Abstract
Objective:
Ziziphus Jujube (Jujube) plant has exhibited numerous medicinal and pharmacological properties including antioxidant and anti-inflammatory effects. This study was carried out to investigate its anti-cancer and pro-apoptotic abilities in human cervical and breast cancer cells in vitro.
Materials and Methods:
The cervical OV2008 and breast MCF-7 cancer cells were incubated with different concentrations of Jujube aqueous extraction (0-3 mg/ml) for various times (0-72 h). Cell viability was assessed by Trypan Blue and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression of two apoptosis-related genes in treated cells evaluated by quantitative Real Time -PCR analysis.
Results:
Jujube significantly inhibited cancer cell viability in a dose- and time- dependent manner. Herb-induced apoptosis was associated with enhanced expression of Bax and decreased Bcl2 gene leading eventually to a time-dependent six fold increase in the Bax/Bcl-2 ratio.
Conclusion:
These results indicated that Jujube may be a natural potential and promising agent to prevent or treat human cancers.
Key Words: Ziziphus Jujube, Cervical cancer, Breast cancer, Apoptosis
Introduction
Cancer is a heterogeneous devastating disease with various biological characteristics (Senapathy et al., 2011 ▶). Breast and cervical cancers are the first and second most common malignancies among women worldwide, respectively (World health organization, 2013). Current therapeutic strategies for cancer such as surgery, radiotherapy and chemotherapy are associated with serious side effects, residual morbidity as well as frequent relapses (Adhvaryu et al., 2008 ▶). A growing body of evidence suggested a promising potential for medicinal plants used as traditional and/or alternative modern medicine. Specifically, there is a considerable interest among oncologists to develop anticancer agents from herbs (Mishra et al., 2011 ▶; Sadiq et al., 2008 ▶; Hoshyar et al., 2015 ▶). Current experiments showed that herbs play anticancer role via induction of program cell death (apoptosis) and cell differentiation, enhancing the immune system potential, inhibiting angiogenesis and reversing multidrug resistance (Liu et al., 2013 ▶). However,mucheffort yet is required to determine the role of herbs in cancer therapy. One of such medicinal plants is Jujube with numerous biological compounds and a long history of use as a remedy for various disorders (Preeti and Tripathi, 2014 ▶).
Apoptosis plays a critical role in the regulation of normal cells homeostasis and cancer cells growth (Kuno et al., 2012 ▶). The present study was designed to shed light on the anti-cancer effects of Jujube on human breast and cervical cancer cells. We found that the Jujube extract decreased the cell viability, a response associated with increased Bax/Bcl-2 genes ratio.
Materials and Methods
Jujube aqueous extract preparation
The semi-dried fruits of Jujube were washed and after seed removal, soft red parts were dried in 50°C and grounded into powder in a mortar. The powder was dissolved in boiling distilled water for 30minute, then filtered by a sterile filter (0.2 μm) and lyophilized (Hoshyar et al., 2015 ▶).
Cell culture and Cell viability assay
Cervical cancer cell line (OV2008) was kindly provided from Doctor Benjamin K. Tsang's laboratory (Department of Obstetrics, Gynecology and Cellular and Molecular Medicine, University of Ottawa, Canada). Breast cancer cell line (MCF-7) and normal cell line (MCF-10A) were purchased from Iranian Biological Resource Center, Iran. OV2008, MCF-7 and MCF-10A cells were cultured in RPMI and DMEM media, respectively (Abedini et al., 2004 ▶, Kobayashi et al., 2013 ▶). The cells were treated with different concentration of Jujube extract (0-3 mg/ml) for various interval times (0-72 hours). The stained cells by Trypan Blue observed and counted via an inverted microscope. MTT assay was used to assess the anti-proliferative effects of Jujube aqueous extract on the cancer cells (Wang et al., 2014 ▶). Using the dose- and time-dependent curves by linear interpolation, the IC50value of Jujube was calculated to analyze its cytotoxic efficiency (Bathaie et al., 2013 ▶).
Quantitative Real Time-PCR analysis
Total RNA was isolated using RNeasy Mini Kit (Qiagene-USA). The extracted RNA was immediately used in RT-PCR to generate first-strand cDNA (cDNA Synthesis Kit, Thermo Scientific, USA). The Quantitative RT-PCR for Bax and Bcl2was carried out using the specific primers (Table 1). Gene amplification was performed in the ABI Step One™ Real-Time PCR System (Applied Biosystems, Foster City, CA) with 40cycles of denaturation at 95°C for 30s, annealing and extension at 60°C for 30s and data collection 80°C for 20s. β-actin gene was used to normalize the relative expression for interested genes calculated by 2ΔΔCT method and SYBR Green kit according to our previous reports (Abedini et al., 2014 ▶).
Table 1.
Real-time primer sequences
| Gene | Sequences |
|---|---|
| β-Actin | 5'TGGCACCCAGCACAATGAA3' (Forward) 5' CTAAGTCATAGTCCGCCTAGAAGCA 3' (Reverse) |
| Bax | 5' TGGAGCTGCAGAGGATGATTG3' (Forward) 5' GAAGTTGCCGTCAGAAAACATG3' (Reverse) |
| Bcl2 | 5'CTGCACCTGACGCCCTTCACC3' (Forward) 5'CACATGACCCCACCGAACTCAAAGA3' (Reverse) |
Statistical analysis
Results are expressed as mean ± SEM for at least three independent experiments (n = 3). Data were analyzed using one-way ANOVA and with Tukey’s post hoc-test to assess differences between experimental groups (PRISM 5.0; Graph- Pad Software Inc.).
Results
Effects of Jujube on cancer cells proliferation
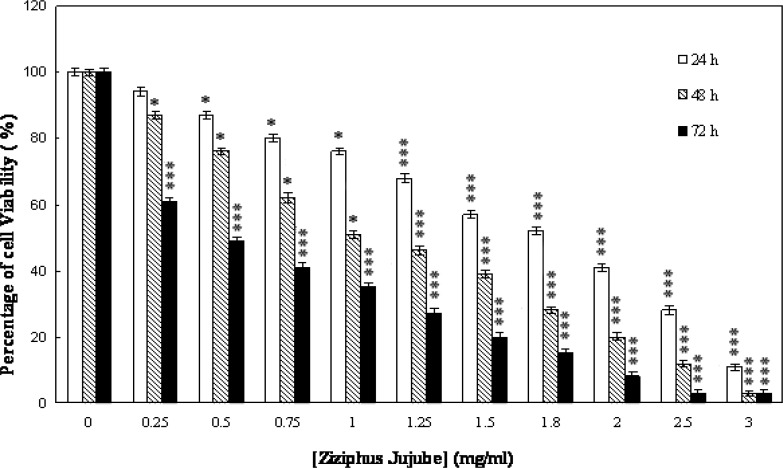
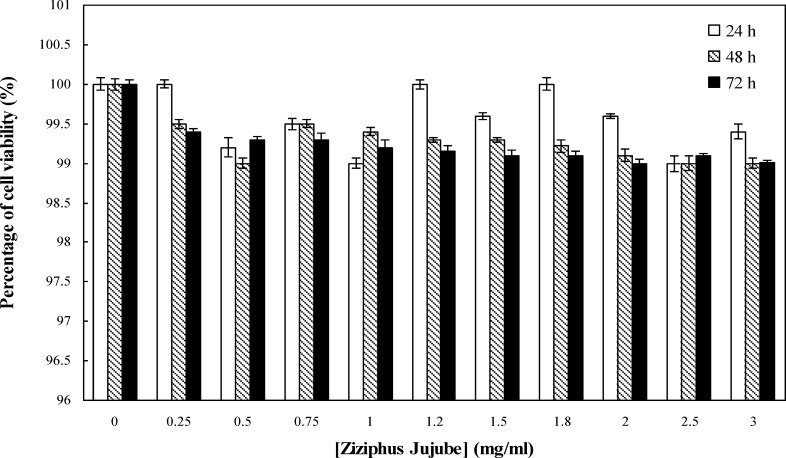
To examine the effect of Jujube on cell proliferation, cells were cultured and treated with Jujube extract (0-3 mg/ml; 0-72 hours). The effect of Jujube aqueous extract on the cancer cell morphology and proliferation were assessed by Trypan Blue staining and MTT assay, respectively. Jujube extract leads to cell shrinkage, blebbing and piknotic nuclei. In addition, Jujube treatments significantly decreased cell proliferation after 24 and 48 hours with 0.25-1 mg/ml (p<0.05) and 1.25-3 mg/ml h (p<0.001) as well as 72 hours with 0.25-3 mg/ml (p<0.001) in a dose- and time-dependent manner when compared with control group without Jujube (Figure 1). As shown in Table 2, the IC50 values of Jujube significantly decreased after different times (24-72 hours) in both cancer cell lines (p<0.01). This response was more evident in MCF-7 cells. In another word, IC50 of Jujube for MCF-7 after different times was higher compared to OV-2008 cells (P<0.05). Analyses of the cell survival showed that OV2008 cells were more sensitive to jujube compared to MCf-7 cells. Parallel treatment of the normal cells with this herb indicted a much less inhibitory effect on the viability of MCF-10A cells (Figure 2). Post hoc test revealed that Jujube at doses of 0.25-3 mg/ml (p=0.4487) did not affect growth of normal cells when compared with control group without Jujube.
Figure 1.
Effect of Jujubeon MCS-7 cell viability. The cells were treated with different concentrations of Jujube for 0-72 hours. Data are expressed as mean ± SEM (n=3). Values are statistically significant at ***p<0.001, *p<0.05 vs. respective control group (One-way ANOVA followed by Tukey’s post hoc test).
Table 2.
IC50 values (mg/ml) of Jujube in both cancer cells.
| Cancer cell line | 24 hours | 48 hours | 72 hours |
|---|---|---|---|
| OV2008 | 1.2 ± 0.03 | 0.5 ± 0.05a | 0.2 ± 0.02a |
| MCF-7 | 1.8 ± 0.08b | 1 ± 0.03a,b | 0.5 ± 0.05a,b |
Figure 2.
Effect of Jujubeon MCF-10A cell viability. The cells treated with different concentrations of Jujube for 0-72 hours. Results are reported as the mean ± SEM (n=3; p>0.05). Values are not statistically significant compared to the control group (One-way ANOVA followed by Tukey’s post hoc test
Data are expressed as the means of triplication ± SEM (n=3). a p<0.01 IC50 for 48 and 72 hours in comparison with 24 hours treatment in both cell lines; b p<0.05 IC50 for MCF-7 cells in comparison with IC50 for OV 2008 cells at different time treatments
Alteration of apoptosis regulating genes expression by Jujube in cancer cells
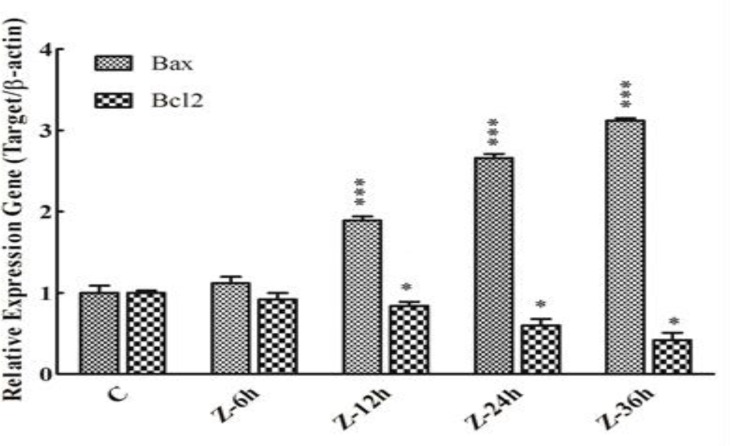
Anti-proliferative effects of Jujube suggested that it may attenuate the cell proliferation through alteration of apoptotic regulating genes. To examine this hypothesis, we investigated Jujube’s effect on expression of Bax and Bcl2 genes. As indicated in Figure 3, herb significantly increased the expression of Bax (p<0.001) and decreased Bcl-2 expression (p<0.05) in cancer cells. Jujube also dramatically increased the Bax/Bcl-2 mRNA ratio as high as six folds in treated cancer cells.
Figure 3.
OV2008 cells treated with 1.2 mg/ml Jujube for 0-36h. Jujube increased the gene expression of Bax and decreased Bcl2 expression in cells. Data represents relative gene expression (Target/β-actin) mean ± SEM of three experiments (n=3). Values are statistically significant at ***p<0.001, *p <0.05 vs. respective control group (One-way ANOVA followed by Tukey’s post hoc test
Discussion
Numerous studies have suggested that herbs exert potent anti-carcinogenic effects due to their ability to induce apoptosis. Some of these medicinal plants are Crocus sativus (Hoshyar et al., 2013 ▶), Allium sativum (Tsubura et al., 2011 ▶), Camellia sinensis (Thakur, 2012 ▶), Aloevera (Rajeswari et al., 2012 ▶), and Curcuma longa (Hashim et al., 2013 ▶). Jujubeis one of the most valuable herbs with terrific medicinal ingredients (Mahajan and Chopda, 2010 ▶).
In the present study, we investigated the anti-proliferative effects of Jujube aqueous extract on MCF-7 and OV2008 cell lines which showed that it significantly inhibited cell growth in a dose- and time-dependent manner. We have also assessed the IC50 of Jujube and shown that its values decreased in a time-dependent manner on both cancer cells. Although there is a significant difference among different times (24-72 hours) for each cancer cell line, the IC50 measures of Jujube for OV2008 were markedly less than the values for MCF-7. This may suggest that OV2008 cells were more sensitive to effective dose of Jujube compared to MCF-7 cells.
In this context, the anti-proliferative effect of de-proteinized polysaccharide (DPP) isolated from jujube on melanoma cells was evaluated and showed that IC50 of DPP was attenuated less than 20% between 24 and 48h treatment (Hung et al., 2012 ▶). However, in the present study, these decreases were 60 and 40% from 24h to 48h for OV2008 and MCF-7 cells, respectively. Moreover, the IC50 values of DPP in melanoma cells at different times were more than three folds in comparison with those of Jujube in OV2008 and MCF-7 (Hung et al., 2012 ▶). Additionally, it has been shown that cisplatin induces cell death at more extent in OV2008 compared to A2780s cells which are both chemosensitive cancer cells (Abedini et al., 2014 ▶). Plastina and coworkers indicted that the three alcoholic extraction of Jujube significantly inhibited proliferation and induced apoptosis in both estrogen receptor alpha (ERα) positive MCF-7 and ERα negative SKBR3 human breast cancer cells. However, they did not provide any result about their mechanism (Plastina et al., 2012 ▶). The present study is in line with their data. Our findings also illustrated that various concentrations of Jujube had no cytotoxic effect on MCF-10Anormal cells. Taken together, it seems that inhibitory effects of Jujube and/or its active metabolites on different cell lines are following the same trends, but illustrated various intensities.
However, the precise mechanism of this response has not been reported yet. We recently have studied the impact of Jujube on gene expression which involves in the cell cycle regulation. We showed that it increased TP53, P27 and P21 mRNA abundance, a response which was associated with a decreased in CD1 mRNA level (Submitted manuscript under review). Moreover, we showed that aqueous Jujube alters the expression of apoptosis regulating genes including Bax and Bcl2 and their ratio. Dysregulation of apoptosis is associated with an imbalance of expression of genes which involvesin cell death and proliferation (Reed, 1999 ▶; Cheung et al., 2012 ▶). The Bcl-2 family genes consists of both pro-apoptotic and anti-apoptotic proteins e.g. Bax and Bcl-2, respectively (Elmore, 2007 ▶; Wong, 2011 ▶). The balance of their expression and distribution are key determinants for cell fate (Piltan et al., 2010 ▶). Bcl-2 located in the membrane of the nucleus and mitochondria and as a pro-survival molecule, sequesters and prevents Bax translocation to mitochondria resulting in apoptosis inhibition (Edlich and Banerjee, 2011 ▶). To further examine the mechanism(s) by which Jujube exerts the cell proliferation, here we evaluated its effects on apoptotic gene expression level. To our knowledge, this is the first quantitative assessment demonstrated that cell treatment with Jujube (1.2 mg/ml; 0-36h) resulted in more than three-fold increase in Bax mRNA level, a response associated with 50% decrease in Bcl-2 mRNA abundance, thereby significantly enhancing the Bax / Bcl-2mRNA ratio as much as 6 folds in treated cells.
In summary these findings support the notion that Jujube exerts selective anti-tumor effect via inhibition of cell growth and induction of apoptosis. It could be apromising strategy to develop a successful treatment for cancer therapy.
Acknowledgments
The authors would like to thank the Research Affairs of Birjand University of Medical Sciences for financially supporting this project.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004 ;23:6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- Abedini MR, Wang PW, Huang YF, Cao M, Chou CY, Shieh DB, Tsang BK. Cell fate regulation by gelsolin in human gynecologic cancers. Proc Natl Acad Sci. 2014;111:14442–14447. doi: 10.1073/pnas.1401166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhvaryu MR, Reddy N, Parabia MH. 2008 Anti-tumor activity of four ayurvedic herbs in dalton lymphoma ascites bearing mice and their short-term In vitro cytotoxicity on DLA-Cell-Line. Afr J Tradit Complement Altern Med. 5:409–418. doi: 10.4314/ajtcam.v5i4.31297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathaie Z, Hoshyar R, Miri H, Sadeghizadeh M. Anticancer effect of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem Cell Biol. 2013;91:1–7. doi: 10.1139/bcb-2013-0014. [DOI] [PubMed] [Google Scholar]
- Cheung HH, Liu X, Rennert OM. Apoptosis: Reprogramming and the Fate of Mature Cells. ISRN Cell Biol. 2012:1–8. [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ. Bcl-x (L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim FJ, Muayad S, Shawkat Abdul Ameer N. The role of Curcuma longa rhizomes ethanolic extract on human lymphocytes treated by bickel by using G banding technique and karyotyping. J Fundam Appl Sci. 2013;9:105–109. [Google Scholar]
- Hoshyar R, Bathaie Z, Sadeghizadeh M. Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol. 2013;32:50–57. doi: 10.1089/dna.2012.1866. [DOI] [PubMed] [Google Scholar]
- Hoshyar R, Mahboob Z, Zarban A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotech, In Press; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshyar R, Mohaghegh Z, Torabi N, Abolghasemi A. Antitumor Activity of Aqueous Extract of Ziziphus Jujube Fruit in Breast Cancer: An In Vitro and In Vivo Study. Asian Pac J Reprod. 2015;4:116–122. [Google Scholar]
- Hung CF, Hsu BY, Chang SC, Chen BH. Antiproliferation of melanoma cells by polysaccharide isolated from Zizyphus jujuba. Nutrition. 2012;28:98–105. doi: 10.1016/j.nut.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Abedini MR, Sakuragi N, Tsang BK. PRIMA-1 increases cisplatin sensitivity in chemoresistant ovarian cancer cells with p53 mutation: a requirement for Akt down-regulation. J Ovarian Res. 2013:6. doi: 10.1186/1757-2215-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T, Tsukamoto T, Haral A, Tanaka T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J Biophys Chem. 2012;3:156–173. [Google Scholar]
- Latest world cancer statistics. World health organization; 2012. [Google Scholar]
- Liu JC, Guan X, Ryan JA, Rivera AG, Mock C, Agrawal V, Letai A. In: High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Stem Cell., editor. Vol. 13. 2013. pp. 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan RT, Chopda MZ. Phyto-Pharmacology of ZZJujube jujuba Mill- A plant review. Pharmacognosy. 2009;3:320–329. [Google Scholar]
- Mishra T, Khullar M, Bhatia A. Anticancer Potential of Aqueous Ethanol Seed Extract of ZZJujube mauritiana against Cancer Cell Lines and Ehrlich Ascites Carcinoma. J Evid Based Complementary Altern Med. 2011;8:1–11. doi: 10.1155/2011/765029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltan A, Totonchi M, Rezazadeh M, Gourabi H, Karimian L, Baghaban Eslaminejad MR, Eftekhari Yazdi P. Quantitative expression of BAG1, BAX and BCL-2 genes in human embryos with different fragmentation grades derived from ART. Yakhteh Med J. 2010;12:257–266. [Google Scholar]
- Plastina P, Bonofiglio D, Vizza D, Fazio A, Rovito D, Giordano C, Barone I, Catalano S, Gabriele B. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J Ethnopharmacol. 2012;27:325–332. doi: 10.1016/j.jep.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Preeti KM, Singh S, Chaudhary N. ZZJujube jujuba: A phyto -pharmacological review. International J Pharmaceutical Res Scholar. 2014;3:514–523. [Google Scholar]
- Rajeswari R, Umadevi M, Sharmila Rahale C, Pushpa R, Selvavenkadesh S, Sampath Kumar KP. Aloe vera: The Miracle Plant Its Medicinal and Traditional Uses in India. Journal of Pharmacognosy and Phytochem. 2012;4:118–124. [Google Scholar]
- Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- Sadiq Y, Alexander AB, Abdulkarim A. Effect of ZZJujube mauritiania (L) seed extracts on spatial recognition memory of rats as measured by the Y-maze test. J Natural Products. 2008;2:31–39. [Google Scholar]
- Senapathy JG, Umadevi P, Kannika PS. The present scenario of cervical cancer control and HPV epidemiology in India: an outline. Asian Pac J Cancer Prev. 2011;12:1107–1115. [PubMed] [Google Scholar]
- Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012;33:377–384. doi: 10.1093/carcin/bgr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubura A, Lai Y, Kuwata M, Uehara N, Yoshizawa K. Anticancer Effects of Garlic and Garlic-derived Compounds for Breast Cancer Control. Anticancer Agent Med Chem. 2011;11:249–253. doi: 10.2174/187152011795347441. [DOI] [PubMed] [Google Scholar]
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87–92. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PW, Abedini MR, Yang LX, Ding AA, Figeys D, Chang JY, Tsang BK, Shieh DB. Gelsolin regulates cisplatin sensitivity in human head‐and‐neck cancer. Int J Cancer. 2014;135:2760–2769. doi: 10.1002/ijc.28928. [DOI] [PubMed] [Google Scholar]