods despite significant differences in time taken to deliver samples.
Discussion
When CSF is collected in appropriate tubes, transferred at room temperature, and processed within 24 hours, neurodegenerative markers can be reliably determined.
Keywords: Cerebrospinal fluid, Biomarkers, Diagnosis, Prehandling
1. Introduction
Cerebrospinal fluid (CSF) measures of amyloid β 1–42 (Aβ1–42), total tau (t-tau), and phosphorylated tau (p-tau) can be used to help diagnose Alzheimer's disease (AD) pathology in individuals with cognitive impairment [1]. These measures are now incorporated into research diagnostic criteria for AD [2], [3] and as an inclusion criterion and outcome measure for clinical trials of disease-modifying drugs (www.clinicaltrials.gov/show/NCT01760005). Measured Aβ1–42, t-tau, and p-tau concentrations are known to be affected by a number of potential confounding factors in preanalytical handling including vessel material and manufacturer [4], aliquot storage volume [5] and number of transfers between vessels [6], and thought to be affected by storage temperature [7], with conflicting evidence about whether time from lumbar puncture (LP) to CSF analysis reduces analyte concentration [7], [8]. This is a particular concern for Aβ1–42 which has a propensity to adsorb to the walls of collection containers, as well as to aggregate with itself and other proteins [8], thus reducing its measured concentration. As CSF Aβ1–42 is reduced in AD, such errors can potentially result in individuals being erroneously diagnosed as having AD pathology [5].
In many centers, CSF samples are transferred to the laboratory on a nonurgent basis, often passing through the hands of porters, specimen reception staff, and laboratory scientists before being aliquoted, frozen, and processed. By contrast, research samples are typically collected according to a standardized operating procedure which sees them rapidly delivered to the laboratory and frozen (see, for example http://www.alzforum.org/sites/default/files/protocol_Biofluid_Sample_Collection_Protocol_for_ADNI_0.pdf). This raises questions about whether data from research studies are applicable to “real-life” clinical cohorts.
The aim of this study was to determine whether the delivery method used to transport CSF from the bedside to the laboratory for aliquoting and freezing, the transfer time, and prompt cooling at the bedside alters the measured concentration of Aβ1–42, t-tau, and p-tau.
2. Methods
2.1. Subjects
We recruited sequential individuals seen at the specialist cognitive disorders clinics at the National Hospital for Neurology and Neurosurgery, referred for diagnostic LP for investigation of a suspected neurodegenerative condition. At the time of clinical sampling, all individuals donated an additional CSF sample for research, and basic demographic data were recorded. The study was reviewed by the local ethics committee (reference: 12/LO/1504), and all participants gave written consent. In those participants who lacked capacity, proxy consent was obtained from a consultee.
2.2. Sample collection and randomization
Individuals who had an LP between the hours of 9 and 10.30 AM performed using a 22-gauge Quincke needle according to locally agreed standard operating procedures. No manometer was used. Four samples of 1-mL volume were collected in polypropylene tubes (Sarstedt Product code 63.9922.254) and numbered sequentially as either 0 (the first sample, discarded) or 1–3.
Three transport methods were used for each individual's samples with the order randomized using a random number generator command in Stata: (1) samples were collected by a designated laboratory technician within 10 minutes of collection and transferred to the laboratory in a cool box containing wet ice at 4°C; (2) samples were collected by a designated laboratory technician who picked up the sample within 10 minutes of collection and transferred to the laboratory at room temperature; and (3) samples were transferred to the laboratory via the routine portering service. (4) A further group of individuals (n = 10) had their third sample deliberately “mistreated,” being quarantined at room temperature for between 24 hours and 1 week. In the laboratory, samples were centrifuged at 1750 relative centrifugal force for 5 minutes at room temperature and frozen at −80°C within 15 minutes of arrival. Each sample was analyzed for Aβ1–42, t-tau, and p-tau using an INNOTEST enzyme-linked immunosorbent assay (ELISA; copyright, Ghent, Belgium). Laboratory staff were blinded to the transfer method.
2.3. Statistics
Sample size calculations were based on prior ELISA-based measures of Aβ1–42, t-tau, and p-tau from 456 individuals with suspected neurodegenerative disease, where the mean (standard deviation) concentration (pg/mL) for Aβ1–42 = 520 (122), t-tau = 556 (442), and p-tau = 70.3 (37.2). Assuming a correlation between results from the transfer methods of >0.9, 30 participants were needed to detect a difference of 20% in sample concentration with 90% power and 5% risk of a type 1 error. Wilcoxon matched-pairs signed-rank tests were used to compare the level of each biomarker concentration between transport methods, and reproducibility was compared between transport methods using Pitman's test of equality of variance for paired samples. Spearman's pairwise correlation coefficient was determined for differences in biomarker concentration and delivery time. All analyses were conducted in Stata version 12.1.
3. Results
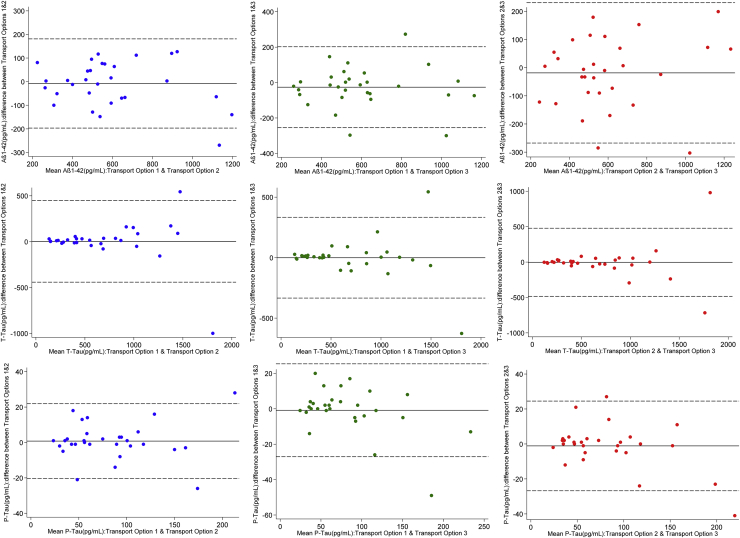
Thirty subjects were included in the initial analysis comparing transfer methods 1–3, including patients with suspected Alzheimer and a range of non-Alzheimer pathologies. Samples randomized to transport options 1 and 2 all arrived simultaneously in the laboratory within 30 minutes of collection. Samples randomized to transport option 3 arrived a median of 24 minutes (range, 13–55) later. There was no significant difference in measured CSF Aβ1–42, t-tau, and p-tau concentrations between any of the transport methods, and no evidence that variance of CSF Aβ1–42 or t-tau differed between the transport methods. There was significant variance of p-tau between transport methods 1 and 2 and between methods 1 and 3, this association being driven by a single data point (Table 1, Fig. 1).
Table 1.
Measured CSF biomarker concentrations for each transport method, results of paired Wilcoxon signed-rank tests used to compare transport methods and Pitman's test for equality of variance
| CSF analyte | Transport option 1∗ | Transport option 2∗ | Transport option 3∗ | Comparison of options 1 and 2 |
Comparison of options 1 and 3 |
Comparison of options 2 and 3 |
|||
|---|---|---|---|---|---|---|---|---|---|
| P different level | P different variance | P different level | P different variance | P different level | P different variance | ||||
| Aβ1-42† | 563 (436–775) | 565 (448–696) | 553 (450–691) | .84 | .24 | .13 | .88 | .52 | .42 |
| T-tau† | 486 (263–874) | 455 (276–842) | 546 (261–999) | .15 | .36 | .59 | .39 | .73 | .79 |
| P-tau† | 61 (42–96) | 56 (43–97) | 61 (43–105) | .68 | .74 | .72 | .04 | .97 | .03 |
Abbreviations: CSF, cerebrospinal fluid; Aβ1–42, amyloid β 1–42; t-tau, total-tau; p-tau, phosphorylated tau.
NOTE. Option 1: Designated courier transported on wet ice; option 2: designated courier at room temperature; and option 3: standard hospital porter.
pg/mL.
Median (interquartile range).
Fig. 1.
Bland-Altman plots for measured CSF Aβ1–42, t-tau, and p-tau concentrations comparing transport options 1 and 2, 1 and 3, and 2 and 3. Solid line indicates mean difference between methods; dashed lines represent 95% reference range for difference between methods. Abbreviations: CSF, cerebrospinal fluid; Aβ1–42, amyloid β 1–42; t-tau, total-tau; p-tau, phosphorylated tau.
Measured t-tau concentration was weakly negatively correlated with transport time for samples transported by porter compared with those sent by courier at room temperature (Spearman's ρ −0.42) and on wet ice (Spearman's ρ −0.39). There was no correlation between Aβ1–42 or p-tau concentration and transport time.
For the 10 individuals who had samples sent by transport method (1) and following quarantine (method 4), the latter samples arrived at the laboratory 1440 minutes (range, 1440–4320) after collection (Table 2).
Table 2.
Measured CSF biomarker concentrations for transport options 1 and 4 (n = 10), results of paired Wilcoxon signed-rank tests used to compare transport methods and Pitman's test for equality of variance
| CSF analyte | Transport option 1∗ | Transport option 4∗ | Comparison of options 1 and 4 | |
|---|---|---|---|---|
| Transfer time (min) |
30 (30–33.5) |
1440 (1440–4320) |
||
|
P different level |
P different level |
|||
| Aβ1-42† | 563 (448–713) | 565 (463–927) | .20 | .39 |
| T-tau† | 471 (273–874) | 345.5 (252–538) | .36 | .50 |
| P-tau† | 60 (39–96) | 50.5 (33–56) | .26 | .73 |
Abbreviations: CSF, cerebrospinal fluid; Aβ1–42, amyloid β 1–42; t-tau, total-tau; p-tau, phosphorylated tau.
NOTE. Option 1: Designated courier transported on wet ice and option 4: Standard hospital porter, where samples were deliberately mistreated at room temperature for >24 h.
pg/mL.
Median (interquartile range).
There was also no significant difference in measured CSF Aβ1–42, t-tau, and p-tau concentrations between transport methods 1 and 4. There was a moderate negative correlation between Aβ1–42 concentration (Spearman's ρ −0.52) and weakly positive correlation with t-tau (0.43) and p-tau concentrations (0.37) and transport time, although these correlations were not statistically significant.
4. Discussion
The clinical utility of CSF biomarkers for diagnosing AD pathology in individuals with cognitive impairment and suspected neurodegeneration is now well established in the research setting [1] but it is less clear to what extent Aβ1–42, t-tau, and p-tau concentrations can be reliably measured and interpreted in “real-life” clinical cohorts where samples cannot always be collected according to gold-standard practices. This is to our knowledge the first prospective, randomized study to show that CSF samples collected in polypropylene vessels can be transferred without cooling, in a time frame and manner appropriate for routine clinical practice, without significantly altering the measured concentration of the most useful neurodegenerative markers, and supports the findings of a prior smaller study suggesting that biomarker concentrations may remain stable at room temperature for up to 24 hours [8]. We found no consistent or significant correlation between transfer time and biomarker concentration for Aβ1–42 or p-tau. P-tau was negatively correlated with delivery time for delivery options 1–3 but this association was not observed for the quarantined samples (option 4) when greater time differences were studied. Although not powered to study changes over this period, this study suggests there may be no effect of transfer time on Aβ1–42, t-tau, or p-tau even when samples were quarantined for up to a week.
The conclusions from this prospective, blinded, randomized study have significant implications for future use of CSF as a clinical diagnostic tool. In many countries, use of CSF sampling in the investigation of dementia is restricted to specialist neurology centers. As biomarkers are increasingly used as part of clinical diagnostic criteria and there is a drive to identify AD in the earliest preclinical phase of the illness, regional hospitals and memory centers are likely to want to make use of CSF sampling to aid early diagnosis, identify individuals for trials, and to improve the likelihood of successful therapeutic intervention. These results show that, provided samples are collected appropriately and in suitable tubes and can reach a laboratory for aliquoting and freezing within a reasonable time frame, robust results can be obtained.
In recent years, there have been moves to improve harmonization in CSF collection and handling methods between centers to help standardize clinical cutpoints and facilitate multicenter observational research studies and trials of disease modifying drugs, yet there is significant variation in the time taken to transfer samples from bedside to laboratory between centers for research biobanking [9]. These data demonstrate that harmonization of this particular variable may be less vital than other preanalytical factors such as test-tube material and brand. A relative weakness of this study is sample size. A significantly larger study could be powered to detect smaller differences between transport groups.
We recommend that clinical CSF is collected according to a standardized operating procedure using polypropylene collection tubes. Our data suggest that samples need not be transferred to the laboratory on ice and that transfer times of up to and beyond 24 hours may not alter the validity of Aβ1–42, t-tau, and p-tau measurement.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using PubMed. Although there are several publications about the importance of cerebrospinal fluid (CSF) prehandling methods, there are none in the clinical setting.
-
2.
Interpretation: Our findings demonstrate that CSF transport methods are not likely to have an effect as significant as was previously thought on neurodegenerative biomarker concentration.
-
3.
Future directions: CSF measurement of neuronal-enriched proteins is feasible outside the context of the research environment.
Acknowledgments
The authors gratefully acknowledge the support of our patients and their families, the Leonard Wolfson Experimental Neurology Centre, Alzheimer's Research UK, and Iceland Foods Ltd.
This work was supported by the NIHR Queen Square Dementia BRU, UCLH Biomedical Research Centre, and the Swedish Research Council. H.Z. is a Wallenberg Academy Fellow.
Footnotes
The authors have no conflicts of interest that are directly relevant to the content of this article.
References
- 1.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: The IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Perret-Liaudet A., Pelpel M., Tholance Y., Dumont B., Vanderstichele H., Zorzi W. Cerebrospinal fluid collection tubes: A critical issue for Alzheimer disease diagnosis. Clin Chem. 2012;58:787–789. doi: 10.1373/clinchem.2011.178368. [DOI] [PubMed] [Google Scholar]
- 5.Toombs J., Paterson R.W., Lunn M.P., Nicholas J.M., Fox N.C., Chapman M.D. Identification of an important potential confound in CSF AD studies: Aliquot volume. Clin Chem Lab Med. 2013;51:2311–2317. doi: 10.1515/cclm-2013-0293. [DOI] [PubMed] [Google Scholar]
- 6.Toombs J., Paterson R.W., Schott J.M., Zetterberg H. Amyloid-beta 42 adsorption following serial tube transfer. Alzheimers Res Ther. 2014;6:5. doi: 10.1186/alzrt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoonenboom N.S., Mulder C., Vanderstichele H., Van Elk E.J., Kok A., Van Kamp G.J. Effects of processing and storage conditions on amyloid beta (1-42) and tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin Chem. 2005;51:189–195. doi: 10.1373/clinchem.2004.039735. [DOI] [PubMed] [Google Scholar]
- 8.Bjerke M., Portelius E., Minthon L., Wallin A., Anckarsater H., Anckarsater R. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teunissen C.E., Tumani H., Bennett J.L., Berven F.S., Brundin L., Comabella M. Consensus Guidelines for CSF and Blood Biobanking for CNS Biomarker Studies. Mult Scler Int. 2011;2011:246412. doi: 10.1155/2011/246412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]