Abstract
Purpose
Patients who develop a strong alliance with their health care providers have been shown to have higher levels of psychosocial well-being and rates of treatment adherence. Young adults with cancer have lower levels of psychosocial well-being and treatment adherence relative to patients with cancer in other age groups. This study sought to evaluate the relationships between the patient-oncologist alliance, psychosocial well-being, and treatment adherence in young adults with advanced cancer.
Patients and Methods
Ninety-five young adults (age 20 to 40 years) with advanced cancer were administered measures of alliance, psychosocial well-being, willingness to adhere to treatment, and treatment adherence. Relationships between alliance and psychosocial well-being were examined bivariately. Multiple linear regression models examined the relationship between alliance and adherence, controlling for confounding influences (eg, psychosocial well-being).
Results
Alliance was significantly (P ≤ .01) and positively associated with greater perceived social support and less severe illness-related grief. After controlling for significant confounding influences (ie, metastases, appraised support, and grief), alliance remained significantly (P ≤ .01) associated with greater willingness to adhere to treatment and greater adherence to oral medication.
Conclusion
By developing a strong alliance, oncologists may enhance psychosocial well-being and increase treatment adherence in young adult patients with advanced cancer.
INTRODUCTION
Therapeutic alliance is the “collaborative and affective bond” (p. 438)1; between a patient and health care provider. The strength of the alliance represents the extent to which patients feel a sense of mutual understanding, caring, and trust with their providers.2 The alliance has been called the “quintessential integrative variable” (p. 449)3 in psychotherapy because it is consistently among the most important influences on psychotherapy outcomes.4,5
An emerging literature has examined the alliance between patients and providers who are not mental health professionals. A stronger alliance in this context is associated with better psychosocial functioning and adjustment to illness. Alliance has been linked to better quality of life, greater satisfaction with treatment, and greater perceived utility of treatment.6–8 In cross-sectional analyses of patients with advanced cancer, a stronger patient-oncologist alliance was associated with better quality of life and greater acceptance of illness.2 In prospective analyses, the patient-oncologist alliance 4 months before death was one of the top nine predictors of quality of life in the last week of life.9
The patient-physician alliance has also been associated with treatment adherence. In medical patient samples, a stronger alliance was associated with enhanced self-efficacy related to adherence and higher levels of adherence.6–8 In addition, higher-quality communication is associated with higher rates of adherence, including adherence to recommendations for cancer screening.10,11 A meta-analysis of patient-physician communication and adherence found that patients whose physicians exhibited poor communication strategies had a 19% higher risk for nonadherence than patients whose physician communicated well.12 In patients with cancer, greater trust in the oncologist has been associated with higher rates of adherence.13 However, the direct relationship between alliance and adherence has not been examined in patients with cancer.
Young adults with cancer are an important group in which to examine alliance, psychosocial well-being, and treatment adherence. First, young adults with cancer have lower levels of well-being than patients with cancer in other age groups.14,15 Young adults with cancer endorse moderate levels of distress,16 and a significant minority meet cutoffs for syndromal depression and anxiety.17 Higher levels of social support are associated with better psychosocial well-being in young adults with advanced cancer.18 Young adults report feeling isolated from their peers during treatment; alliance with oncologists may be an especially important source of support in this population.19,20
Second, rates of nonadherence in adolescents and young adults with cancer are high, ranging from 27% to 60%,21–23 and are stable over time.21 Comparing adherence rates in young adults with those in pediatric and older adult samples is difficult because of differing methodologies and the wide range of adherence estimates in adult cancer samples (16% to 100% adherence).24 However, evidence suggests that nonadherence rates are higher in young adults than in other oncology samples.24–27 Nonadherence in young adults is associated with higher rates of morbidity22 and lower survival rates, after controlling for illness severity.28 As a result, poor adherence is a possible contributor to the deficit in survival improvements in young adults relative to other age groups.29 Young adults' attitudes toward adherence are also important. In patients with cancer age 16 to 24 years, 39% considered stopping treatment.30
Young adulthood is characterized by unique developmental characteristics, including increased autonomy from the family of origin, heightened influence of peers, and openness to risk-taking. These characteristics may account for documented differences in the alliance between mental health providers and youth and adult patients.31 There is increasing recognition of the need for oncologists to be sensitive to the developmental issues of young adults.32 Adolescents and young adults rank “availability of health providers who know about treating young adults with cancer” as their second most important health care need (p. 141).33 Yet, approximately one third of adolescents and young adults report an unmet need for approachable health care providers.34
Research on the patient-provider alliance and psychosocial well-being in patients with cancer is limited and has not been conducted in young adults. In addition, the relationship between alliance and adherence in patients with cancer has not been examined. We hypothesize that a stronger alliance will be associated with greater psychosocial well-being, greater willingness to adhere to treatment, and higher rates of treatment adherence. On the basis of a meta-analytic study indicating that depression reduces treatment adherence,35 we hypothesize that psychosocial well-being will be a confounding factor in the relationship between alliance and adherence.
PATIENTS AND METHODS
Participants and Procedures
Eligible patients were identified through a review of electronic medical records by a master's-degree–level research assistant or a licensed clinical psychologist at a single tertiary cancer care center. Eligible patients were 20 to 40 years of age and had a diagnosis of incurable, recurrent, or metastatic cancer (ie, advanced cancer). Participants were excluded if they were not fluent in English, were too weak to complete the interview, and/or had scores of 5 or above on the Short Portable Mental Status Questionnaire indicating cognitive impairment likely to invalidate a participant's responses. Approval was obtained from the institutional review board, and all enrolled participants provided written informed consent.
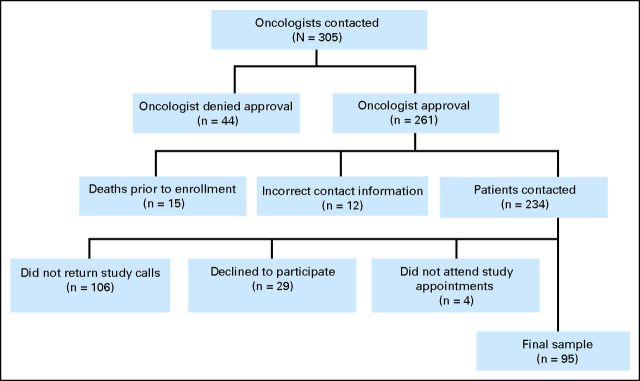
Oncologists for 305 patients were contacted to request permission to recruit patients for the study; 261 patients were approved for contact, 15 patients died before study enrollment, and 12 had incorrect contact information. Of the 234 patients contacted, 29 (12%) declined participation. Ninety-five patients completed study procedures, resulting in a participation rate of 41% (Fig 1).
Fig 1.
Flow chart for participant recruitment.
Face-to-face interviews were conducted. Each participant completed a single interview; interviews were conducted between April 2010 and May 2012 by a master's- or doctoral-degree–level interviewer. Interviews lasted 50 to 90 minutes, and participants were compensated $25.
Measures
Demographic and disease characteristics.
Demographic characteristics were based on participant self-report and included age, sex, race, marital status, parental status, education level, health insurance status, and income. Disease characteristics were obtained from participants' medical records and included cancer diagnosis, stage at diagnosis, presence of metastatic disease, time since diagnosis, and whether the patient was participating in a drug trial.
Therapeutic alliance.
The Human Connection (THC) scale is a 16-item measure of the alliance between a patient and oncologist (current sample: Cronbach's α = .89).2 The THC scale was developed to measure the extent to which patients feel a sense of mutual understanding, caring, and trust with their oncologists. The THC has been validated in older patients with advanced cancer.2
Performance status.
Participants' physical performance status was assessed with the Karnofsky performance scale, a clinician's rating scale from 0 (death) to 100 (normal; no evidence of disease), completed by the trained study interviewer.36
Psychosocial well-being.
The 12-item version of the Interpersonal Support Evaluation List (ISEL)37 was used to assess perceived availability of social resources in three areas: appraisal support or someone to talk to about problems (Cronbach's α = .52), tangible support or material aid (Cronbach's α = .38), and belonging support or someone with whom to engage in activity (Cronbach's α = .62), in addition to a total social support score (Cronbach's α = .72). Higher scores indicate greater social support. The ISEL has demonstrated adequate reliability in the general population37 and has been validated in women with metastatic breast cancer,38 bereaved caregivers,39 and a similar sample of young adults with advanced cancer.18
The McGill Quality-of-Life Questionnaire (MQOL) is a 16-item self-report measure of quality of life over the previous 2 days that has been validated in individuals with life-threatening illness40 and advanced cancer.41,42 The psychological (Cronbach's α = .86) and existential (Cronbach's α = .82) QOL subscales were included. The physical QOL subscale was not included because of overlap with the Karnofsky performance scale. The social support subscale was excluded because of conceptual overlap with the ISEL.
The Prolonged Grief Disorder Scale (Cronbach's α = 0.77) was developed as a measure of grief in the context of bereavement43; it has been adapted to assess grief due to illness-related losses. The PG-13 has been validated in bereaved individuals.44 The PG-12 (which omits the “time from loss” item in the PG-13) was validated in patients with advanced cancer45 and young adults with advanced cancer.46
Illness acceptance was assessed with the 7-item Struggle with Illness subscale of the Peace, Equanimity, and Acceptance of the Cancer Experience (PEACE) scale (Cronbach's α = .74).47 The PEACE scale demonstrated adequate reliability and validity in a sample of older adults with advanced cancer.47
Willingness to adhere to treatment.
Willingness to adhere to treatment was assessed with two items from the Cancer Therapy Satisfaction Questionnaire.48 The items evaluated patient perception that cancer treatment was worth the adverse effects (“In the last four weeks, how often did you feel that cancer therapy was worth taking even with the adverse effects”) and thoughts about stopping cancer treatment (“In general, in the last four weeks, how often did you think about stopping your cancer therapy”). Each item is rated on a 5-point scale (1, never; 5, always).
Treatment adherence.
Treatment adherence was assessed with a single self-report item from the Cancer Therapy Satisfaction Questionnaire48 (“In the last four weeks, how often did you take your cancer therapy exactly as directed by your doctor?”). Participants were instructed to respond on the basis of their adherence to cancer therapy pills by using a 5-point scale (1, never; 5, always). This item was administered only to patients taking oral medications (n = 59). The item did not indicate which medications patients were adherent with.
Statistical Analysis
Relationships between alliance and sample characteristics (ie, disease and demographic characteristics) and psychosocial variables (ie, social support, quality of life, illness acceptance, and grief) were examined by using t tests, one-way analysis of variance, and Pearson correlation coefficients. Linear regression analyses were used to examine the relationship between alliance (independent variable) and willingness to adhere to treatment and treatment adherence (dependent variables). Dependent variables were individually regressed on the measure of alliance. Items assessing willingness to adhere to treatment were analyzed in separate regression models. Demographic and disease characteristics and psychosocial variables significantly (P ≤ .01) associated with alliance were included in each regression model as confounding factors. We specifically sought to determine whether statistically significant effects of alliance on treatment adherence became insignificant with the inclusion of psychosocial confounders. An alpha level of P ≤ .01 was used as the threshold for statistical significance.
RESULTS
Sample Characteristics
Sample characteristics are provided in Table 1. The sample was primarily white (86.3%) and female (68.4%), with a mean age of 33.4 years (standard deviation [SD], 5.51). Approximately half the sample was married (56.8%), and more than one third had dependent children (40.0%). One third of the sample was patients with breast cancer (33.7%). Other diagnoses included brain tumors, leukemia/lymphoma, soft tissue cancers, and colon cancer. Mean time since diagnosis was 3.40 years (SD, 2.99). All participants had advanced disease at the time of the interview.
Table 1.
Sample Characteristics
| Demographic/Disease Characteristic | Total Sample (N = 95) |
|||
|---|---|---|---|---|
| No. | % | Mean | SD | |
| Sex | ||||
| Female | 65 | 68.4 | ||
| Male | 30 | 31.6 | ||
| Race | ||||
| White | 82 | 86.3 | ||
| Hispanic | 5 | 5.3 | ||
| Asian American/Pacific Islander/American Indian | 4 | 4.2 | ||
| African American | 4 | 4.2 | ||
| Marital status | ||||
| Married | 54 | 56.8 | ||
| Other | 41 | 43.2 | ||
| Dependent children | ||||
| Yes | 38 | 40.0 | ||
| No | 57 | 60.0 | ||
| Health insurance | ||||
| Yes | 93 | 97.9 | ||
| No | 2 | 2.1 | ||
| Income, $ | ||||
| 0-10,999 | 2 | 2.1 | ||
| 11,000-20,999 | 6 | 6.3 | ||
| 21,000-30,999 | 5 | 5.3 | ||
| 31,000-50,999 | 10 | 10.5 | ||
| 51,000-99,999 | 32 | 33.7 | ||
| 100,000 or more | 29 | 30.5 | ||
| Don't know | 11 | 11.6 | ||
| Cancer diagnosis | ||||
| Breast | 32 | 33.7 | ||
| Brain tumor | 15 | 15.8 | ||
| Leukemia/lymphoma | 10 | 10.5 | ||
| Soft tissue | 8 | 8.4 | ||
| Colon | 4 | 4.2 | ||
| Other | 26 | 27.4 | ||
| Stage at diagnosis | ||||
| I | 5 | 5.3 | ||
| II | 10 | 10.5 | ||
| III | 24 | 25.3 | ||
| IV | 25 | 26.3 | ||
| Unknown | 31 | 32.6 | ||
| Metastasis | ||||
| Yes | 50 | 52.6 | ||
| No | 45 | 47.4 | ||
| Drug trial | ||||
| Yes | 27 | 28.4 | ||
| No | 68 | 71.6 | ||
| Age, years | 33.4 | 5.51 | ||
| Education, years | 15.87 | 2.52 | ||
| Years since diagnosis | 3.40 | 2.99 | ||
| Performance status | 80.11 | 11.06 | ||
| Social support (total scale score) | 42.38 | 4.39 | ||
| Appraisal | 13.97 | 1.89 | ||
| Tangible | 14.57 | 1.66 | ||
| Belonging | 13.85 | 2.11 | ||
| Quality of life | ||||
| Psychological | 25.78 | 9.86 | ||
| Existential | 44.33 | 9.90 | ||
| Illness acceptance | 19.91 | 4.32 | ||
| Grief | 24.24 | 7.16 | ||
| Treatment worth adverse effects* | 4.57 | 0.69 | ||
| Think about stopping treatment* | 1.51 | 0.92 | ||
| Treatment adherence* | 4.63 | 0.67 | ||
| Alliance | 57.06 | 6.35 | ||
Abbreviation: SD, standard deviation.
Response scale: 1, never; 5, always.
Associations of sample characteristics with alliance are provided in Table 2. Participants with metastatic disease at the time of the interview reported a stronger alliance (mean, 58.80; SD, 4.38) than participants without metastatic disease (mean, 55.1; SD, 7.59; t [93] = 2.92; P < .001). Younger age was associated with more frequent thoughts about stopping cancer therapy (r [93] = −.31; P = .004), and higher income was positively associated with treatment adherence (F [4,54] = 5.30; P = .001). No additional significant relationships among sample characteristics and alliance, willingness to adhere to treatment, and adherence emerged.
Table 2.
Bivariate Relationships of Sample Characteristics and Psychosocial Well-Being With Alliance
| Characteristic | Alliance |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | t (df = 93) | F | r* | P | |
| Sex | −.76 | .45 | ||||
| Male | 56.33 | 6.01 | ||||
| Female | 57.40 | 6.52 | ||||
| Race | −.37 | .71 | ||||
| White | 57.16 | 6.45 | ||||
| Other | 56.46 | 5.90 | ||||
| Marital status | 1.32 | .19 | ||||
| Married | 56.31 | 7.20 | ||||
| Other | 58.05 | 4.93 | ||||
| Dependent children | 2.06 | .04 | ||||
| Children | 58.68 | 5.69 | ||||
| No children | 55.98 | 6.58 | ||||
| Cancer diagnosis | −.92 | .36 | ||||
| Breast | 57.90 | 7.13 | ||||
| Other | 56.63 | 5.93 | ||||
| Metastasis | 2.92† | .00 | ||||
| Yes | 58.80 | 4.38 | ||||
| No | 55.13 | 7.59 | ||||
| Drug trial | 0.01 | .99 | ||||
| Yes | 57.07 | 5.81 | ||||
| No | 57.06 | 6.60 | ||||
| Stage at diagnosis | (3,60) = 2.11 | .11 | ||||
| I | 54.60 | 2.88 | ||||
| II | 56.50 | 6.82 | ||||
| III | 55.33 | 7.60 | ||||
| IV | 59.36 | 4.41 | ||||
| Income, $ | (5,89) = 2.42 | .04 | ||||
| 0-10,999 | 44.50 | 16.26 | ||||
| 11,000-20,999 | 59.00 | 2.10 | ||||
| 21,000-30,999 | 54.54 | 8.79 | ||||
| 31,000-50,999 | 55.95 | 7.22 | ||||
| 51,000-99,999 | 57.44 | 6.29 | ||||
| 100,000 or more | 58.38 | 4.11 | ||||
| Age | 0.01 | .94 | ||||
| Education | 0.08 | .43 | ||||
| Years since diagnosis | −.01 | .95 | ||||
| Performance status | 0.10 | .32 | ||||
| Social support (total score) | 0.25 | .02 | ||||
| Appraisal | 0.32† | .00 | ||||
| Tangible | 0.20 | .06 | ||||
| Belonging | 0.07 | .50 | ||||
| Quality of life | ||||||
| Psychological | 0.22 | .03 | ||||
| Existential | 0.22 | .04 | ||||
| Illness acceptance | 0.22 | .04 | ||||
| Grief | −.28† | .01 | ||||
NOTE. Stage at diagnosis: “Unknown” not included in analysis; Income: 11.6% (n = 11) reported “don't know.” Median value entered for “don't know” responses. Health insurance status was not examined because 97.9% of the sample had health insurance coverage.
Sample size ranges from 88 to 94 because of missing data.
P < .01.
Psychosocial Well-Being
Associations of psychosocial variables with alliance are provided in Table 2. Appraisal support was the only social support subscale significantly positively associated with alliance (r [88] = .32; P = .002). Alliance was also associated with less severe illness-related grief (r [92] = −.28; P = .01).
Associations of psychosocial variables with adherence were also examined. More frequent thoughts of stopping cancer treatment was associated with more severe grief (r [85] = .28; P = .009) and lower levels of psychological quality of life (r [86] = −.35; P = .001). In addition, better adherence to cancer therapy was associated with better psychological quality of life (r [59] = .38; P = .003). No other significant relationships between psychosocial well-being and adherence emerged.
Alliance and Adherence
Associations of alliance and adherence are presented in Table 3. Because of their relationship with alliance, metastases, appraisal support, and grief were included in adjusted analyses. A stronger alliance was associated with more frequent thoughts that treatment is worth the adverse effects in unadjusted analyses (P = .002). However, this relationship was not significant after controlling for the confounding variables (P = .038). A stronger alliance was also associated with less frequent thoughts of stopping cancer treatment in unadjusted analyses (P < .001). The relationship remained significant (P < .001) after controlling for the confounders of metastases (β = .11; P = .57), appraisal support (β = −.03; P = .63), and grief (β = .02; P = .26). Finally, a stronger alliance was associated with better adherence to cancer treatment in unadjusted analyses (P = .01). The relationship remained significant (P = .005) after controlling for metastases (β = .16; P = .36), appraisal support (β = −.07; P = .16), and grief (β = −.02; P = .14). Thus, psychosocial well-being did not account for the relationship between alliance and adherence.
Table 3.
Regression Analyses With Alliance Predicting Willingness to Adhere to Treatment and Treatment Adherence
| Outcome | Alliance |
||||
|---|---|---|---|---|---|
| β | SE | df | 95% CI | P | |
| Treatment worth adverse effects | 0.04 | 0.01 | 86 | 0.01 to 0.06 | .002 |
| Adjusted* | 0.03 | 0.01 | 81 | 0.002 to 0.06 | .038 |
| Think about stopping treatment | −.07 | 0.01 | 85 | −.10 to −.05 | .00 |
| Adjusted* | −.06 | 0.02 | 80 | −.09 to −.03 | .00 |
| Treatment adherence | 0.03 | 0.01 | 58 | 0.01 to 0.06 | .01 |
| Adjusted* | 0.04 | 0.01 | 58 | 0.01 to 0.07 | .005 |
NOTE. β, unstandardized regression coefficients.
Controlling for metastasis, appraisal support, and grief.
DISCUSSION
This study examined the relationship between the patient-oncologist alliance and psychosocial well-being, willingness to adhere to treatment, and treatment adherence in young adults with advanced cancer. A stronger alliance was associated with greater perceived social support and less severe grief due to cancer-related losses. In addition, a stronger alliance was associated with greater willingness to adhere to treatment and better adherence to oral medication after controlling for confounding sample characteristics and psychosocial well-being. These results indicate that the role of the oncologist extends beyond the prescription of medication and includes patients' perception of social support, psychological well-being, and attitudes toward and actual behaviors regarding treatment adherence.
Although the relationship between a stronger alliance and greater well-being is consistent with research on alliance in older adults with advanced cancer,2 these cross-sectional data preclude concluding that a causal relationship exists. A strong alliance may provide comfort that improves young adults' well-being. Developing a strong alliance may also be easier with young adults who are less distressed. Notably, alliance was associated only with a single subscale of the social support measure, appraisal support or the availability of someone to talk to about problems. This finding suggests that providing someone for young adults to talk to is an important component of the alliance.
A strong alliance in this study was associated with greater willingness to adhere to treatment and better adherence to oral medication. Poor adherence is common in young adults with cancer, is associated with greater morbidity and lower survival rates, and has been identified as a potential contributor to the lag in improved survival rates in young adults relative to other age groups.21,22,29 The patient-oncologist alliance may be a powerful mechanism for improving treatment adherence and associated medical outcomes in young adults. Given that psychosocial well-being did not explain away the association between alliance and adherence, these effects were independent of one another. In other words, improving alliance may improve both psychosocial well-being and adherence. Longitudinal analyses are needed to confirm these hypothesized causal relationships.
Medical professionals are often not aware of the issues unique to young adults and may benefit from training on how to work effectively with this patient population.49 In addition, young adults are generally unfamiliar with the health care system, which may complicate development of a strong alliance. Training young adults and oncologists on developing an alliance may be an effective mechanism for improving treatment adherence. In a meta-analysis of physician communication across diseases, patients whose providers did not receive communication skills training had a 12% higher risk of nonadherence relative to patients whose physicians were trained.12
Communication skills training for oncologists shows initial promise, but additional research is needed.50,51 Communication is only one aspect of the alliance, and the degree to which communication training improves the patient-oncologist alliance is unclear. Skills-based resources that teach oncologists and young adults strategies for developing a strong alliance may improve patient care, adherence, and treatment outcomes. Guidelines for developing a therapeutic alliance have been published for patients with pediatric cancer and their families.52 Similar guidelines for young adult patients may help oncologists, young adults, and their families form effective relationships.
Oncologists who want to foster a therapeutic alliance with their patients should strive to listen attentively to their concerns, convey respect, offer empathic support, and promote trust in working together toward shared goals of care. Developing a strong alliance with young adult patients with cancer requires consideration of the maturity level, independence, and cognitive and emotional development of the patient.53,54 Young adults prefer direct communication that is positive, respectful, and nonjudgmental,32,55 whereas communication styles that are punitive, aloof, and over-controlling are problematic.56 In addition, a flexible approach to treatment and emphasis of the importance of treatment adherence may be particularly important for this age group.53,56
Limitations and Future Directions
The measure of alliance used in this study was developed in older adults with advanced cancer. Although the measure demonstrated good reliability and validity in this sample, the alliance between a young adult and his/her oncologist may have unique characteristics not captured in a measure developed in older samples. Assessing young adults' and oncologists' experiences of the alliance through qualitative interviews would inform development of young adult–specific measures of the patient-provider alliance.
Adherence and willingness to adhere to treatment in this study were assessed with self-report items, which may be susceptible to over-reporting.57 Further, the adherence measure was a single item that assessed adherence only to oral medications. Cancer treatment is often multifaceted and may include dietary restrictions, limitations on activities, and attendance at follow-up appointments. Use of multi-item measures that combine objective and subjective items will provide a more comprehensive and accurate assessment of adherence. In addition, the adherence item did not assess the medications with which patients were nonadherent. Future research is needed to determine which specific treatments patients fail to adhere to.
This study is also limited by a cross-sectional design. Longitudinal studies that examine alliance and adherence over time will identify changes in these constructs and determine whether oncologists can improve adherence through the alliance. Finally, the sample consisted of young adults with advanced cancer; the results cannot be generalized to other age groups, diseases, or disease stages.
CONCLUSION
In this study, a strong alliance between the young adult and oncologist was associated with greater psychosocial well-being, greater willingness to adhere to treatment, and better treatment adherence. Development of guidelines and skills-based training for oncologists, young adults, and their families that foster a strong alliance may improve the psychosocial functioning of young adult patients with cancer, improve their treatment adherence, and promote the positive outcomes that follow from better mental health and treatment adherence.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Karen Fasciano, Holly G. Prigerson
Financial support: Holly G. Prigerson
Administrative support: Holly G. Prigerson
Provision of study materials or patients: Holly G. Prigerson
Collection and assembly of data: Kelly M. Trevino, Holly G. Prigerson
Data analysis and interpretation: Kelly M. Trevino, Holly G. Prigerson
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: A meta-analytic review. J Consult Clin Psychol. 2000;68:438–450. [PubMed] [Google Scholar]
- 2.Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: The Human Connection Scale. Cancer. 2009;115:3302–3311. doi: 10.1002/cncr.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe BE, Goldfried MR. Research on psychotherapy integration: Recommendations and conclusions from an NIMH workshop. J Consult Clin Psychol. 1988;56:448–451. doi: 10.1037//0022-006x.56.3.448. [DOI] [PubMed] [Google Scholar]
- 4.Horvath AO, Bedi RP. The alliance. In: Norcross JC, editor. Psychotherapy Relationships That Work: Therapist Contributions and Responsiveness to Patients. New York, NY: Oxford University Press; 2002. pp. 37–69. [Google Scholar]
- 5.Norcross JC. Psychotherapy Relationships That Work: Therapist Contributions and Responsiveness to Patients. New York, NY: Oxford University Press; 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bennett JK, Fuertes JN, Keitel M, et al. The role of patient attachment and working alliance on patient adherence, satisfaction, and health-related quality of life in lupus treatment. Patient Educ Couns. 2011;85:53–59. doi: 10.1016/j.pec.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Fuertes JN, Mislowack A, Bennett J, et al. The physician-patient working alliance. Patient Educ Couns. 2007;66:29–36. doi: 10.1016/j.pec.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Fuertes JN, Boylan LS, Fontanella JA. Behavioral indices in medical care outcome: The working alliance, adherence, and related factors. J Gen Intern Med. 2009;24:80–85. doi: 10.1007/s11606-008-0841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Nilsson ME, Prigerson HG. Factors important to patients' quality of life at the end of life. Arch Intern Med. 2012;172:1133–1142. doi: 10.1001/archinternmed.2012.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox SA, Heritage J, Stockdale SE, et al. Cancer screening adherence: Does physician-patient communication matter? Patient Educ Couns. 2009;75:178–184. doi: 10.1016/j.pec.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39:555–563. doi: 10.1177/1090198111421800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: A meta-analysis. Med Care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillen MA, de Haes HC, Smets EM. Cancer patients' trust in their physician: A review. Psychooncology. 2011;20:227–241. doi: 10.1002/pon.1745. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke CH, Rosner B, Chen WY, et al. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22:1849–1856. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 15.Mazanec SR, Daly BJ, Douglas SL, et al. The relationship between optimism and quality of life in newly diagnosed cancer patients. Cancer Nurs. 2010;33:235–243. doi: 10.1097/NCC.0b013e3181c7fa80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter J, Sonoda Y, Baser RE, et al. A 2-year prospective study assessing the emotional, sexual, and quality of life concerns of women undergoing radical trachelectomy versus radical hysterectomy for treatment of early-stage cervical cancer. Gynecol Oncol. 2010;119:358–365. doi: 10.1016/j.ygyno.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisseling KC, Kondalsamy-Chennakesavan S, Bekkers RL, et al. Depression, anxiety and body image after treatment for invasive stage one epithelial ovarian cancer. Aust N Z J Obstet Gynaecol. 2009;49:660–666. doi: 10.1111/j.1479-828X.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 18.Trevino KM, Fasciano K, Block S, et al. Correlates of social support in young adults with advanced cancer. Support Care Cancer. 2013;21:421–429. doi: 10.1007/s00520-012-1536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zebrack B, Hamilton R, Smith AW. Psychosocial outcomes and service use among young adults with cancer. Semin Oncol. 2009;36:468–477. doi: 10.1053/j.seminoncol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Zebrack BJ. Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117:2289–2294. doi: 10.1002/cncr.26056. [DOI] [PubMed] [Google Scholar]
- 21.Butow P, Palmer S, Pai A, et al. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4800–4809. doi: 10.1200/JCO.2009.22.2802. [DOI] [PubMed] [Google Scholar]
- 22.Kondryn HJ, Edmondson CL, Hill J, et al. Treatment non-adherence in teenage and young adult patients with cancer. Lancet Oncol. 2011;12:100–108. doi: 10.1016/S1470-2045(10)70069-3. [DOI] [PubMed] [Google Scholar]
- 23.Festa RS, Tamaroff MH, Chasalow F, et al. Therapeutic adherence to oral medication regimens by adolescents with cancer: I. Laboratory assessment. J Pediatr. 1992;120:807–811. doi: 10.1016/s0022-3476(05)80256-2. [DOI] [PubMed] [Google Scholar]
- 24.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 25.Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: Adherence companion study 60104. J Clin Oncol. 2010;28:2418–2422. doi: 10.1200/JCO.2009.26.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruddy KJ, Pitcher BN, Archer LE, et al. Persistence, adherence, and toxicity with oral CMF in older women with early-stage breast cancer (Adherence Companion Study 60104 for CALGB 49907) Ann Oncol. 2012;23:3075–3081. doi: 10.1093/annonc/mds133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 28.Kennard BD, Stewart SM, Olvera R, et al. Nonadherence in adolescent oncology patients: Preliminary data on psychological risk factors and relationships to outcome. J Clin Psychol Med Settings. 2004;11:31–39. [Google Scholar]
- 29.Bleyer A. Young adult oncology: The patients and their survival challenges. CA Cancer J Clin. 2007;57:242–255. doi: 10.3322/canjclin.57.4.242. [DOI] [PubMed] [Google Scholar]
- 30.Kondryn HJ, Edmondson CL, Hill JW, et al. Treatment non-adherence in teenage and young adult cancer patients: A preliminary study of patient perceptions. Psychooncology. 2009;18:1327–1332. doi: 10.1002/pon.1541. [DOI] [PubMed] [Google Scholar]
- 31.Zack SE, Castonguay LG, Boswell JF. Youth working alliance: A core clinical construct in need of empirical maturity. Harv Rev Psychiatry. 2007;15:278–288. doi: 10.1080/10673220701803867. [DOI] [PubMed] [Google Scholar]
- 32.Morgan S, Davies S, Palmer S, et al. Sex, drugs, and rock ‘n’ roll: Caring for adolescents and young adults with cancer. J Clin Oncol. 2010;28:4825–4830. doi: 10.1200/JCO.2009.22.5474. [DOI] [PubMed] [Google Scholar]
- 33.Zebrack BJ, Mills J, Weitzman TS. Health and supportive care needs of young adult cancer patients and survivors. J Cancer Surviv. 2007;1:137–145. doi: 10.1007/s11764-007-0015-0. [DOI] [PubMed] [Google Scholar]
- 34.Millar B, Patterson P, Desille N. Emerging adulthood and cancer: How unmet needs vary with time-since-treatment. Palliat Support Care. 2010;8:151–158. doi: 10.1017/S1478951509990903. [DOI] [PubMed] [Google Scholar]
- 35.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 36.Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale: An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Mermelstein R, Kamark T, et al. Measuring the functional components of social support. In: Sarason IG, Sarason B, editors. Social Support: Theory, Research, and Applications. The Netherlands, Martinus Nijhoff: The Hague; 1985. pp. 73–94. [Google Scholar]
- 38.Turner-Cobb JM, Sephton SE, Koopman C, et al. Social support and salivary cortisol in women with metastatic breast cancer. Psychosom Med. 2000;62:337–345. doi: 10.1097/00006842-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Tomarken A, Holland J, Schachter S, et al. Factors of complicated grief pre-death in caregivers of cancer patients. Psychooncology. 2008;17:105–111. doi: 10.1002/pon.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen SR, Mount BM, Bruera E, et al. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: A multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med. 1997;11:3–20. doi: 10.1177/026921639701100102. [DOI] [PubMed] [Google Scholar]
- 41.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarakeshwar N, Vanderwerker LC, Paulk E, et al. Religious coping is associated with the quality of life of patients with advanced cancer. J Palliat Med. 2006;9:646–657. doi: 10.1089/jpm.2006.9.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prigerson HG, Maciejewski PK, Reynolds CF, 3rd, et al. Inventory of Complicated Grief: A scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995;59:65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- 44.Prigerson HG, Horowitz MJ, Jacobs SC, et al. Prolonged grief disorder: Psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS Med. 2009;6:e1000121. doi: 10.1371/journal.pmed.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobsen JC, Zhang B, Block SD, et al. Distinguishing symptoms of grief and depression in a cohort of advanced cancer patients. Death Stud. 2010;34:257–273. doi: 10.1080/07481180903559303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trevino KM, Maciejewski P, Fasciano K, et al. Grief and life disruption in young adults with advanced cancer. J Adolesc Young Adult Oncol. 2011/2012;1:168–172. [Google Scholar]
- 47.Mack JW, Nilsson M, Balboni T, et al. Peace, Equanimity, and Acceptance in the Cancer Experience (PEACE): Validation of a scale to assess acceptance and struggle with terminal illness. Cancer. 2008;112:2509–2517. doi: 10.1002/cncr.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abetz L, Coombs JH, Keininger DL, et al. Development of the cancer therapy satisfaction questionnaire: Item generation and content validity testing. Value Health. 2005;8(suppl 1):S41–S53. doi: 10.1111/j.1524-4733.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 49.Hayes-Lattin B, Mathews-Bradshaw B, Siegel S. Adolescent and young adult oncology training for health professionals: A position statement. J Clin Oncol. 2010;28:4858–4861. doi: 10.1200/JCO.2010.30.5508. [DOI] [PubMed] [Google Scholar]
- 50.Kissane DW, Bylund CL, Banerjee SC, et al. Communication skills training for oncology professionals. J Clin Oncol. 2012;30:1242–1247. doi: 10.1200/JCO.2011.39.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singy P, Bourquin C, Sulstarova B, et al. The impact of communication skills training in oncology: A linguistic analysis. J Cancer Educ. 2012;27:404–408. doi: 10.1007/s13187-012-0385-5. [DOI] [PubMed] [Google Scholar]
- 52.Masera G, Spinetta JJ, Jankovic M, et al. Guidelines for a therapeutic alliance between families and staff: A report of the SIOP Working Committee on Psychosocial Issues in Pediatric Oncology. Med Pediatr Oncol. 1998;30:183–186. doi: 10.1002/(sici)1096-911x(199803)30:3<183::aid-mpo12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari A, Thomas D, Franklin AR, et al. Starting an adolescent and young adult program: Some success stories and some obstacles to overcome. J Clin Oncol. 2010;28:4850–4857. doi: 10.1200/JCO.2009.23.8097. [DOI] [PubMed] [Google Scholar]
- 54.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Zebrack B, Chesler MA, Kaplan S. To foster healing among adolescents and young adults with cancer: What helps? What hurts? Support Care Cancer. 2010;18:131–135. doi: 10.1007/s00520-009-0719-y. [DOI] [PubMed] [Google Scholar]
- 56.D'Agostino NM, Penney A, Zebrack B. Providing developmentally appropriate psychosocial care to adolescent and young adult cancer survivors. Cancer. 2011;117:2329–2334. doi: 10.1002/cncr.26043. [DOI] [PubMed] [Google Scholar]
- 57.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]