Abstract
Purpose
To investigate the prognostic value of the Hematopoietic Cell Transplantation–Specific Comorbidity Index (HCT-CI) in patients who received transplantation admitted to the intensive care unit (ICU).
Patients and Methods
We investigated the association of HCT-CI with inpatient mortality and overall survival (OS) among 377 patients who were admitted to the ICU within 100 days of allogeneic stem-cell transplantation (ASCT) at our institution. HCT-CI scores were collapsed into four groups and were evaluated in univariate and multivariate analyses using logistic regression and Cox proportional hazards models.
Results
The most common pretransplantation comorbidities were pulmonary and cardiac diseases, and respiratory failure was the primary reason for ICU admission. We observed a strong trend for higher inpatient mortality and shorter OS among patients with HCT-CI values ≥ 2 compared with patients with values of 0 to 1 in all patient subsets studied. Multivariate analysis showed that patients with HCT-CI values ≥ 2 had significantly higher inpatient mortality than patients with values of 0 to 1 and that HCT-CI values ≥ 4 were significantly associated with shorter OS compared with values of 0 to 1 (hazard ratio, 1.74; 95% CI, 1.23 to 2.47). The factors associated with lower inpatient mortality were ICU admission during the ASCT conditioning phase or the use of reduced-intensity conditioning regimens. The overall inpatient mortality rate was 64%, and the 1-year OS rate was 15%. Among patients with HCT-CI scores of 0 to 1, 2, 3, and ≥ 4, the 1-year OS rates were 22%, 17%, 18%, and 9%, respectively.
Conclusion
HCT-CI is a valuable predictor of mortality and survival in critically ill patients after ASCT.
INTRODUCTION
Complications associated with allogeneic hematopoietic stem-cell transplantation (ASCT) can lead to critical illness with multiple-organ failure.1 Eleven percent to 24% of patients who undergo transplantation require admission to the intensive care unit (ICU).2–6 Although the mortality rate of ASCT patients admitted to the ICU has been decreasing,1,3,7–9 their short-term mortality is still greater than 50%.6,10–12
Accurate estimation of prognosis after ICU admission is indispensable for physicians to evaluate the potential benefits or futility of ICU care and to advise the patients and their families on the decisions about life-supportive therapies. Mechanical ventilation and vasopressor administration are two of the most significant prognostic factors of survival of ASCT recipients among the various life-supportive interventions provided in the ICU3,5,6,8,13,14; however, these are of little value in prognostication before ICU admission. Other factors such as hemodynamic instability, multiorgan system failure, graft-versus-host disease (GVHD), hyperbilirubinemia, and type of transplantation (allogeneic v autologous) have been described as important prognostic factors of survival.3,6,8,14 Well-known instruments used to predict survival of patients admitted to the ICU, such as the Acute Physiology and Chronic Health Evaluation (APACHE) II,3,5,8,13,15 APACHE III,14,16 and the Sequential Organ Failure Assessment,4,17 have limited prognostic value in ASCT recipients.
Because the presence and the severity of comorbidities affect the outcomes of patients admitted to the ICU, these are included in ICU prognostic models of mortality.15,18 Comorbidity is defined as any illness unrelated to a patient's principal diagnosis, and such comorbidities influence the outcomes of the disease under evaluation.19,20 The Charlson comorbidity index (CCI), which was initially developed to estimate mortality in longitudinal studies by classifying comorbidities according to the International Classification of Diseases, Ninth Revision, has been found to significantly improve outcome prediction compared with the chronic health component of APACHE II in critically ill patients admitted to the ICU.21 The Hematopoietic Cell Transplantation–Specific Comorbidity Index (HCT-CI), which was derived from the CCI, was initially developed to predict outcomes after ASCT22 and was found to be a strong predictor of survival and mortality associated with nonrelapse in single-center and multi-institutional studies.23,24 We investigated the HCT-CI measured at the time of ASCT as a predictive instrument for mortality during hospitalization and overall survival (OS) among patients admitted to our institution's ICU early (< 100 days) after ASCT.
PATIENTS AND METHODS
This study was conducted with the approval of the Institutional Review Board of The University of Texas MD Anderson Cancer Center. We included patients older than 18 years who underwent ASCT and were admitted to the ICU, during the conditioning period or within the first 100 days of the transplantation, between June 2001 and December 2010. Only the first ICU admission of patients was included in the study. Demographics, pretransplantation comorbidities and test results, Karnofsky performance status (KPS) at the time of transplantation, disease and transplantation characteristics, date of transplantation, date and survival status at last follow-up, and dates of ICU admission and discharge were gathered from the institutional registries. Reason for ICU admission, date of hospital discharge, survival status at the time of hospital discharge, and the place/institution to where the patients were discharged were gathered from individual patient medical records.
The decision to transfer patients to the ICU was taken following institutional policies and in conjunction with the intensive care physician on call. Our ICU admission policies are based on the Society of Critical Care Medicine Admission, Discharge, and Triage Guidelines.25 We classified conditioning dose-intensities into ablative and nonablative based on published criteria.26 Engraftment day was defined as the first day of peripheral-blood neutrophil count ≥ 500/μL. Donor-recipient HLA matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, -Cw, -DQB1, and -DRB1 loci. Acute GVHD was diagnosed clinically and confirmed pathologically whenever possible. Patients were clinically managed according to MD Anderson Cancer Center standard guidelines including prophylaxis for Pneumocystis carinii, herpes viruses, and fungal infections. Patients received no cytomegalovirus (CMV) -specific antiviral prophylaxis and were monitored for CMV reactivation by CMV polymerase chain reaction or pp65 antigenemia assay of peripheral blood.
HCT-CI score was calculated as previously described by Sorror et al,22 and the score values were based on the pretransplantation body mass index, comorbidities, laboratory values, and test results. The definitions of comorbidities included in the HCT-CI are listed in Table 1. The diffusion capacity of carbon monoxide was measured following the American Thoracic Society and European Respiratory Society guidelines and using the Cotes' formula as routinely performed in our pulmonary laboratory.27 HCT-CI scores were collapsed into four groups (0 to 1, 2, 3, and ≥ 4) to facilitate our analyses. Inpatient mortality was defined as death from any cause in the hospital before discharge or in hospice within 7 days of hospital discharge. OS was defined as the time from ICU admission to death or last follow-up.
Table 1.
Definitions and Weighted Scores of the Pretransplantation Comorbidities Included in the HCT-CI and Their Prevalence Among the Allogeneic Transplantation Patients Admitted to the Intensive Care Unit
| Comorbidity | Definitions | HCT-CI Weighted Score | Patients (N = 377) |
|
|---|---|---|---|---|
| No. | % | |||
| Arrhythmia | Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmia | 1 | 16 | 4 |
| Cardiac | Coronary artery disease, congestive heart failure, myocardial infarction, or EF ≤ 50% | 1 | 55 | 15 |
| Cerebrovascular disease | Transient ischemic attack or cerebrovascular accident | 1 | 3 | 1 |
| Diabetes | Requiring treatment with insulin or oral hypoglycemic but not diet alone | 1 | 47 | 12 |
| Hepatic, mild | Chronic hepatitis, bilirubin > ULN to 1.5× ULN, or AST/ALT > ULN to 2.5× ULN | 1 | 56 | 15 |
| Infection | Requiring continuation of antimicrobial treatment after day 0 | 1 | 10 | 3 |
| Inflammatory bowel disease | Crohn's disease or ulcerative colitis | 1 | 4 | 1 |
| Obesity | Patients with a body mass index > 35 kg/m2 | 1 | 41 | 11 |
| Psychiatric disturbance | Depression or anxiety requiring psychiatric consult or treatment | 1 | 40 | 11 |
| Renal, moderate/severe | Serum creatinine > 2 mg/dL, on dialysis, or prior renal transplantation | 2 | 3 | 1 |
| Rheumatologic | SLE, RA, polymyositis, mixed CTD, or polymyalgia rheumatic | 2 | 10 | 3 |
| Peptic ulcer | Requiring treatment | 2 | 11 | 3 |
| Pulmonary, moderate | DLCO and/or FEV1 66% to 80% or dyspnea on slight activity | 2 | 123 | 33 |
| Heart valve disease | Except mitral valve prolapse | 3 | 9 | 2 |
| Hepatic, moderate/severe | Liver cirrhosis, bilirubin > 1.5× ULN, or AST/ALT > 2.5× ULN | 3 | 16 | 4 |
| Prior solid tumor | Treated at any time point in the patient's past history, excluding nonmelanoma skin cancer | 3 | 20 | 5 |
| Pulmonary, severe | DLCO and/or FEV1 ≤ 65% or dyspnea at rest or requiring oxygen | 3 | 179 | 47 |
Abbreviations: CTD, connective tissue disease; DLCO, diffusion capacity of carbon monoxide; EF, ejection fraction; FEV1, forced expiratory volume in 1 second; HCT-CI, Hematopoietic Cell Transplantation–Specific Comorbidity Index; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; ULN, upper limit of normal.
Incidence of the inpatient mortality was analyzed using univariate and multivariate logistic regression models. The Kaplan-Meier method was used to estimate median OS. Univariate and multivariate Cox proportional hazards models were used to estimate hazard ratios while evaluating the impact of clinically important factors on OS. The following variables were included in the logistic models and survival models: age at transplantation, primary diagnosis, conditioning dose intensity, donor match and relation, graft source, manifestation of acute GVHD at ICU admission, and HCT-CI score groups. Finally, the effect of HCT-CI scores ≥ 2 on inpatient mortality and OS was evaluated in various patient subsets using univariate logistic regression and univariate Cox proportional hazards models. SAS 9.3 software (SAS Institute, Cary, NC) was used for statistical analyses. NCSS 2007 (NCSS, Kaysville, UT) was used to draw the forest plot in subset analysis of inpatient mortality.
RESULTS
Patient Characteristics
Of 3,039 patients who underwent ASCT at MD Anderson Cancer Center between June 2001 and December 2010, 389 (13%) were admitted to the ICU within 100 days of transplantation. Of these, 12 patients had incomplete pretransplantation comorbidity data and were excluded from the analyses. Eighty-three patients (21%) were admitted to the ICU more than once. Demographic and clinical characteristics of the patients are listed in Table 2. Median age was 53 years (range, 19 to 80 years). Approximately half of the patients had acute leukemia or myelodysplastic syndrome. Matched related and matched unrelated grafts were used in 121 patients (32%) and 156 patients (41%), respectively, whereas 100 patients (27%) received HLA-mismatched transplantations. Three percent of patients were admitted to the ICU before the cell infusion, and 62% of patients were admitted between neutrophil engraftment and transplantation day 100. One hundred eight patients (29%) had a diagnosis of acute GVHD at the time of ICU admission. The most common reasons for ICU admission were respiratory failure (n = 230), septic shock (n = 44), and altered mental status (n = 33).
Table 2.
Demographics and Clinical Characteristics of the Patients Who Receive Allogeneic Transplantation Admitted to the ICU
| Characteristic | No. of Patients (N = 377) | % |
|---|---|---|
| Age at transplantation, years | ||
| Median | 53 | |
| Range | 19-80 | |
| > 55 | 156 | 41 |
| Diagnosis | ||
| Acute leukemias and MDS | 194 | 51 |
| Myeloproliferative disease | 29 | 8 |
| Lymphoma | 124 | 33 |
| Multiple myeloma | 13 | 3 |
| Solid tumors and others | 17 | 5 |
| Conditioning dose-intensity | ||
| Nonablative | 178 | 47 |
| Ablative | 199 | 53 |
| Donor HLA match | ||
| Mismatch | 100 | 27 |
| Match | 277 | 73 |
| Donor relation | ||
| Unrelated | 224 | 59 |
| Related | 153 | 41 |
| Graft source | ||
| Peripheral blood | 224 | 59 |
| Umbilical cord | 42 | 11 |
| Bone marrow | 111 | 29 |
| Day of transplantation at ICU admission | 25 | |
| Median | 25 | |
| Range | −5 to 100 | |
| Transplantation period at ICU admission | ||
| During preparative regimen | 13 | 3 |
| Before engraftment | 130 | 35 |
| After engraftment | 234 | 62 |
| Acute GVHD at the time of ICU admission | ||
| No | 269 | 71 |
| Yes | 108 | 29 |
| HCT-CI score distribution | ||
| 0 | 35 | 9 |
| 1 | 21 | 6 |
| 2 | 60 | 16 |
| 3 | 112 | 30 |
| 4 | 75 | 20 |
| 5 | 28 | 7 |
| 6 | 16 | 4 |
| 7 | 17 | 5 |
| 8 | 7 | 2 |
| 9 | 5 | 1 |
| 10 | 1 | 0 |
| Reason for ICU admission | ||
| Respiratory failure | 230 | 61 |
| Septic shock | 44 | 12 |
| Altered mental status | 33 | 9 |
| Arrhythmia | 20 | 5 |
| Non-GI, non-CNS bleeding | 15 | 4 |
| GI bleeding | 13 | 3 |
| Myocardial infarction | 6 | 2 |
| Upper airway compromise | 5 | 1 |
| Chest pain | 4 | |
| ARF requiring SLED | 4 | |
| Anaphylactic/drug/cell reaction | 3 | |
| Hypertension | 3 | |
| Thrombotic thrombocytopenic purpura | 3 | |
| Unknown | 3 | |
| Veno-occlusive disease | 2 | |
| Mucositis | 2 | |
| Hypotension | 1 | |
| Diabetic emergency | 1 | |
| Dehydration due to GI fluid loss | 1 | |
| For leukapheresis | 1 | |
Abbreviations: ARF, acute renal failure; GVHD, graft-versus-host disease; HCT-CI, Hematopoietic Cell Transplantation–Specific Comorbidity Index; ICU, intensive care unit; MDS, myelodysplastic syndrome; SLED, sustained low-efficiency dialysis.
HCT-CI Score
The prevalence of pretransplantation comorbidities included in the HCT-CI score and the distribution of HCT-CI score values among patients are listed in Tables 1 and 2, respectively. The most common pretransplantation comorbidities were pulmonary (n = 302), cardiac (n = 73), and hepatic (n = 72). Patients' HCT-CI score values ranged between 0 and 10, with a median score of 3. One hundred forty-nine patients (39%) and 13 patients (3%) had HCT-CI score values of ≥ 4 and ≥ 8, respectively (Table 3). The frequency of HCT-CI score values ≥ 2 did not differ between age groups (≤ v > 55 years) and conditioning regimen used (ablative v reduced intensity).
Table 3.
Inpatient Mortality and 1-Year OS Rates According to Patient HCT-CI Scores With Their Respective Univariate ORs and HRs
| HCT-CI Score | Patients (N = 377) |
Inpatient Mortality |
Univariate OR for Inpatient Mortality |
1-Year OS | Univariate HR for OS |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | 95% CI | HR | 95% CI | ||
| 0-1 | 56 | 15 | 26 | 46 | 1.00 | 22.2 | 1.00 | ||
| 2 | 60 | 16 | 40 | 67 | 2.31 | 1.09 to 4.89 | 16.7 | 1.37 | 0.92 to 2.04 |
| 3 | 112 | 30 | 70 | 63 | 1.92 | 1.004 to 3.68 | 17.7 | 1.36 | 0.96 to 1.94 |
| ≥ 4 | 149 | 39 | 104 | 69 | 2.67 | 1.42 to 5.01 | 9.3 | 1.68 | 1.20 to 2.35 |
Abbreviations: HCT-CI, Hematopoietic Cell Transplantation–Specific Comorbidity Index; HR, hazard ratio; OR, odds ratio; OS, overall survival.
Inpatient Mortality
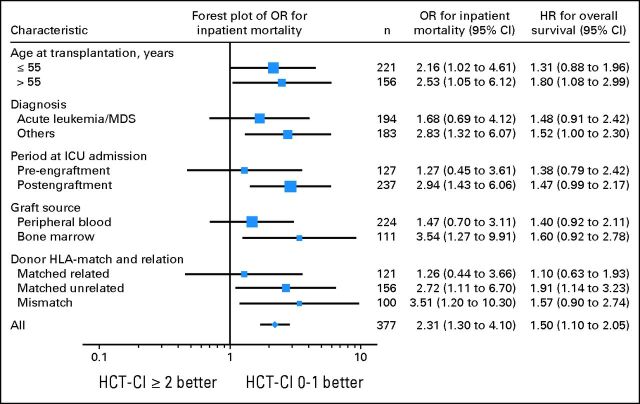
Overall, 240 (64%) of 377 patients admitted to the ICU died in the hospital. Table 3 demonstrates the odds of inpatient mortality according to the HCT-CI scores. Inpatient mortality was significantly higher among patients with HCT-CI scores ≥ 2 than among patients with scores of 0 to 1. A multivariate logistic regression model demonstrated that HCT-CI score ≥ 2 was significantly associated with higher odds of inpatient mortality (Table 4). Conditioning regimen intensity, ICU admission during the conditioning phase, and presence of acute GVHD at the time of ICU admission were the only other independent factors found to affect the inpatient mortality. A second logistic regression model, additionally including KPS and with less power as a result of missing KPS values (n = 309), demonstrated that HCT-CI score ≥ 4 was significantly associated with increased inpatient mortality (P = .003). Finally, HCT-CI scores ≥ 2 were associated with significantly higher odds of inpatient mortality in the following subsets of patients: older than 55 years, with a diagnosis other than acute leukemia or myelodysplastic syndrome, admitted to ICU after engraftment, received bone marrow grafts, and with matched unrelated or mismatched donors (Fig 1).
Table 4.
Multivariate Analyses of Inpatient Mortality and OS
| Factor | Inpatient Mortality |
Multivariate OR for Inpatient Mortality |
Median OS (days) | Multivariate HR for OS |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | OR | 95% CI | P | HR | 95% CI | P | ||
| Age at transplantation, years | |||||||||
| ≤ 55 | 139 | 63 | Reference | 37 | Reference | ||||
| > 55 | 101 | 65 | 1.17 | 0.72 to 1.90 | .53 | 28 | 1.27 | 0.99 to 1.62 | .05 |
| Diagnosis | |||||||||
| Acute leukemia/MDS | 127 | 65 | Reference | 33 | Reference | ||||
| Lymphoma | 80 | 65 | 0.92 | 0.55 to 1.54 | .76 | 32 | 1.00 | 0.78 to 1.29 | .98 |
| Myeloproliferative disease | 14 | 47 | 0.44 | 0.18 to 1.07 | .07 | 89 | 0.79 | 0.51 to 1.22 | .29 |
| Multiple myeloma | 9 | 69 | 1.43 | 0.38 to 5.30 | .60 | 14 | 1.52 | 0.84 to 2.77 | .17 |
| Solid tumors/others | 10 | 59 | 1.27 | 0.41 to 3.90 | .68 | 48 | 1.05 | 0.59 to 1.88 | .86 |
| Year when transplantation was performed | |||||||||
| 2000-2005 | 95 | 61 | Reference | 37 | Reference | ||||
| 2006-2010 | 145 | 66 | 1.23 | 0.77 to 1.97 | .39 | 33 | 1.01 | 0.79 to 1.27 | .96 |
| Transplantation period at ICU admission | |||||||||
| During preparative regimen | 2 | 15 | 0.12 | 0.03 to 0.59 | .009 | 126 | 0.60 | 0.31 to 1.16 | .13 |
| Before engraftment | 84 | 65 | 1.08 | 0.64 to 1.84 | .77 | 37 | 1.09 | 0.83 to 1.42 | .55 |
| After engraftment | 154 | 66 | Reference | 29 | Reference | ||||
| Graft source | |||||||||
| Peripheral blood | 140 | 63 | Reference | 32 | Reference | ||||
| Umbilical cord | 31 | 74 | 1.77 | 0.63 to 4.95 | .28 | 37 | 1.25 | 0.78 to 2 | .36 |
| Bone marrow | 69 | 62 | 1.20 | 0.67 to 2.15 | .54 | 38 | 1.05 | 0.79 to 1.40 | .72 |
| HLA match status | |||||||||
| Mismatch | 64 | 67 | Reference | 33 | Reference | ||||
| Match | 176 | 63 | 1.09 | 0.56 to 2.11 | .80 | 34 | 0.95 | 0.69 to 1.31 | .74 |
| Donor relation | |||||||||
| Unrelated | 144 | 64 | Reference | 38 | Reference | ||||
| Related | 96 | 63 | 0.98 | 0.59 to 1.65 | .94 | 26 | 1.06 | 0.82 to 1.36 | .68 |
| Conditioning dose-intensity | |||||||||
| Reduced intensity | 107 | 60 | Reference | 37 | Reference | ||||
| Ablative | 133 | 67 | 1.64 | 1.01 to 2.68 | .05 | 31 | 1.26 | 0.99 to 1.61 | .07 |
| Acute GVHD at the time of ICU admission | |||||||||
| No | 164 | 61 | Reference | 40 | Reference | ||||
| Yes | 76 | 70 | 1.85 | 1.02 to 3.35 | .04 | 21 | 1.28 | 0.96 to 1.61 | .09 |
| HCT-CI score | |||||||||
| 0-1 | 26 | 46 | Reference | 83 | Reference | ||||
| 2 | 40 | 67 | 2.24 | 1.02 to 4.93 | .05 | 32 | 1.35 | 0.90 to 2.04 | .15 |
| 3 | 70 | 63 | 2.13 | 1.07 to 4.25 | .03 | 34 | 1.38 | 0.96 to 1.97 | .08 |
| ≥ 4 | 104 | 70 | 2.92 | 1.49 to 5.72 | .002 | 26 | 1.74 | 1.23 to 2.47 | .002 |
Abbreviations: HCT-CI, Hematopoietic Cell Transplantation–Specific Comorbidity Index; GVHD, graft-versus-host disease; HR, hazard ratio; ICU, intensive care unit; MDS, myelodysplastic syndrome; OR, odds ratio; OS, overall survival.
Fig 1.
Subgroup analyses of inpatient mortality and overall survival comparing patients with Hematopoietic Cell Transplantation–Specific Comorbidity Index (HCT-CI) scores ≥ 2 versus patients with scores of 0 to 1. Forest plot demonstrates the odds ratios (OR) for inpatient mortality. HR, hazard ratio; ICU, intensive care unit; MDS, myelodysplastic syndrome.
OS
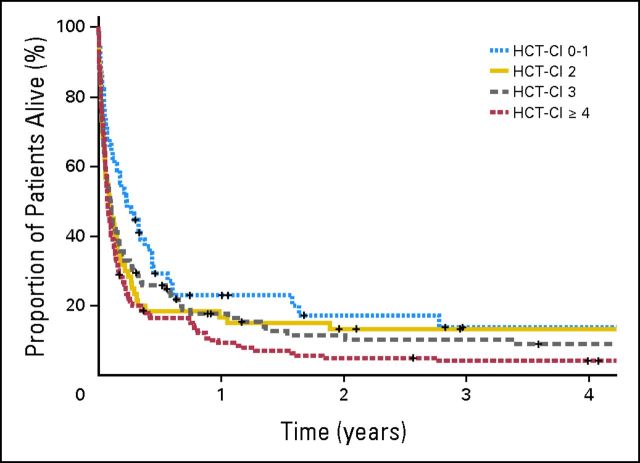
Twenty-eight patients (7%) died within 24 hours of ICU admission. Overall, 320 patients (85%) died during the first year of follow-up. The median OS was 34 days (95% CI, 27 to 41 days), and the corresponding cumulative survival rates at 30 days and 1 year were 52% and 15%, respectively. Among patients with HCT-CI scores of 0 to 1, 2, 3, and ≥ 4, the 1-year OS rates were 22%, 17%, 18%, and 9% respectively (Table 3 and Fig 2). The difference in OS between HCT-CI scores of ≥ 4 and 0 to 1 was significant in both univariate and multivariate analyses (Tables 3 and 4). No other significant prognostic factor for OS was found, although a strong trend of worse OS was observed in patients older than 55 years (Table 4). A second Cox proportional hazards model including KPS (n = 309) again demonstrated that patients with HCT-CI scores of ≥ 4 had a significantly worse survival (P = .001). HCT-CI score ≥ 2 was associated with a significant survival disadvantage in patients older than 55 years, patients who had a diagnosis other than acute leukemia or myelodysplastic syndrome, and patients with a matched unrelated HLA donor (Fig 1).
Fig 2.
Kaplan-Meier plots of overall survival in patients with Hematopoietic Cell Transplantation–Specific Comorbidity Index (HCT-CI) scores of 0 to 1, 2, 3, and ≥ 4.
DISCUSSION
This study demonstrated the utility of HCT-CI in predicting inpatient mortality and the OS of patients who received allogeneic transplantations admitted to the ICU within 100 days of ASCT. We found that HCT-CI scores ≥ 2 and ≥ 4 were associated with significantly higher inpatient mortality and reduced OS, respectively, independent of other factors. Patient subset analyses confirmed the prognostic value of HCT-CI. The conditioning regimen intensity, the admission to the ICU during the conditioning phase, and presence of acute GVHD at the time of ICU admission were other independent factors associated with inpatient mortality.
Although some comorbidities and worsening organ function at the time of ICU admission are known to predict poorer outcomes and are included in various ICU prognostic models, the effect of patients' baseline comorbidities on ICU outcomes has seldom been studied. The comorbidities are independent of performance status and may decrease the physiologic reserve of patients, rendering them vulnerable to critical illness. Accordingly, the CCI has been found to be useful in predicting mortality in general ICU patients.21 Similarly, we found that HCT-CI was predictive of inpatient mortality and OS in patients who received allogeneic transplantation admitted early to ICU, even after controlling for confounding factors. HCT-CI is a modification of the CCI to be used in the ASCT setting and was initially developed to predict survival after ASCT. Subsequent studies, with the exception of a few studies with small patient cohorts or with cohorts including transplantations performed over long periods of time,28–30 confirmed its predictive value after various different types of transplantation, including transplantations with reduced-intensity conditioning, autologous transplantation, and transplantations in pediatric population.22–24,31–35 Our results suggest that the reported association between HCT-CI and transplantation-related mortality may be partly a result of the worse outcomes after intensive care in patients with higher HCT-CI scores.
Indices of various organ functions and various ICU prognostic scores measured at the time of ICU admission have been found to predict ICU outcomes3,4,6,14; however, their availability solely at times when patients have already received ASCT and are already critically ill limits their utility in the discussions that occur with the patient and family members at the time of considering the ASCT procedure or admission to ICU. In contrast, HCT-CI can be calculated at the time of planning the ASCT, and it may help physicians and patients to make informed decisions regarding the care options available beforehand.
At the MD Anderson Cancer Center, 13% of patients who received allogeneic transplantation required admission to ICU within 100 days of the procedure, compared with 14% to 20% of patients reported from other centers.4,6 Compared with the previous findings for patients with acute myeloid leukemia in first remission who underwent ASCT at our center between 1990 and 2001,24 patients with acute myeloid leukemia in our current study had a higher prevalence of pretransplantation comorbidities (HCT-CI score ≥ 3 in 76% in current study v 58% in previous study) as a result of the development of reduced-intensity conditioning regimens designed to allow older and medically infirm patients to receive ASCT and likely higher propensity of patients with baseline comorbidities to require intensive care after transplantation. Our observations that the most common pretransplantation comorbidities and reason for ICU admission were pulmonary diseases and respiratory failure, respectively, may support the latter deduction.
Outcomes of patients who received allogeneic transplantations admitted to the ICU remain poor in our study group, with only 36% of patients surviving throughout the hospital stay and a 1-year OS of 15%. Similarly, in recent literature regarding ICU patients, survival at the time of hospital discharge was reported to range between 22% and 41% among allogeneic transplantation recipients,3,6,14 whereas 1-year OS ranged between 11% and 16%.4,6 The inpatient mortality rate at our institution remained essentially the same at 63% to 64% among allogeneic transplantation recipients admitted to ICU between 1994 and 1996 and between 2001 and 2010.8 Although the results of intensive care have improved over the last decade,9,14 the population receiving transplantations had a higher prevalence of comorbidities, explaining the persistent high overall mortality. Future studies could help clarify this question by stratifying patients according to their mortality risks with HCT-CI and similar tools.
The limitations of our study include the retrospective nature of the analysis. Scoring of comorbidities was based on review of the medical records, and some comorbidities may not have been noted. However, because comorbidity definitions in HCT-CI are based on both historical and laboratory data (the latter being easily extractable from medical records by registry data managers), comorbidity data still should be sufficiently complete. Furthermore, the prevalence of comorbidities was relatively high in our cohort. Our cohort also did not include patients who had required critical care but were not transferred to the ICU because of perceived futility by the attending physician or patient/family wishes, introducing a selection bias. Finally, after the completion of our study, it has been suggested to use Dinakara's formula36 for correction of the diffusion capacity of carbon monoxide for hemoglobin.37,38 However, to change current practices based on the American Thoracic Society recommendations, it is necessary to properly study and validate these formulas in the hematopoietic stem-cell transplantation population.
In summary, our results demonstrated the value of the HCT-CI score in predicting inpatient mortality and the OS of critically ill patients after ASCT admitted to the ICU. HCT-CI seems to be a useful tool to improve the risk assessment of patients before and after ASCT as well as to refine the survival-predicting capabilities necessary to make better ICU resource allocation decisions. We advocate consideration of HCT-CI scores when making therapeutic decisions regarding the use of ASCT in patients with comorbidities and the management of major complications that may occur. A high HCT-CI score alone should not preclude an admission to ICU. Further studies are required to identify other prognostic factors assessable before ICU admission and to model a prognostication system able to pinpoint subsets of patients who would be better served by comfort rather than critical care.
Glossary Terms
- Comorbidity:
Having two or more diseases at the same time.
- HLA (human leukocyte antigen):
The human major histocompatibility complex, which is expressed as two sets of highly polymorphic cell surface molecules, termed HLA class I and HLA class II. HLA class I molecules are expressed on all nucleated cells and are encoded by diverse alleles of the HLA-A, HLA-B, or HLA-C genes (eg, HLA-A1 [HLA molecule encoded by the A1 allele of the HLA-A gene] and HLA-B7 [HLA molecule encoded by the B7 allele of the HLA-B gene]). HLA class I molecules bind peptides derived from cellular proteins upon processing. Cytotoxic T lymphocytes, expressing the CD8 coreceptor, recognize cell-bound peptides in association with HLA class I molecules on target cells.
Footnotes
Presented in part at the 53rd Annual Meeting of the American Society of Hematology, December 10-13, 2011, San Diego, CA.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ulas D. Bayraktar, Elizabeth J. Shpall, Joseph L. Nates
Collection and assembly of data: Ulas D. Bayraktar, Ping Liu, Joseph L. Nates
Data analysis and interpretation: Ulas D. Bayraktar, Elizabeth J. Shpall, Ping Liu, Stefan O. Ciurea, Gabriela Rondon, Marcos de Lima, Marylou Cardenas-Turanzas, Kristen J. Price, Richard E. Champlin, Joseph L. Nates
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.McArdle JR. Critical care outcomes in the hematologic transplant recipient. Clin Chest Med. 2009;30:155–167. doi: 10.1016/j.ccm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive-care unit support. Mayo Clin Proc. 1992;67:117–122. doi: 10.1016/s0025-6196(12)61310-x. [DOI] [PubMed] [Google Scholar]
- 3.Soubani AO, Kseibi E, Bander JJ, et al. Outcome and prognostic factors of hematopoietic stem cell transplantation recipients admitted to a medical ICU. Chest. 2004;126:1604–1611. doi: 10.1378/chest.126.5.1604. [DOI] [PubMed] [Google Scholar]
- 4.Gilli K, Remberger M, Hjelmqvist H, et al. Sequential Organ Failure Assessment predicts the outcome of SCT recipients admitted to intensive care unit. Bone Marrow Transplant. 2010;45:682–688. doi: 10.1038/bmt.2009.220. [DOI] [PubMed] [Google Scholar]
- 5.Jackson SR, Tweeddale MG, Barnett MJ, et al. Admission of bone marrow transplant recipients to the intensive care unit: Outcome, survival and prognostic factors. Bone Marrow Transplant. 1998;21:697–704. doi: 10.1038/sj.bmt.1701158. [DOI] [PubMed] [Google Scholar]
- 6.Pène F, Aubron C, Azoulay E, et al. Outcome of critically ill allogeneic hematopoietic stem-cell transplantation recipients: A reappraisal of indications for organ failure supports. J Clin Oncol. 2006;24:643–649. doi: 10.1200/JCO.2005.03.9073. [DOI] [PubMed] [Google Scholar]
- 7.Kache S, Weiss IK, Moore TB. Changing outcomes for children requiring intensive care following hematopoietic stem cell transplantation. Pediatr Transplant. 2006;10:299–303. doi: 10.1111/j.1399-3046.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 8.Price KJ, Thall PF, Kish SK, et al. Prognostic indicators for blood and marrow transplant patients admitted to an intensive care unit. Am J Respir Crit Care Med. 1998;158:876–884. doi: 10.1164/ajrccm.158.3.9711076. [DOI] [PubMed] [Google Scholar]
- 9.Naeem N, Reed MD, Creger RJ, et al. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: Does it really matter? Bone Marrow Transplant. 2005;37:119–133. doi: 10.1038/sj.bmt.1705222. [DOI] [PubMed] [Google Scholar]
- 10.Afessa B, Azoulay E. Critical care of the hematopoietic stem cell transplant recipient. Crit Care Clin. 2010;26:133–150. doi: 10.1016/j.ccc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Kew AK, Couban S, Patrick W, et al. Outcome of hematopoietic stem cell transplant recipients admitted to the intensive care unit. Biol Blood Marrow Transplant. 2006;12:301–305. doi: 10.1016/j.bbmt.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Naeem N, Eyzaguirre A, Kern JA, et al. Outcome of adult umbilical cord blood transplant patients admitted to a medical intensive care unit. Bone Marrow Transplant. 2006;38:733–738. doi: 10.1038/sj.bmt.1705502. [DOI] [PubMed] [Google Scholar]
- 13.Trinkaus MA, Lapinsky SE, Crump M, et al. Predictors of mortality in patients undergoing autologous hematopoietic cell transplantation admitted to the intensive care unit. Bone Marrow Transplant. 2008;43:411–415. doi: 10.1038/bmt.2008.336. [DOI] [PubMed] [Google Scholar]
- 14.Afessa B, Tefferi A, Dunn WF, et al. Intensive care unit support and Acute Physiology and Chronic Health Evaluation III performance in hematopoietic stem cell transplant recipients. Crit Care Med. 2003;31:1715–1721. doi: 10.1097/01.CCM.0000065761.51367.2D. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Johnston JA, Wagner DP, Timmons S, et al. Impact of different measures of comorbid disease on predicted mortality of intensive care unit patients. Med Care. 2002;40:929–940. doi: 10.1097/00005650-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield S, Aronow HU, Elashoff RM, et al. Flaws in mortality data: The hazards of ignoring comorbid disease. JAMA. 1988;260:2253–2255. [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Poses RM, McClish DK, Smith WR, et al. Prediction of survival of critically ill patients by admission comorbidity. J Clin Epidemiol. 1996;49:743–747. doi: 10.1016/0895-4356(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farina L, Bruno B, Patriarca F, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23:1131–1138. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- 24.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: Combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Guidelines for intensive care unit admission, discharge, and triage. Crit Care Med. 1999;27:633–638. [PubMed] [Google Scholar]
- 26.Bacigalupo A. Second EBMT Workshop on reduced intensity allogeneic hemopoietic stem cell transplants (RI-HSCT) Bone Marrow Transplant. 2002;29:191–195. doi: 10.1038/sj.bmt.1703355. [DOI] [PubMed] [Google Scholar]
- 27.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 28.Castagna L, Fürst S, Marchetti N, et al. Retrospective analysis of common scoring systems and outcome in patients older than 60 years treated with reduced-intensity conditioning regimen and alloSCT. Bone Marrow Transplant. 2011;46:1000–1005. doi: 10.1038/bmt.2010.227. [DOI] [PubMed] [Google Scholar]
- 29.Patel P, Sweiss K, Nimmagadda S, et al. Comorbidity index does not predict outcome in allogeneic myeloablative transplants conditioned with fludarabine/i.v. busulfan (FluBu4) Bone Marrow Transplant. 2011;46:1326–1330. doi: 10.1038/bmt.2010.293. [DOI] [PubMed] [Google Scholar]
- 30.Guilfoyle R, Demers A, Bredeson C, et al. Performance status, but not the hematopoietic cell transplantation comorbidity index (HCT-CI), predicts mortality at a Canadian transplant center. Bone Marrow Transplant. 2009;43:133–139. doi: 10.1038/bmt.2008.300. [DOI] [PubMed] [Google Scholar]
- 31.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka K, Nannya Y, Ueda K, et al. Differential prognostic impact of pretransplant comorbidity on transplant outcomes by disease status and time from transplant: A single Japanese transplant centre study. Bone Marrow Transplant. 2010;45:513–520. doi: 10.1038/bmt.2009.194. [DOI] [PubMed] [Google Scholar]
- 33.Pollack SM, Steinberg SM, Odom J, et al. Assessment of the hematopoietic cell transplantation comorbidity index in non-Hodgkin lymphoma patients receiving reduced-intensity allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:223–230. doi: 10.1016/j.bbmt.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labonté L, Iqbal T, Zaidi MA, et al. Utility of comorbidity assessment in predicting transplantation-related toxicity following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2008;14:1039–1044. doi: 10.1016/j.bbmt.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 36.Dinakara P, Blumenthal WS, Johnston RF, et al. The effect of anemia on pulmonary diffusing capacity with derivation of a correction equation. Am Rev Respir Dis. 1970;102:965–969. doi: 10.1164/arrd.1970.102.6.965. [DOI] [PubMed] [Google Scholar]
- 37.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffey DG, Pollyea DA, Myint H, et al. Adjusting DLCO for Hb and its effects on the Hematopoietic Cell Transplantation-specific Comorbidity Index. Bone Marrow Transplant. 2013;48:1253–1256. doi: 10.1038/bmt.2013.31. [DOI] [PubMed] [Google Scholar]