Introduction
Giant-cell tumor of bone (GCTB) primarily occurs in young adults between the ages of 20 and 40 years and comprises approximately 5% of primary bone tumors.1 Pediatric cases of GCT are even less frequent and are believed to comprise only 1.7% of all cases of GCTB.2 Although usually a benign tumor, GCTB frequently recurs locally after surgical resection.1,3,4 Approximately 3% of GCTB metastasize, primarily to the lungs5; metastatic disease at presentation is uncommon. Histologically, GCTB has two cellular components: neoplastic mononuclear cells that are evenly scattered among osteoclast precursors and osteoclast-like giant cells.3,6 Radiographically, these tumors usually appear as lytic destructive lesions, often in the proximal femur or distal tibia.3,6 Clinically, patients present with pain and often have deformities at the tumor site, without constitutional symptoms. About two decades ago, the receptor activator of NF-kappaB ligand (RANKL) signaling pathway was discovered, and its importance in the regulation of bone growth and turnover became apparent. For instance, RANKL knockout mice (with absence of the ligand) demonstrate osteopetrotic bone changes as a result of impaired osteoclast differentiation and subsequent decreased bone resorption. Because denosumab inhibits RANKL (and therefore osteoclast activity), it is used in the treatment of postmenopausal osteoporosis, in which there is a state of increased bone resorption. Furthermore, RANKL is thought to participate in the growth of the tumor cells, possibly as a result of production of growth factors by osteoclast-like giant cells through a paracrine loop.7 Recently, a phase II study in adults with GCTs has demonstrated significant clinical response to the anti-RANKL monoclonal antibody denosumab.6 There is also histologic confirmation of the treatment effects of denosumab on GCTs.8 However, we are unaware of published data regarding the safety and efficacy of this drug in pediatric patients and the impact it may have on bone growth and health.
Case Report
A 10-year-old white girl presented to her primary pediatrician with a chief complaint of right knee pain in January of 2010. She was a competitive ice skater, and her usual routines had become progressively more difficult. She was diagnosed with “runner's knee” and prescribed nonsteroidal anti-inflammatory drugs and rest for her pain. She did not seek additional medical care, despite progression of her knee pain to the point that it limited her walking.
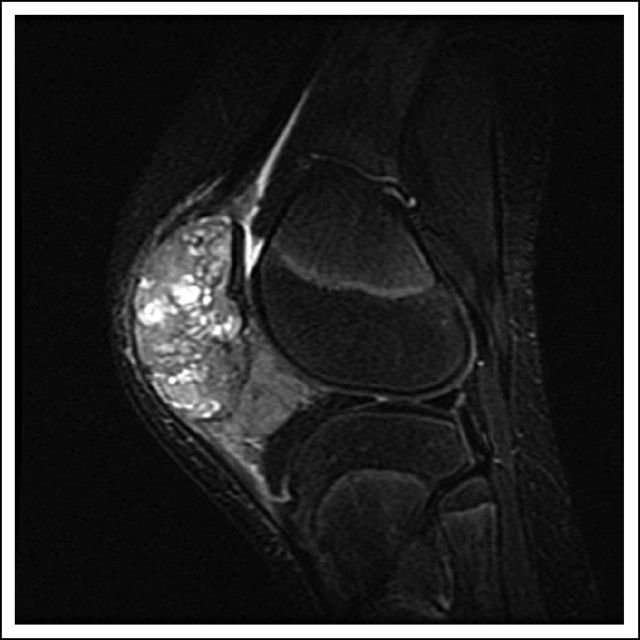
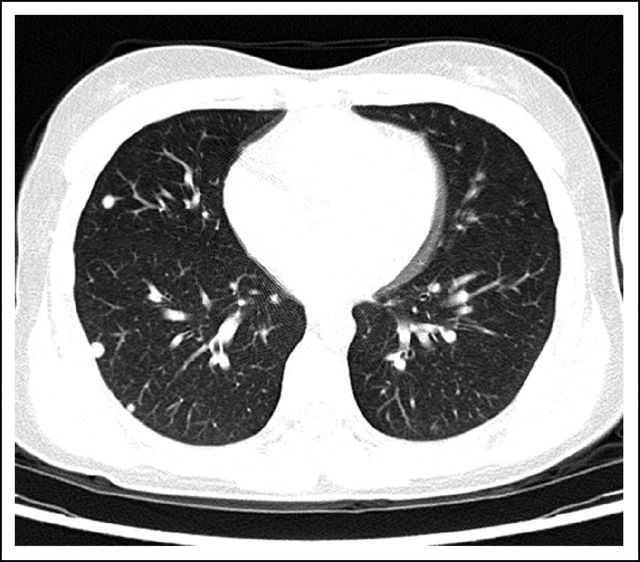
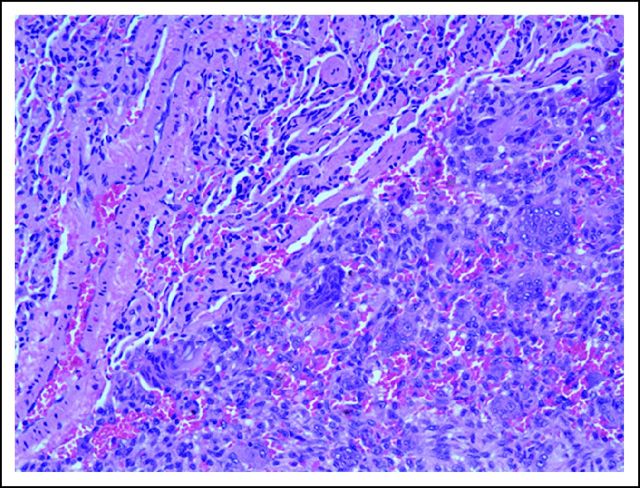
In July 2010, she fell on her right knee and was taken to a local emergency room, where a severely swollen knee was noted. A radiograph of her knee showed destruction of the patella. She was referred to an orthopedic surgeon, who unsuccessfully attempted an arthrocentesis. A magnetic resonance imaging scan demonstrated a 5.9 × 4.8 × 4.9-cm osseous and soft tissue mass centered in the patella (Fig 1). There was marrow extension and three similar subcutaneous lesions were observed around the knee. She was referred to orthopedic surgery at our tertiary care center, where the patellar tumor was biopsied. After use of special stains and review by an expert consultant, a diagnosis of GCTB was established (Fig 2A, multinucleated osteoclast giant cells with large numbers of nuclei are evenly scattered among mononuclear tumor cells; Fig 2B, mononuclear tumor cells display nuclear reactivity for P63). In addition, a positron emission tomography–computed tomography scan demonstrated hypermetabolic activity of the patellar mass, the three subcutaneous nodules, and innumerable (> 30) pulmonary nodules (Fig 3). The patient underwent resection of a pulmonary nodule that confirmed metastatic GCTB (Fig 4, metastatic GCTB [lower right] and adjacent lung parenchyma [upper left]). She was subsequently started on denosumab with induction dosing of 120 mg subcutaneously, once per week for 3 weeks, followed by 120 mg denosumab subcutaneously once per month. She is currently 20 months into treatment.
Fig 1.
Fig 2.
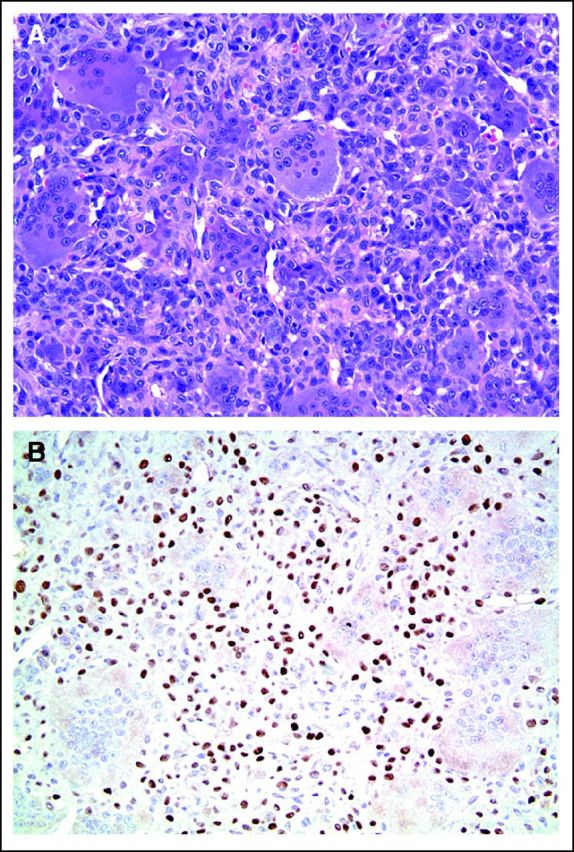
Fig 3.
Fig 4.
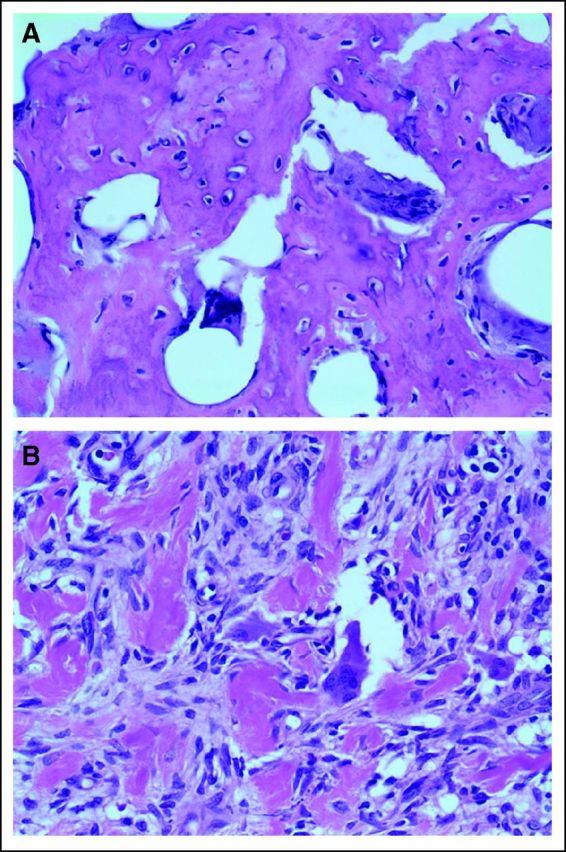
Initially, our patient was relying on a wheelchair at school and was unable to perform any physical activities because of pain and immobility of her knee. Within 4 months of beginning the treatment with denosumab, her pain dramatically improved, and she did not require regular pain medications. Approximately 6 to 7 months into treatment she was back to her regular activities, including ice skating (with a protective knee guard). In light of the excellent clinical response, we opted for local control of her patellar tumor and subcutaneous nodules to debulk the tumor and to improve local function, given that her patella was greatly enlarged and restricted knee flexion. As a secondary goal, this procedure would also provide material to evaluate histologic response. The patella and subcutaneous nodules were removed a year after diagnosis. The patient is currently able to ice skate, run, and bike. Pathologic examination of the tumor after treatment with denosumab demonstrated significant response, as evidenced by the paucity or disappearance of giant cells and the production of bone and fibrous tissue (Figs 5A and 5B, GCT after denosumab treatment; Fig 5A shows extensive bone deposition and rare apoptotic osteoclast giant cells; Fig 5B shows fibrosis and residual multinucleated giant cells with sparse nuclei. Numerous fields were completely devoid of giant cells). She has no pulmonary symptoms; subsequent chest computed tomography scans have demonstrated significant response in the size of the nodules; however, they are still present.
Fig 5.
The patient has required 4,000 IU per day of oral vitamin D supplementation to achieve a low normal vitamin D level; her most recent 25-hydroxy vitamin D level was 34 μg/L. She is also receiving calcium and phosphorus supplementation because of the development of hypocalcemia and hypophosphatemia from decreased bone turnover during her treatment with denosumab; her most recent serum calcium level was 9.1 mg/dL, and her phosphorus level was 3.6 mg/dL. Serum markers of bone resorption and bone formation were suppressed with treatment (C-telopeptide, 268 pg/mL [normal, 503-2,077 pg/mL] and bone-specific alkaline phosphatase, 24.6 ng/mL [normal, 44 to 163 ng/mL]). The patient has had no evidence of necrosis of her jaw. Furthermore, her growth velocity since starting denosumab treatment is 6 cm per year, and her height is at the 54th percentile for her age and gender. Interestingly, as a result of the denosumab therapy, she has developed dense metaphyseal bands in her long bones on radiographs, as well as increased total body and lumbar spine bone mineral density by dual-energy x-ray absorptiometry (Z-scores, 2.7 and 4, respectively). This is believed to be an adverse effect of the denosumab treatment and raises potential concerns for worsening osteopetrotic bone disease and increased risk of fracture as treatment continues.
Discussion
GCTB is rare in children; historically, surgery was the only therapy available. However, only 80% of tumors are amenable to surgery, and of those that are surgically resected, recurrence rates vary between 10% and 75%.9 Thomas et al6 completed an open-label, phase II study with single-agent denosumab in patients who were older than age 18 years with recurrent GCTB or unresectable tumors. Of the 35 patients who were evaluable for efficacy, 86% met tumor response criteria at 25 weeks of treatment. A similar percentage reported clinical benefits, including decreased pain and improvement in functional status. Denosumab works by binding to RANKL and thus blocking binding to RANK on osteoclasts and osteoclast precursors, therefore inhibiting differentiation of osteoclasts and osteoclast-mediated bone reabsorption. Because the giant cells in GCTB also express RANK, denosumab is an attractive target-specific therapy for this tumor.1,6,10 Denosumab has also been used to treat bone metastases, such as in prostate cancer.11
A number of factors make our patient informative and unique, most notably her young age and metastatic disease at presentation. We have successfully shown that denosumab is tolerable and effective in a peripubertal female. Furthermore, despite our initial concerns that therapy with denosumab might stunt her growth, she continues to have a normal growth velocity for her age and gender. She has had minimal adverse effects, mostly related to her bone health, that have affected her daily life. However, she has developed dense metaphyseal bands, generalized increased bone mineral density, and suppressed biomarkers of bone remodeling; these changes are concerning for a negative alteration in bone quality and potential increased risk of fracture, as has been described in osteopetrotic rickets.12,13 Interestingly, a case has been reported in which osteopetrosis was induced by treatment of a peripubertal child with bisphosphonates.14
Because of her innumerable lung nodules, the consensus plan is to continue treating her with denosumab monthly for the near future. It is probable that she will require long-term treatment with denosumab to prevent osteoclast and giant cells from reactivating and thus to prevent progression of her pulmonary disease. Whether intermittent or continuous denosumab is preferable in metastatic GCTB is unknown and can only be determined by a randomized trial. The possibility of removing her pulmonary nodules with the intent of complete cure has been proposed; at this time, the patient's family is considering this option.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.Thomas DM, Skubitz KM. Giant cell tumor of bone. Curr Opin Oncol. 2009;21:338–344. doi: 10.1097/CCO.0b013e32832c951d. [DOI] [PubMed] [Google Scholar]
- 2.Picci P, Manfrini M, Zucchi V, et al. Giant-cell tumor of bone in skeletally immature patients. J Bone Joint Surg Am. 1983;65:486–490. [PubMed] [Google Scholar]
- 3.Gong L, Liu W, Sun X, et al. Histological and clinical characteristics of malignant giant cell tumor of bone. Virchows Arch. 2012;460:327–334. doi: 10.1007/s00428-012-1198-y. [DOI] [PubMed] [Google Scholar]
- 4.Miller IJ, Blank A, Yin SM, et al. A case of recurrent giant cell tumor of bone with malignant transformation and benign pulmonary metastases. Diagn Pathol. 2010;5:62. doi: 10.1186/1746-1596-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campanacci M, Baldini N, Boriani S, et al. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 6.Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol. 2010;11:275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 7.Skubitz KM, Cheng EY, Clohisy DR, et al. Gene expression in giant-cell tumors. J Lab Clin Med. 2004;144:193–200. doi: 10.1016/j.lab.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Branstetter DG, Nelson SD, Manivel JC, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18:4415–4424. doi: 10.1158/1078-0432.CCR-12-0578. [DOI] [PubMed] [Google Scholar]
- 9.Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135:149–158. doi: 10.1007/s00432-008-0427-x. [DOI] [PubMed] [Google Scholar]
- 10.Lipton A, Jacobs I. Denosumab: Benefits of RANK ligand inhibition in cancer patients. Curr Opin Support Palliat Care. 2011;5:258–264. doi: 10.1097/SPC.0b013e328349731c. [DOI] [PubMed] [Google Scholar]
- 11.Miller RE, Roudier M, Jones J, et al. RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther. 2008;7:2160–2169. doi: 10.1158/1535-7163.MCT-08-0046. [DOI] [PubMed] [Google Scholar]
- 12.Demirel F, Esen I, Tunc B, et al. Scarcity despite wealth: Osteopetrorickets. J Pediatr Endocrinol Metab. 2010;23:931–934. doi: 10.1515/jpem.2010.149. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan FS, August CS, Fallon MD, et al. Osteopetrorickets: The paradox of plenty—Pathophysiology and treatment. Clin Orthop Relat Res. 1993;294:64–78. [PubMed] [Google Scholar]
- 14.Whyte MP, Wenkert D, Clements KL, et al. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]