SUMMARY
Poyang Lake is situated within the East Asian Flyway, a migratory corridor for waterfowl that also encompasses Guangdong Province, China, the epicenter of highly pathogenic avian influenza (HPAI) H5N1. The lake is the largest freshwater body in China and a significant congregation site for waterfowl; however, surrounding rice fields and poultry grazing have created an overlap with wild waterbirds, a situation conducive to avian influenza transmission. Reports of HPAI H5N1 in healthy wild ducks at Poyang Lake have raised concerns about the potential of resilient free-ranging birds to disseminate the virus. Yet the role wild ducks play in connecting regions of HPAI H5N1 outbreak in Asia is hindered by a lack of information about their migratory ecology. During 2007–08 we marked wild ducks at Poyang Lake with satellite transmitters to examine the location and timing of spring migration and identify any spatiotemporal relationship with HPAI H5N1 outbreaks. Species included the Eurasian wigeon (Anas penelope), northern pintail (Anas acuta), common teal (Anas crecca), falcated teal (Anas falcata), Baikal teal (Anas formosa), mallard (Anas platyrhynchos), garganey (Anas querquedula), and Chinese spotbill (Anas poecilohyncha). These wild ducks (excluding the resident mallard and Chinese spotbill ducks) followed the East Asian Flyway along the coast to breeding areas in northern China, eastern Mongolia, and eastern Russia. None migrated west toward Qinghai Lake (site of the largest wild bird epizootic), thus failing to demonstrate any migratory connection to the Central Asian Flyway. A newly developed Brownian bridge spatial analysis indicated that HPAI H5N1 outbreaks reported in the flyway were related to latitude and poultry density but not to the core migration corridor or to wetland habitats. Also, we found a temporal mismatch between timing of outbreaks and wild duck movements. These analyses depend on complete or representative reporting of outbreaks, but by documenting movements of wild waterfowl, we present ecological knowledge that better informs epidemiological investigations seeking to explain and predict the spread of avian influenza viruses.
Keywords: Anatidae, waterfowl, Poyang Lake, Brownian bridge movement model, satellite telemetry, highly pathogenic avian influenza, H5N1, East Asian Flyway
Poyang Lake, located 50 km northeast of Nanchang in Jiangxi Province, is the largest freshwater lake in China with a total area of about 3585 km2 (39). Over 520,000 birds are found at Poyang Lake during the nonbreeding period (43), many of which are long-distance migrants including over 2700 Siberian cranes (Grus leucogeranus), representing 95% of the global population (24,28). Among the 300 bird species recorded, a significant proportion are migratory Anatidae, including the whooper swan (Cygnus cygnus), greater white-fronted goose (Anser albifrons), greylag goose (Anser anser), Chinese spotbill (Anas poecilohyncha), Eurasian wigeon (Anas penelope), and mallard (Anas platyrhynchos) (28,43). Poultry grazing in surrounding croplands maximizes overlap between wild bird and domestic poultry, thereby increasing chances of transmission of avian influenza viruses (AIVs). In addition, a relatively recent farming practice at the Poyang Lake region involves rearing wild waterfowl in captivity, which is preferred by some Chinese consumers over domestic fowl. Migratory swan geese (Anser cygnoides) as well as nonmigratory Chinese spotbills are now raised in captivity for sale in markets. Many of these farm-raised waterfowl are allowed to feed or swim in the wetlands, facilitating direct interaction of farmed and free-ranging wild birds.
Areas such as Poyang Lake in which large numbers of domestic ducks and geese are raised in areas with little or no biosafety measures (9) are conducive to the emergence of novel highly pathogenic avian influenza (HPAI) subtypes. For example, phylogenetic analyses of AIVs by Mukhtar et al. (30) showed that reassortment of Nanchang and Hokkaido subtypes at Poyang Lake may have resulted in the evolution of HPAI Gs/Gd/1/96 of subtype H5N1. Subsequent introductions of new AIVs from domestic to wild birds are also probable, especially because domestic and wild ducks are intermixed and poultry outnumber wild waterfowl by more than 25 to 1 (43). Evidence for introduction of novel AIVs into wild bird populations exists in the detection of two HPAI H5N1 genotypes (‘Z’ and ‘V ’) from Poyang Lake between October 2004 and March 2005 by Chen et al. (6). While the species sampled were identified (mallard, falcated teal [Anas falcata], and Chinese spotbill), the results were pooled into a mixed “migratory duck” category that combined species with contrasting ecology and behavior, limiting ecological interpretation of these findings (12,31,45). Both Mukhtar et al. and Chen et al. have implicated wild waterfowl at Poyang Lake in HPAI H5N1 transmission, and both proposed a possible link to Qinghai Lake, where the largest recorded wild bird epizootic occurred in 2005 (6,29). However, little empirical information was available at that time on the migratory movements of wild ducks from the Poyang and Qinghai regions to ecologically establish an association with HPAI H5N1 outbreaks.
In this paper, we present results from eight wild duck species including the Eurasian wigeon (Anas penelope), northern pintail (Anas acuta), common teal (Anas crecca), falcated teal, Baikal teal (Anas formosa), mallard, garganey (Anas querquedula), and Chinese spotbill, marked with satellite transmitters at Poyang Lake, Wuhan, China during 2007–08. The infection status of birds marked at the onset of the project in March 2007 was diagnosed using both reverse transcriptase-polymerase chain reaction (RT-PCR) and serological testing. We used telemetry locations to develop movement models and utilization distributions of the East Asian Flyway migration routes. We compared these migratory pathways with regional HPAI H5N1 outbreaks reported from this region in poultry and wild birds to determine if there were spatial relationships between wild duck movements and outbreak areas. Finally, we examined relationships between the timing of outbreaks and wild duck life-cycle stages to see if they were correlated.
MATERIALS AND METHODS
Study sites
Ducks were captured at Poyang Lake (29°09′N, 116°13′E) located 50 km northeast of Nanchang in Jiangxi Province, China. Poyang Lake is fed by the Gan and Xiu rivers, and its shallow basin is connected to the Yangtze River. The lake is part of a large alluvial floodplain with seasonal rainfall (between May and September) that expands its area to about 4000 km2 (38). Severe flooding has been common since 1950s because of land reclamation, levee construction, and El Niño events (38). The surrounding land use is dominated by rice agriculture, as Poyang Lake is in the center of China’s rice production region. The lake basin also supports a rich benthic community of at least 58 invertebrate taxa, which exceeds resources available to waterfowl in the adjacent river systems (41). Satellite telemetry locations for ducks marked at Poyang Lake were used to describe the spring migration range of waterfowl in the East Asian Flyway, extending northward from Poyang Lake to northeast China and southeastern Siberia.
Capture and marking
We captured ducks between March 8 and 20, 2007, and November 27 and December 7, 2007, on the western side of Poyang Lake, China. We captured one duck in a line of monofilament leg nooses, but most ducks were captured by local cooperators with large mistnets erected across open water. Upon capture, birds were placed in individual cloth bags and promptly processed. We recorded species identity, mass, flat wing chord, diagonal or short tarsus (8), sex, and age for each bird.
Ducks were marked with 12 or 18 g solar-powered, 30 g GPS-solar-powered, or 26 g battery-implant Platform Terminal Transmitters (PTT-100 and solar-GPS PTT-100, Microwave Telemetry, Inc., Columbia, MD, USA). Solar transmitters were secured to birds with a teflon harness (Bally Ribbon Mills, Bally, PA), and the implant transmitters were surgically secured in the abdominal cavity (26) by a veterinarian with the antenna protruding dorsally. Transmitter packages averaged <3% of the bird’s body weight. Birds were released near capture locations as soon as possible after processing. Procedures for capture, handling, and marking were approved by the USGS Patuxent Wildlife Research Center Animal Care and Use Committee.
Satellite telemetry locations
PTTs were programmed to transmit for 10-hr periods every 2 days, and GPS-PTTs were scheduled to record 6–12 GPS locations each day. Transmissions were received by the Argos satellite tracking system (CLS America Inc., Largo, MD). CLS calculated PTT locations from the perceived Doppler-effect shifts in transmission frequency during a satellite overpass. Each Doppler-derived PTT location was accompanied by a location quality class index. CLS reports a 1-sigma error radius of >1000 m, 350–1000 m, 150–350 m, and ≤150 m, for location classes indices 0, 1, 2, and 3, respectively. Auxiliary location classes A, B, and Z are not assigned accuracy estimates by CLS. We used a filtering algorithm (D. Douglas, Version 7.03, http://alaska.usgs.gov/science/biology/spatial/) to identify and remove implausible auxiliary Doppler locations based on distance moved, movement rate, and turning angle. We used ArcGIS 9.2 (Environmental Systems Research Institute, Inc., Redlands, CA) and Google Earth 5.0 (Google, Mountain View, CA) to plot telemetry locations. Migratory stopover sites were defined as areas where birds moved less than 20 km ≥ 24 hr time period.
Environmental data layers
We coanalyzed telemetry locations with a variety of digital thematic maps. For habitat features we used the Moderate-resolution Imaging Spectroradiometer (MODIS)/Terra Land Cover Classification, distributed by the Land Processes Distributed Active Archive Center, U.S. Geological Survey Center for Earth Resources Observation and Science (http://LPDAAC.usgs.gov). The MODIS/Terra Land Cover Classification contains a primary thematic that delineates 17 land cover classes defined by the International Geosphere-Biosphere Programme (IGBP). We used a University of Maryland modification of the IGBP scheme (Land Cover Type 2), which we generalized into five broad land cover categories: 1) water, 2) upland or natural vegetation, including barren or sparsely vegetated areas, 3) wetland, 4) cropland or cropland mosaic, and 5) urban.
Poultry density data
Poultry density data were obtained from the United Nations Food and Agriculture Organization (FAO) via the Geonetwork (http://www.fao.org/geonetwork). Methodology and sources of the estimates are described in the FAO’s Gridded Livestock of the World (44). Briefly, for each country the most recent available livestock census data were converted into densities to produce “observed” data and then disaggregated based on statistical relations with environmental variables in similar agro-ecological zones to produce “predicted” poultry distributions. The data files were disseminated in raster format (0.0833° resolution), with pixel values containing estimated poultry densities (head/km2).
HPAI H5N1 outbreak data
Information about HPAI H5N1 outbreaks were obtained from the Emergency Prevention System for Transboundary Animal and Plant Pests and Diseases (EMPRES) Database (11) for the period December 2003–February 2009. The 7-yr time frame for outbreaks was used to maximize the number of cases included in statistical analyses, providing a more complete outbreak pattern. Variables associated with each outbreak included date, location (country, administrative region, locality, latitude and longitude), reliability of the field veterinarian’s diagnosis and the laboratory approach that was used to confirm viral subtype, and whether the outbreak occurred in wild birds or poultry. We restricted our analyses to records considered reliable according to the FAO EMPRES Program and the World Organisation for Animal Health (OIE).
Spatial analyses
We pooled species into four groups according to body mass: 1) falcated teal, 2) small teal (common teal, garganey, Baikal teal), 3) Eurasian wigeon, and 4) Chinese spotbill. We used all tracking locations that described the spring migration route of each waterfowl group (March to July 2007) for statistical analyses. We applied a fixed kernel home range analysis (19) of each bird’s migration route to identify stopover sites. The fixed kernel analysis was performed using Animal Space Use (v. 1.2 Beta) software (20) and applied a least squares cross-validation method to obtain the kernel smoothing parameter. We applied Brownian bridge movement models (21) to create utilization distributions for spring migration pathways of each species group. We used Animal Space Use (v. 1.2) to create the Brownian bridge utilization distributions (BBUDs) and assumed the distribution of location errors was circular normal.
For coanalysis with virus outbreak data, we used the 99% BBUDs for each species group to avoid location outliers. We averaged the BBUDs of all species groups to describe a population-level spring migration route (21) and extracted the upper 60% utilization distribution, partitioned into six 10% intervals (i.e., 40–99%). We included 29 FAO-OIE confirmed HPAI H5N1 outbreak events from 2003 to 2009 involving wild and domestic birds that occurred within the population-level BBUD spring migration corridor. Wild bird mortalities were not analyzed according to taxon because most outbreak cases from this region were identified as “waterbirds” (32). We spatially intersected each HPAI H5N1 outbreak location with the land cover and poultry density maps. An equal number of random locations from within the overall BBUD were also intersected for comparison with the outbreak locations.
Statistical analyses
An information theoretic approach (Akaike Information Criterion [AIC]) was used to compare outbreak and random locations under 21 a priori defined logistic regression models. Covariates for the logistic models included latitude, habitat type (water, upland, wetland, cropland, and urban), poultry density, and utilization distribution (BBUD). Models within 2.0 ΔAIC of the top-ranked model were considered to have biological significance (3). All analyses were conducted in Program-R (36) using the “glm” function (family = binomial, link = logit).
We divided the annual cycle of wild ducks into four seasonal stages in their life cycle on the basis of the area, scale of movement, and arrival and departure dates. These stages were 1) breeding and post-breeding (including annual moult): May 1–October 2; 2) fall migration: October 3–November 18; 3) nonbreeding: November 19–February 20; and 4) spring migration: February 21–April 30. We examined the null hypothesis of equal probability of an outbreak within season by comparing likelihood ratios during the four seasonal stages. To evaluate temporal variation, we weighted each PTT-marked duck equally and calculated average poultry exposure estimates throughout the annual cycle. We used a G-statistic (46) to detect if there was a significant difference between the number of observed (reported in EMPRES database) and expected (random distribution in time) outbreaks for each of the four seasonal stages.
AIV testing
Virological analyses were performed on samples collected from birds marked during March 2007, but not performed for samples collected during recapture. We obtained cloacal and tracheal swabs and blood from each bird following standard sampling and transport procedures (10). For all birds three analyses were performed. The first test, a sandwich enzyme-linked immunosorbent assay (ELISA) kit (KeQian Biology Technology Ltd, China), was used to detect the presence of type A influenza virus antigen with optical density recorded at 630 nm wavelength (OD630). An OD630 value above 0.23 was regarded as positive. The second test, an rRT-PCR assay, targeted the Matrix gene (13) and was used to screen for type A influenza virus RNA. The third test, an indirect ELISA kit (KeQian Biology Technology), was used to detect the presence of AIV H5 antibody with an OD630 value above 0.40 regarded as positive. Tests were conducted according to OIE standards (33). Laboratory analyses were conducted by the Chinese Academy of Sciences, Wuhan Institute of Virology, China.
RESULTS
Capture and marking
We obtained a total of 13,699 locations from 33 ducks marked with PTTs at Poyang Lake in 2007 including the Chinese spotbill (13 adults: 6 males, 7 females), falcated teal (5 adults: 4 males, 1 female; 1 first-year female), Eurasian wigeon (5 adults: 1 male, 4 females), common teal (3 adult males; 1 first-year male), Baikal teal (2 adult males), garganey (1 adult male), northern pintail (1 first-year female), and mallard (1 adult male; Table 1). The total number of locations obtained per duck varied widely (2–909) as did the duration over which signals were received (14–617 days). We obtained few locations for the northern pintail, so it was excluded from analyses. Similarly, the mallard was excluded from analyses because the individual we captured was a resident. The Chinese spotbills were nonmigratory and year-round residents at Poyang Lake. We recovered spring migration tracks for 12 of the other 18 migratory ducks.
Table 1.
Performance of satellite transmitter for ducks marked in 2007–08 at Poyang Lake, China. Species include Baikal teal (BATE), common teal (CMTE), Eurasian wigeon (EUWI), falcated teal (FATE), garganey (GARG), mallard (MALL), northern pintail (NOPI), and Chinese spotbill duck (SBDU). Estimated accuracy of location codes (from best to least, left to right) are described in the Methods.
| PTT | Species | Age | Sex | Last message | Working days | Total locations | Location class
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 2 | 1 | 0 | A | B | Z | |||||||
| 73290 | BATE | A | M | Apr. 17, 2007 | 29 | 36 | 2 | 1 | 6 | 1 | 11 | 13 | 2 |
| 74827 | BATE | A | M | Mar. 31, 2007 | 14 | 9 | 0 | 0 | 1 | 1 | 2 | 5 | 0 |
| 74826 | CMTE | A | M | Oct. 1, 2007 | 198 | 163 | 3 | 12 | 17 | 13 | 43 | 69 | 6 |
| 74828 | CMTE | A | M | Nov. 24, 2008 | 617 | 614 | 8 | 27 | 44 | 118 | 135 | 266 | 16 |
| 74829 | CMTE | A | M | Jan. 3, 2008 | 59 | 291 | 1 | 1 | 1 | 2 | 4 | 13 | 2 |
| 73020 | CMTE | J | M | Nov. 3, 2007 | 231 | 488 | 22 | 74 | 111 | 118 | 70 | 78 | 15 |
| 39571 | EUWI | A | F | Jun. 16, 2008 | 200 | 6 | 0 | 0 | 0 | 0 | 2 | 4 | 0 |
| 40217 | EUWI | A | F | Nov. 3, 2008 | 340 | 221 | 74 | 50 | 19 | 17 | 34 | 27 | 0 |
| 40754 | EUWI | A | F | Oct. 17, 2008 | 323 | 156 | 13 | 26 | 25 | 22 | 28 | 40 | 2 |
| 73007 | EUWI | A | F | Dec. 25, 2007 | 283 | 909 | 56 | 162 | 285 | 218 | 68 | 104 | 16 |
| 73031 | EUWI | A | M | Jul. 1, 2007 | 104 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 73025 | FATE | A | M | Jun. 10, 2008 | 451 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 73034 | FATE | A | M | Sep. 12, 2007 | 179 | 282 | 10 | 23 | 47 | 38 | 73 | 84 | 7 |
| 73288 | FATE | A | M | Nov. 6, 2007 | 234 | 222 | 5 | 10 | 19 | 31 | 72 | 80 | 5 |
| 73289 | FATE | A | M | Oct. 9, 2008 | 570 | 795 | 15 | 24 | 64 | 146 | 175 | 335 | 36 |
| 74830 | FATE | A | F | Jul. 31, 2007 | 134 | 17 | 0 | 1 | 2 | 3 | 6 | 5 | 0 |
| 73017 | FATE | J | F | Jul. 29, 2008 | 500 | 239 | 9 | 15 | 39 | 38 | 59 | 75 | 4 |
| 74831 | GARG | A | M | Mar. 29, 2008 | 376 | 341 | 23 | 37 | 45 | 41 | 75 | 111 | 9 |
| 73294 | MALL | A | M | May 4, 2008 | 414 | 1002 | 5 | 12 | 20 | 17 | 42 | 106 | 14 |
| 73032 | NOPI | J | F | Jan. 24, 2008 | 54 | 35 | 0 | 1 | 6 | 2 | 9 | 16 | 1 |
| 45972 | SBDU | A | F | Jan. 14, 2009 | 412 | 856 | 20 | 33 | 31 | 32 | 80 | 69 | 15 |
| 46082 | SBDU | A | F | Dec. 9, 2007 | 10 | 19 | 0 | 0 | 1 | 1 | 1 | 4 | 0 |
| 46083 | SBDU | A | M | Active | 424 | 2252 | 50 | 76 | 95 | 71 | 109 | 143 | 15 |
| 46126 | SBDU | A | F | Dec. 23, 2007 | 22 | 58 | 0 | 1 | 0 | 7 | 3 | 4 | 0 |
| 46127 | SBDU | A | F | Dec. 19, 2007 | 18 | 12 | 0 | 0 | 0 | 2 | 2 | 4 | 0 |
| 46128 | SBDU | A | M | Nov. 9, 2008 | 342 | 1668 | 52 | 63 | 46 | 42 | 80 | 113 | 7 |
| 46129 | SBDU | A | M | Aug. 7, 2008 | 250 | 936 | 17 | 32 | 33 | 32 | 63 | 88 | 13 |
| 46130 | SBDU | A | M | Aug. 23, 2008 | 266 | 916 | 8 | 21 | 31 | 17 | 78 | 113 | 10 |
| 73022 | SBDU | A | F | May 13, 2007 | 62 | 66 | 10 | 7 | 7 | 5 | 12 | 21 | 4 |
| 73043 | SBDU | A | M | Jan. 14, 2008 | 301 | 708 | 15 | 15 | 20 | 12 | 68 | 84 | 14 |
| 73044 | SBDU | A | F | Apr. 9, 2007 | 21 | 76 | 0 | 0 | 2 | 5 | 5 | 7 | 1 |
| 73296 | SBDU | A | M | Jan. 2, 2009 | 655 | 833 | 23 | 28 | 60 | 49 | 67 | 109 | 14 |
| 74815 | SBDU | A | F | Active | 422 | 471 | 2 | 6 | 12 | 38 | 72 | 81 | 13 |
Migration from Poyang Lake
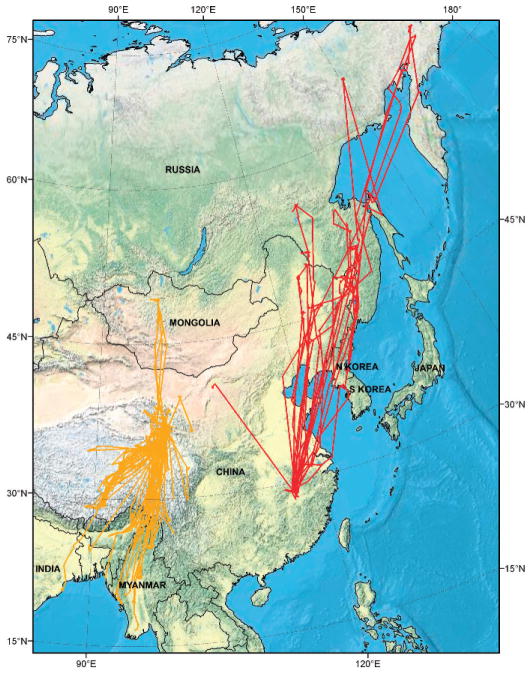
None of the birds migrated from the East Asian nonbreeding area at Poyang Lake to the Qinghai Lake breeding area in the Central Asian Flyway (Fig. 1). A single falcated teal migrated northwest toward the Central Asian Flyway, but it stopped 700 km northeast of Qinghai Lake on the eastern edge of the Gobi Desert, a region that presents a large ecological barrier to waterfowl migration.
Fig. 1.
General movement paths for more than 111 waterbirds marked with satellite transmitters in the East Asian Flyways depicted in red (this study) and Central Asian Flyways depicted in yellow (author’s unpublished data), 2007–08.
Falcated teal
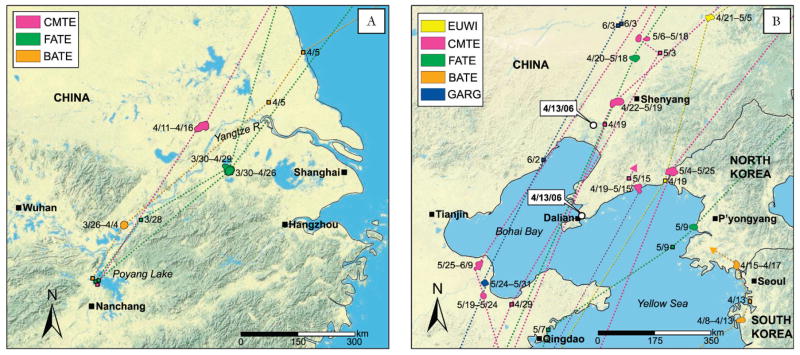
We obtained 1557 total locations for migrating falcated teal (Table 1). The falcated teal departed from Poyang Lake from March 28 to April 29. From late March through late April, they used migration sites in the Lower Yangtze River in Anhui and Zhejiang Provinces to the southwest of Shanghai (Table 2). In late April they migrated to sites on Bohai Bay (Fig. 2B) including a stopover site on the south side near Qingdao in Shandong Province. One falcated teal migrated to the northwest, but all other birds migrated along the coast to northern China and southeast Siberia. The core of their spring migration corridor was in the northeastern region of Manchuria in China (Fig. 3A).
Table 2.
Spring migration areas in 2007 for wild ducks migrating from Poyang Lake, China. Species include Baikal teal (BATE), common teal (CMTE), falcated teal (FATE), garganey (GARG), and Eurasian wigeon (EUWI).
| Date range | Country | Site name | Coordinates (latitude, longitude) | Distance from Poyang Lake (km) | Length of stay (range in days) | Number | Total locations | Species |
|---|---|---|---|---|---|---|---|---|
| Mar. 30–May 18 | China | Lower Yangtze River | 31.11°N, 117.81°E | 330 | 11 (1–37) | 7 | 61 | 1 BATE, 4 CMTE, 2 FATE, 1 GARG |
| Apr. 29–Jun. 9 | China | Lower Huang River | 37.80°N, 118.82°E | 1210 | 10 (1–21) | 3 | 31 | 2 CMTE, 1 GARG |
| Apr. 15–Jun. 2 | China, N. Korea | North Yellow Sea Coastal Wetlands | 39.85°N, 124.26°E | 1600 | 7 (1–27) | 7 | 56 | 1 BATE, 2 FATE |
| Apr. 20–Jun. 3 | China | Upper Liao River Basin | 42.72°N, 123.26°E | 2060 | 12 (1–28) | 5 | 51 | 2 CMTE, 1 EUWI, 1 FATE, 1 GARG |
| May 19–Jun. 11 | China | Songhua River Wetlands | 45.97°N, 112.12°E | 2680 | 7 (1–20) | 3 | 30 | 2 CMTE, 1 FATE |
| Apr. 27–May 12 | Russia | Lower Amur River Basin | 52.94°N, 141.17°E | 4540 | 4 (1–10) | 3 | 7 | 3 EUWI |
| May 14–May 29 | Russia | Shelikhov Gulf, Sea of Okhotsk | 60.26°N, 158.73°E | 7000 | 12 (10–14) | 2 | 41 | 2 EUWI |
Fig. 2.
Stopover and staging sites for ducks during the 2007 spring migration including (A) the Yangtzee River and (B) Bohai Bay. Stopover sites are represented by 95% fixed kernel home ranges and dates indicate length of stopover periods. Two HPAI H5N1 outbreaks in wild birds from 2003 to 2009 are indicated (○) with the date. Species include Eurasian wigeon (EUWI), common teal (CMTE), falcated teal (FATE), Baikal teal (BATE), and garganey (GARG).
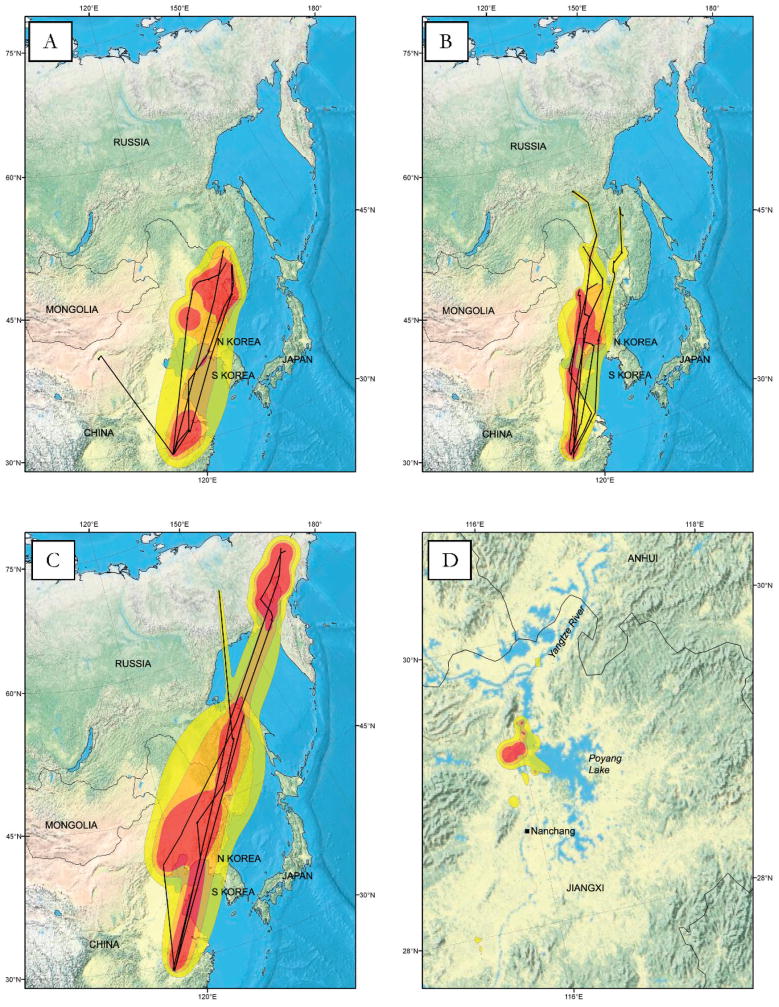
Fig. 3.
Brownian bridge utilization distribution for the spring migration of ducks captured and marked at Poyang Lake, China. Groupings include (A) falcated teal, (B) teal (common teal, garganey, Baikal teal), (C) Eurasian wigeon, and (D) Chinese spotbill. Shading from darker to lighter indicates isopleths incorporating 70–99% of total locations.
Common teal
We obtained 1942 total locations for the species group of small teal that included common teal (4), Baikal teal (2), and garganey (1; Table 1). These teal departed from Poyang Lake from March 26 to May 3. Like the larger falcated teal, they used migration sites in the Lower Yangtze River in Anhui and Zhejiang Provinces to the west of Shanghai (Table 2; Fig. 3B). In late April they migrated to coastal wetland staging areas to the south of Bohai Bay on the Lower Huang River, and to the north of Bohai Bay off the coasts of South Korea, North Korea, and China (Fig. 2B). The small teal migrated farther north than the falcated teal into eastern Siberia south of the Sea of Okhotsk. Their BBUD was similar to that of falcated teal with a more southerly concentration.
Eurasian wigeon
We obtained 1294 total locations for Eurasian wigeon (Table 1). Eurasian wigeon seemed to depart Poyang Lake later than the teal, but their first recorded postdeparture locations were not until late April in northeast China (Figs. 2A, 3C). We obtained fewer locations for the wigeon, so it is difficult to determine if the seemingly late timing of their migration was due to inferior PTT performance (three of the five birds had implant battery-powered transmitters). Eurasian wigeon migrated farther northeast than other ducks, crossing the Sea of Okhotsk and concentrating in the Magadan Oblast of northeast Siberia (Fig. 3C).
Chinese spotbill
In contrast to the teal and Eurasian wigeon, Chinese spotbills are a nonmigratory species (4) that resides year-round in the Poyang Lake region. We obtained 8871 total locations (Table 1). As expected, the Chinese spotbill ducks remained within Jiangxi Province throughout the spring (Fig. 3D).
Spatial relationship of migration pathways and outbreaks
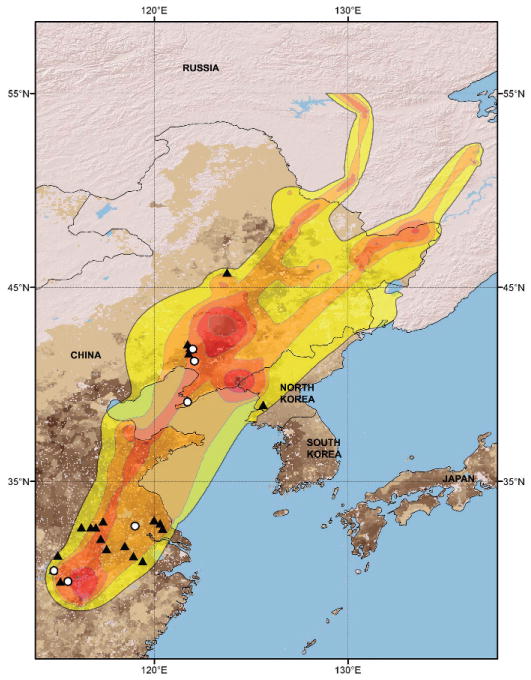
We examined waterfowl movements in relation to land cover, poultry density, and virus outbreaks within a rectangular study area bounded to the northeast at 55°N, 140°E and the southwest at 25°N, 110°E. We averaged the three BBUD isopleths for falcated teal, common and other small teal, and Eurasian wigeon to describe the East Asian Flyway pathway for wild ducks from Poyang Lake (Fig. 4). Although the migratory corridor extended east to west across most of the Bohai Bay, there was a stopover region used by most ducks in China just north of Bohai Bay. We overlaid the overall BBUD migration pathway on a grid of poultry density and plotted the documented outbreak locations. The AIC analysis indicated that two models fit the data (Table 3): these included the top-ranked model with covariates latitude+poultry density, and the second-ranked model with latitude+poultry density+BBUD. However, a comparative examination of each covariate’s contribution to the model (Table 4) indicated that BBUD magnitude was only weakly and inversely related to the outbreak location (i.e., fewer wild bird locations where there were more outbreaks). Thus, we concluded that the top AIC ranked model (latitude+poultry density) was the single most parsimonious model in explaining variation in spatial outbreak locations.
Fig. 4.
Brownian Bridge utilization distribution (BBUD) for ducks from Poyang Lake, China, 2007–08. Shading, from darker to lighter, indicates isopleths incorporating 70–99% of total locations. Darker brown background shading indicates greater density of poultry. Outbreaks of HPAI H5N1 restricted to within the BBUD and occurring 2003–09 are indicated in poultry (▲) and wild birds (○).
Table 3.
Best-fitting models under Akaike Information Criterion (AIC) model selection. Outbreak locations from 2003 to 2009 (n = 26) are compared with random points (n = 26). Variables include outbreak latitude (Lat), poultry density (Dens), utilization distribution for satellite-marked wild ducks (BBUD), urban areas (Urban), cropland (Crop), water, and wetlands. Number of parameters (k), AIC score, AIC difference (ΔAIC), likelihood, and AIC weights are reported. Models with biologically significant AIC scores are indicated (*).
| Model | Description | k | AIC | ΔAIC | Likelihood | AIC weight |
|---|---|---|---|---|---|---|
| 1 | Lat+Dens* | 3 | 66.48 | 0.00 | 1.00 | 0.36 |
| 2 | Lat+Dens+BBUD* | 4 | 67.85 | 1.38 | 0.50 | 0.18 |
| 3 | Lat+Urban+Crop+BBUD | 5 | 69.04 | 2.57 | 0.28 | 0.10 |
| 4 | Lat+Crop+Dens+BBUD | 5 | 69.39 | 2.91 | 0.23 | 0.08 |
| 5 | Lat+Water+Wetland+Dens | 5 | 69.97 | 3.49 | 0.17 | 0.06 |
| 6 | Lat+Urban+Crop+Dens+BBUD | 6 | 70.05 | 3.57 | 0.17 | 0.06 |
| 7 | Dens | 2 | 71.62 | 5.14 | 0.08 | 0.03 |
| 8 | Lat+Water+Urban+Crop+Dens+BBUD | 7 | 71.64 | 5.16 | 0.08 | 0.03 |
| 9 | Lat | 2 | 71.71 | 5.23 | 0.07 | 0.03 |
| 10 | Lat+Water+Wetland+Crop +Dens | 6 | 71.85 | 5.37 | 0.07 | 0.03 |
| 11 | Lat+UD | 3 | 72.59 | 6.11 | 0.05 | 0.02 |
| 12 | Lat+Water+Wetland+Urban+Crop+Dens+BBUD | 8 | 73.51 | 7.03 | 0.03 | 0.01 |
| 13 | Lat+Urban+Crop+UD | 5 | 74.14 | 7.66 | 0.02 | 0.01 |
| 14 | Full model: Lat+Water+Upland+Wetland+Urban+Crop+Dens+BBUD | 9 | 74.20 | 7.72 | 0.02 | 0.01 |
| 15 | Lat+Water+Wetland+Crop | 5 | 76.70 | 10.21 | 0.01 | 0.00 |
| 16 | Urban+Crop+Dens+BBUD | 5 | 77.58 | 11.10 | 0.00 | 0.00 |
| 17 | Null model | 0 | 82.41 | 15.93 | 0.00 | 0.00 |
Table 4.
Top competing AIC models within 2.0 ΔAIC encompassing the variables: intercept, outbreak latitude, poultry density, and wild bird utilization distribution. Statistics include the β coefficients, SE, z-value, significance value, and a χ2 test comparison to the null model.
| Model | Variables | β | SE | z | Pr (>|z|) | Pr (>|χ2|) |
|---|---|---|---|---|---|---|
| 1 | Intercept | 4.3660 | 2.2050 | 1.98 | 0.05 | – |
| Outbreak latitude | −0.1440 | 0.0572 | −2.52 | 0.01 | <0.001 | |
| Poultry density | 0.0001 | 0.0001 | 2.23 | 0.03 | 0.007 | |
| 2 | Intercept | 0.4621 | 5.4200 | 0.09 | 0.93 | – |
| Outbreak latitude | −0.1572 | 0.0601 | −2.62 | 0.01 | <0.001 | |
| Poultry density | 0.0001 | 0.0001 | 2.18 | 0.03 | 0.007 | |
| Utilization distribution | 4.8080 | 6.1880 | 0.78 | 0.44 | 0.429 |
Outbreaks were positively correlated with poultry density and inversely correlated with latitude, indicating that greater frequencies of outbreaks occurred at lower latitudes and with higher poultry densities. When we examined outbreaks in relation to both latitude and date, most confirmed outbreaks occurred at latitudes less than 40°N, and outbreaks began by October before most ducks had reached nonbreeding areas in early November. The spring migration utilization distribution (Fig. 4) had a weak positive relationship with outbreaks, and the χ2 test indicated that utilization distribution was nonsignificant when compared with the null model (Table 4). None of the land cover class covariates were helpful in explaining the occurrence of outbreak events.
When we examined the outbreaks that occurred within the overall BBUD on a seasonal basis (Fig. 5), we found that the number of observed and expected outbreaks differed by life-cycle period (G = 19.98, χ20.05,3 = 7.82, P < 0.001). Outbreaks occurred before many wild ducks had migrated to their nonbreeding areas (Fig. 5). More outbreaks occurred than were expected during the fall (61-day period) and the nonbreeding period (121-day period), whereas fewer occurred during the breeding season (122-day period) and spring migration (61-day period; Fig. 6).
Fig. 5.
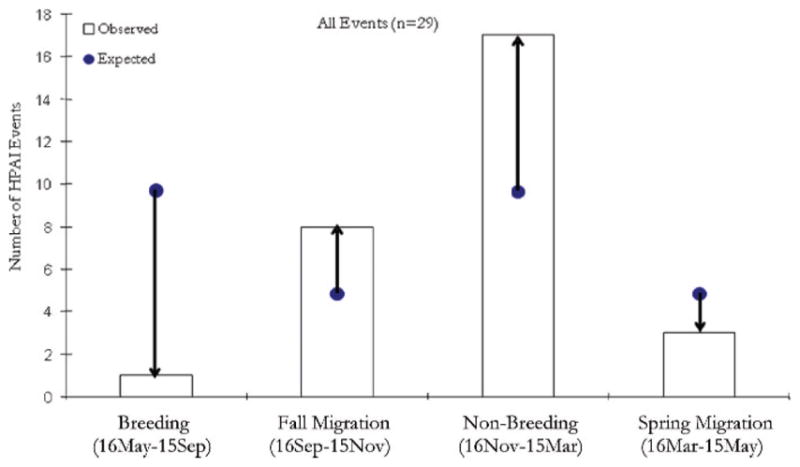
Comparison of observed (□) and expected (●) HPAI H5N1 outbreak events in the East Asian Flyway by seasonal period in the life cycle of wild ducks. Expected numbers are calculated under the assumption that outbreaks should be proportional to the number of days of a particular life stage. Arrows indicate the direction of the expected from the observed values. Observed and expected values differed (Gstat = 19.98, χ20.05,3 = 7.82, P < 0.001).
Fig. 6.
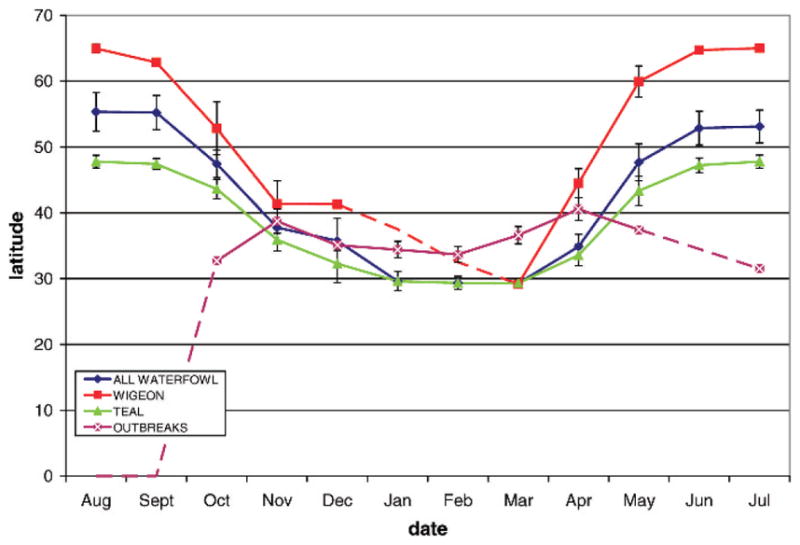
Relationship between location of ducks and HPAI H5N1 outbreaks according to latitude and seasonal period in the life cycle of wild ducks. Lines indicate the location of all waterfowl, Eurasian wigeon, teal, and outbreaks, where dashed lines indicate missing data. Outbreaks are limited to lower latitudes <40° and seem to be detected beginning in September before most wild ducks have migrated south of 40°.
AIV results
In total, 20 birds representing seven species were sampled for AIV, including Eurasian wigeon (n = 2), falcated teal (n = 6), common teal (n = 4), Baikal teal (n = 2), mallard (n = 1), garganey (n = 1), and Chinese spotbill (n = 4). The results of the sandwich ELISA and RT-PCR revealed that all 20 ducks were negative for type A influenza virus (Table 5). However, the serology results of the indirect ELISA test for AIV H5 antibody indicated that the only mallard was positive (Table 5). These results indicate that no bird was actively infected by HPAI H5N1; however, the presence of antibody in the blood of the mallard suggested prior exposure to H5N1. All birds that we handled appeared clinically healthy and showed no obvious symptoms of influenza infection.
Table 5.
Summary of virological analyses for the 20 birds marked during March 2007.
| PTT | Species | RT-PCR
|
Sandwich ELISA AIV-Ag (OD630)A
|
Indirect ELISA AIV-H5-Ab (OD630)B
|
||
|---|---|---|---|---|---|---|
| Tracheal swab | Cloacal swab | Tracheal swab | Cloacal swab | Serum | ||
| 73290 | BATE | Negative | Negative | 0.15 | 0.23 | 0.30 |
| 74827 | BATE | Negative | Negative | 0.18 | 0.21 | 0.26 |
| 73020 | COTE | Negative | Negative | 0.23 | 0.29 | 0.17 |
| 74826 | COTE | Negative | Negative | 0.16 | 0.29 | 0.32 |
| 74828 | COTE | Negative | Negative | 0.25 | 0.26 | 0.33 |
| 74829 | COTE | Negative | Negative | 0.19 | 0.26 | 0.21 |
| 73007 | EUWI | Negative | Negative | 0.22 | 0.18 | 0.14 |
| 73031 | EUWI | Negative | Negative | 0.22 | 0.29 | 0.38 |
| 73017 | FATE | Negative | Negative | 0.25 | 0.31 | 0.31 |
| 73025 | FATE | Negative | Negative | 0.23 | 0.28 | 0.19 |
| 73034 | FATE | Negative | Negative | 0.28 | 0.22 | 0.27 |
| 73288 | FATE | Negative | Negative | 0.23 | 0.23 | 0.14 |
| 73289 | FATE | Negative | Negative | 0.18 | 0.25 | 0.20 |
| 74830 | FATE | Negative | Negative | 0.12 | 0.13 | 0.20 |
| 74831 | GARG | Negative | Negative | 0.15 | 0.21 | 0.22 |
| 73294 | MALL | Negative | Negative | 0.27 | 0.22 | 0.50C |
| 73022 | SBDU | Negative | Negative | 0.24 | 0.25 | 0.24 |
| 74043 | SBDU | Negative | Negative | 0.22 | 0.28 | 0.23 |
| 74044 | SBDU | Negative | Negative | 0.27 | 0.29 | 0.22 |
| 73296 | SBDU | Negative | Negative | 0.20 | 0.21 | 0.19 |
An optical density (at 630 nm) ≥0.30 was considered positive
An optical density (at 630 nm) ≥0.40 was considered positive.
Denotes positive sample.
DISCUSSION
Our ecological studies have been directed at improving understanding of the potential role of wild birds in the transmission of avian influenza. Prior to our satellite telemetry studies, little data were available on migratory movements of wild birds during the nonbreeding season at Poyang Lake, a region that has been suggested as an important source of HPAI H5N1 (6,30). Because sample size in satellite telemetry projects is limited by the cost of the technology along with battery life and performance, it is often difficult to generalize the sequential movements of individuals to broader migratory patterns of populations. However, application of the recently developed Brownian bridge movement model (21) allowed us to describe the flyway migration corridor in a probabilistic manner. As waterfowl demonstrate a high degree of seasonal fidelity to sites within and between years (1), we believe our BBUD analysis of spring 2007 migration tracks provided a reliable representation of the general migration route for the East Asian Flyway.
We predicted that spatial and temporal concordance of HPAI H5N1 outbreaks with wild duck movements should occur if wild ducks were directly involved in spread of the virus. Lack of correlation between the core flyway corridors of wild birds as indicated by the BBUD analysis and sites of reported outbreaks did not support this hypothesis (Table 3). However, this type of spatial analysis depends on complete or representative reporting of outbreaks. If outbreaks are not fully reported, it could result in erroneous conclusions (22,23). Thus, our analysis highlights the importance of transparency in outbreak reporting to better understand the spread of disease. In addition, our analyses would have been improved with larger samples of the different species. By grouping species with different ecological characteristics, we may have missed individual differences in their movement patterns that may have been spatially correlated more closely to outbreaks.
We found no relationship among HPAI H5N1 outbreaks and land cover characteristics typically associated with waterfowl, such as water or wetland habitats (Table 3). However, a biologically significant relationship between poultry density and sites of HPAI H5N1 outbreaks was detected (Table 4), consistent with modeling studies of the virus in Southeast Asia (14). The relationship of outbreaks with latitude (Fig. 6) could stem from the higher density of poultry farms in southern, compared to northern China. In addition, the relationship of outbreaks to season (Fig. 6) may be due to the higher poultry stocking rates prior to Chinese New Year in January or February, a phenomenon that also governs the incidence of Newcastle disease (16).
Our examination of HPAI H5N1 outbreaks in relation to stages in the annual life cycle of migratory birds suggests a temporal mismatch (Fig. 5). Similar to earlier studies of whooper swans in the East Asian Flyway (26), we found fewer outbreaks than expected during the breeding season, when AIV prevalence typically reaches a maximum in wild bird populations (37). Instead, the peak in observed outbreaks coincided with the nonbreeding season of migratory birds. During the breeding season, birds migrate to latitudes higher than 40°N (Fig. 6), precluding contact with poultry farms. However, the possibility exists that persistence of AIV H5 subtype particles in the environment (2) may be responsible for the lag between the breeding season and the peak in infections two months later. Our data also indicate that migration between nonbreeding and breeding areas is prolonged, with many intervening stopover sites. If AIV particles survive in the environment with increased residence time in colder climates (2), wild birds may act as agents of transmission, “seeding” areas along their migration routes such as rice fields. Larger waterfowl such as geese may defecate as often as every 3.7 minutes (47) and therefore if infected have the potential to contaminate the environment with high loads of virus. Some duck species, such as the mallard, may shed virus for several days before the onset of clinical signs (25), a period of time in which they could potentially travel several hundred kilometers assuming that the infection did not affect their movements (40). Opportunities for poultry to acquire AIV shed by wild birds at Poyang Lake are created by the practice of herding domestic ducks onto the harvested rice fields to consume waste grains, as well as foraging in wetlands frequented by migratory birds.
Our study identified that most ducks from Poyang Lake migrated across Bohai Bay, a body of water bordering Hebei Province in Northeast China. This area may represent a migratory “thoroughfare” of regional importance for several species of waterbirds such as whooper swans (32), bar-tailed godwits (Limosa limosa) (15,35), and swan geese (author’s unpublished data). Studies have identified sites that attract a diversity of wild bird species as important for interspecific transmission of low pathogenic avian influenza virus (7,17,18). Similarly, the convergence of numerous waterbird species from different flyways at Bohai Bay may facilitate exchange of AIVs of different geographic origin. Regional migration stopover sites where wild bird species congregate and intermix are commonplace along other flyways, such as Qinghai Lake in the Central Asian Flyway. These high-density regional stopover areas may serve as catalysts for spread of disease in wild birds (27), just as live poultry or “wet” markets facilitate transmission by bringing together multiple species of domestic fowl drawn from populations of different geographic regions (5,42).
Our tracking results did not show any evidence of a migratory pathway for waterfowl between Poyang Lake in the East Asian Flyway and Qinghai Lake in the Central Asian Flyway (Fig. 1), even when we included all 111 waterbirds marked with satellite transmitters in 2007–08 (author’s unpublished data). In addition to the tracking data for eight species of migratory ducks from Poyang Lake, this included two species (bar-headed geese, ruddy shelduck) involved in the large 2005 outbreak at Qinghai Lake (6,29). Although most waterbirds migrate in a north-south direction, migration of some species may be east-west such as pochards (Aythya ferina) (1) and whooper swans (32). It is possible that a larger sample of more species would result in detection of a migratory pathway connecting the East and Central Asian Flyways, but our tracking results to date indicate that, at best, it is not a commonly used route by waterfowl. Alternative possibilities for the movement of HPAI H5N1 from Poyang Lake to Qinghai Lake include that other species may follow that migration route, some species overlap at staging areas such as Dong Ting Lake to the west or Bohai Bay to the north and serve as relay agents, or migration routes from other regions might be involved. For example, our telemetry work in the Central Asian Flyway documented a previously unknown migration route connecting Qinghai Lake to a 2005 outbreak site in Mongolia (34). Another alternative explanation is the trade of poultry or poultry products between these two wetlands. However, because poultry production is largely absent in this migration corridor and we found no connection between the flyways, the movement of HPAI H5N1 from the Poyang Lake and Qinghai Lake regions remains a mystery.
Acknowledgments
This work was supported by the U.S. Geological Survey (Western Ecological Research Center, Patuxent Wildlife Research Center, Alaska Science Center, and Avian Influenza Program), the United Nations Food and Agriculture Organization AGAH-EMPRES Wildlife Unit, National Institutes of Health Fogarty International Center (7R01TW007869-04), the Government of Sweden financial support through donation OSRO/GLO/GO1/SWE, and the Chinese Academy of Sciences (No. 2007FY6210700, INFO-115-D02, KSCX2-YW-N-063, and 2005CB523007). We thank S. Haseltine, R. Kearney, P. Bright, S. Schwarzbach, and J. Howell of USGS and E. Moncada, Q. Gao, V. Martin, F. Guo, J. Lubroth, and J. Domenech of the FAO for support of this project. We thank the staff at Poyang Lake National Nature Reserve, including Liu Guanhua, Manager Zhao, Manager Wu, and Biologist Wu. Ding Changqing and Yin Zuohua (CAS IOZ) provided excellent field assistance, and G. Olsen and B. Siraroonrat assisted with implant surgeries. We are grateful to the Qinghai Lake National Nature Reserve staff, Qinghai Forestry Bureau (S. Li), Chinese Academy of Sciences (X. Hu, L. Hu, N. Kong, Z. Luo), and the U.S. Embassy (W. Chang, D. Jassem) for logistical and field support. We are also grateful to W. Perry for GIS webpage support, C. Hamilton and J. Pinto of FAO for outbreak data from the EMPRES database. and J. Horne for assisting with technical questions on Program Animal Space Use. N. Hill and S. De La Cruz provided helpful to strengthen earlier versions of this manuscript. The use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Abbreviations
- AIC
Akaike Information Criterion
- AIV
avian influenza virus
- BBUD
Brownian bridge utilization distribution
- ELISA
enzyme-linked immunosorbent assay
- EMPRES
Emergency Prevention System for Transboundary Animal and Plant Pests and Diseases
- FAO
Food and Agriculture Organization
- GPS
global positioning system
- HPAI
highly pathogenic avian influenza
- IGBP
International Geosphere-Biosphere Programme
- MODIS
Moderate-resolution imaging spectroradiometer
- OD
optical density
- OIE
Office International des Epizooties
- PTT
platform terminal transmitter
- RT-PCR
reverse transcriptase-polymerase chain reaction
References
- 1.Boere G, Stroud D. The flyway concept: what it is and what it isn’t. In: Boere G, Galbraith CA, Stroud D, Bridge LK, editors. Waterbirds around the world: a global overview of the conservation, management and research of the world’s waterbird flyways. Stationery Office; Edinburgh, Scotland: 2006. p. 940. [Google Scholar]
- 2.Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 2007;51:285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- 3.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information theoretic approach. Springer-Verlag; New York: 1998. [Google Scholar]
- 4.Carboneras C. Spot-billed duck. In: del Hoyo J, Elliott A, Sargatal J, editors. Handbook of the birds of the world, vol. 1, Ostrich to ducks. Lynx Edicions; Barcelona, Spain: 1992. [Google Scholar]
- 5.Cardona C, Yee K, Carpenter T. Are live bird markets reservoirs of avian influenza? Poult Sci. 2009;88:856–859. doi: 10.3382/ps.2008-00338. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Li Y, Li Z, Shi J, Shinya K, Deng G, Qi Q, Tian G, Fan S, Zhao H, Sun Y, Kawaoka Y. Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J Virol. 2006;80:5976–5983. doi: 10.1128/JVI.00110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R, Holmes EC. Frequent inter-species transmission and geographic subdivision in avian influenza viruses from wild birds. Virology. 2009;383:156–161. doi: 10.1016/j.virol.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzubin A, Cooch EG. Measurement of geese: general field methods. California Waterfowl Association; Sacramento: 1992. [Google Scholar]
- 9.Food and Agricultural Organization (FAO) Farming systems and poverty: improving farmers’ livelihoods in a changing world. Food and Agricultural Organization of the United Nations and World Bank; Rome, Italy: 2001. [Google Scholar]
- 10.Food and Agricultural Organization (FAO) Wild birds and avian influenza: an introduction to applied field research and disease sampling techniques. Food and Agricultural Organization of the United Nations and World Bank; Rome, Italy: 2007. [Google Scholar]
- 11.Food and Agricultural Organization (FAO) EMPRIS-i global animal health information system of FAO’s Emergency Prevention Programme for Transboundary Animal Diseases (EMPRES) EMPRES Animal Health Programme, Animal Health Service, Animal Production and Health Division; Rome, Italy: 2009. Available from: http://empres-i.fao.org/empres-i/home. [Google Scholar]
- 12.Feare CJ. The role of wild birds in the spread of HPAI H5N1. Avian Dis. 2007;51:440–447. doi: 10.1637/7575-040106R1.1. [DOI] [PubMed] [Google Scholar]
- 13.Fouchier RA, Bestebroer TM, Herfst S, Van Der Kemp L, Rimmelzwaan GF, Osterhaus AD. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–4101. doi: 10.1128/jcm.38.11.4096-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert M, Xiao X, Pfeiffer DU, Epprecht M, Boles S, Czarnecki C, Chaitaweesub P, Kalpravidh W, Minh PQ, Otte MJ, Martin V, Slingenbergh J. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc Natl Acad Sci U S A. 2008;105:4769–4774. doi: 10.1073/pnas.0710581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill RE, Jr, Tibbitts TL, Douglas DC, Handel CM, Mulcahy DM, Gottschalck JC, Warnock N, McCaffery BJ, Battley PF, Piersma T. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc R Soc B. 2009;279:447–457. doi: 10.1098/rspb.2008.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins DA, Shortridge KF. Newcastle disease in tropical and developing countries. In: Alexander DJ, editor. Newcastle disease. Springer; New York: 1988. pp. 273–302. [Google Scholar]
- 17.Hinshaw VS, Webster RG, Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can J Microbiol. 1980;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- 18.Hinshaw VS, Wood JM, Webster RG, Deibel R, Turner B. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull WHO. 1985;63:711–719. [PMC free article] [PubMed] [Google Scholar]
- 19.Horne JS, Garton EO. Likelihood cross-validation versus least squares cross-validation for choosing the smoothing parameter in kernel home-range analysis. J Wildl Manag. 2006;70:641–648. [Google Scholar]
- 20.Horne JS, Garton EO. Animal space use 1.2. 2007 [cited 6 Mar 2009]. Available from: http://www.cnr.uidaho.edu/population_ecology/animal_space_use.htm.
- 21.Horne JS, Garton EO, Krone SM, Lewis JS. Analyzing animal movements using Brownian bridges. Ecology. 2007;88:2354–2363. doi: 10.1890/06-0957.1. [DOI] [PubMed] [Google Scholar]
- 22.John K, Kazwala R, Mfinanga GS. Knowledge of causes, clinical features and diagnosis of common zoonoses among medical practitioners in Tanzania. BMC Infect Dis. 2008;8:162. doi: 10.1186/1471-2334-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen P, an der Heiden M, Kern P, Schoneberg I, Krause G, Alpers K. Underreporting of human alveolar echinococcosis, Germany. Emerg Infect Dis. 2008;14:935–937. doi: 10.3201/eid1406.071173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanai Y, Uetaa M, Germogenov N, Nagendran M, Mitad N, Higuchie H. Migration routes and important resting areas of Siberian cranes (Grus leucogeranus) between northeastern Siberia and China as revealed by satellite tracking. Biol Conserv. 2002;106:339–346. [Google Scholar]
- 25.Keawcharoen J, van Riel D, van Amerongen G, Bestebroer T, Beyer WE, van Lavieren R, Osterhaus AD, Fouchier RA, Kuiken T. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korschgen CE, Kenow KP, Gendron-Fitzpatrick A, Green WL, Dein FJ. Implanting intra-abdominal radio transmitters with external whip antennas in ducks. J Wildl Manag. 1996;60:132–137. [Google Scholar]
- 27.Krapu GL, Reinecke KJ, Jorde DG, Simpson SG. Spring-staging ecology of midcontinent greater white-fronted geese. J Wildl Manag. 1995;59:736–746. [Google Scholar]
- 28.Li ZWD, Mundkhur T. Numbers and distribution of waterbirds and wetlands in the Asia-Pacific region: results of the Asian waterbird census 2002–2004. Wetlands International; Kuala Lumpur: 2007. [Google Scholar]
- 29.Liu J, Xiao H, Lei F, Zhu Q, Qin K, Zhang XW, Zhang XL, Zhao D, Wang G, Feng Y, Ma J, Liu W, Wang J, Gao GF. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005;309:1206. doi: 10.1126/science.1115273. [DOI] [PubMed] [Google Scholar]
- 30.Mukhtar MM, Rasool ST, Song D, Zhu C, Hao Q, Zhu Y, Wu J. Origin of highly pathogenic H5N1 avian influenza virus in China and genetic characterization of donor and recipient viruses. J Gen Virol. 2007;88:3094–3099. doi: 10.1099/vir.0.83129-0. [DOI] [PubMed] [Google Scholar]
- 31.Muzaffar SB, Ydenberg RC, Jones IL. Avian influenza: an ecological and evolutionary perspective for waterbird scientists. Waterbirds. 2006;29:243–406. [Google Scholar]
- 32.Newman SH, Iverson SA, Takekawa JY, Gilbert M, Prosser DJ, Batbayar N, Natsagdorj T, Douglas DC. Migration of whooper swans and outbreak of highly pathogenic avian influenza H5N1 virus in eastern Asia. PLoS One. 2009;4:e5729. doi: 10.1371/journal.pone.0005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Office des Internationales Epizootes. Manual of diagnostic tests and vaccines for terrestrial animals. World Organization of Animal Health; Paris: 2007. Avian influenza. [Google Scholar]
- 34.Prosser DJ, Takekawa JY, Newman SH, Yan B, Douglas DC, Hou Y, Xing Z, Li T, Li Y, Zhao D, Perry WM, Palm EC, Zhang D. Satellite-marked waterfowl reveal migratory connection between H5N1 outbreak areas in China and Mongolia. Ibis. 2009;151:568–576. [Google Scholar]
- 35.Qing-Quan B. The finding of Australian satellite tracing Bar-tailed Godwit in Dandong, Liaoning. China Crane News. 2008;12:55–57. [Google Scholar]
- 36.R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [cited 6 Mar 2009]. Available from: http://www.R-project.org. [Google Scholar]
- 37.Rundstadler JA, Happ GM, Slemons RD, Sheng Z-M, Gundlach N, Petrula M, Senne D, Nolting J, Evers DL, Modrell A, Huson H, Hills S, Rothe T, Marr T, Taubenberger JK. Using RRT-PCR analysis and virus isolation to determine the prevalence of avian influenza virus infections in ducks in Minto Flats State Game Refuge, Alaska, during August 2005. Arch Virol. 2007;152:1901–1910. doi: 10.1007/s00705-007-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankman D, Keim BD, Song H. Flood frequency in China’s Poyang Lake region: trends and teleconnections. Int J Climatol. 2006;26:1255–1266. [Google Scholar]
- 39.Shankman D, Liang Q. Landscape changes and increasing flood frequency in China’s Poyang Lake region. Prof Geogr. 2003;55:434–445. [Google Scholar]
- 40.van Gils JA, Munster VJ, Radersma R, Liefhebber D, Fouchier RA, Klaassen M. Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza A virus. PLoS ONE. 2007;2:e184. doi: 10.1371/journal.pone.0000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H-Z, Xu Q-Q, Cui Y-D, Liang Y-L. Macrozoo-benthic community of Poyang Lake, the largest freshwater lake of China, in the Yangtze floodplain. Limnology. 2007;8:65–71. [Google Scholar]
- 42.Webster RG. Wet markets—a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weitao J, Yun-Bao W. Survey on species and their number of wintering waterbirds in surrounding Poynag Lake area in 2006. China Crane News. 2007;11:37–38. [Google Scholar]
- 44.Wint W, Robinson T. Gridded livestock of the world—2007. Food and Agriculture Organization; Rome, Italy: 2007. [PubMed] [Google Scholar]
- 45.Yasue M, Feare CJ, Bennun L, Fiedler W. The epidemiology of H5N1 avian influenza in wild birds: why we need better ecological data. BioScience. 2006;56:923–929. [Google Scholar]
- 46.Zar JH. Biostatistical analysis. 4. Prentice Hall; Upper Saddle River, NJ: 1998. [Google Scholar]
- 47.Zhang JX, Liu JJ. Feeding ecology of two wintering geese species at Poyang Lake, China. Freshwater Ecol. 1999;14:439–445. [Google Scholar]