Abstract
Purpose
Inotuzumab ozogamicin (INO) is an antibody-targeted chemotherapy agent composed of a humanized anti-CD22 antibody conjugated to calicheamicin, a potent cytotoxic agent. We performed a phase I/II study to determine the maximum-tolerated dose (MTD), safety, efficacy, and pharmacokinetics of INO plus rituximab (R-INO) for treatment of relapsed/refractory CD20+/CD22+ B-cell non-Hodgkin lymphoma (NHL).
Patients and Methods
A dose-escalation phase to determine the MTD of R-INO was followed by an expanded cohort to further evaluate the efficacy and safety at the MTD. Patients with relapsed follicular lymphoma (FL), relapsed diffuse large B-cell lymphoma (DLBCL), or refractory aggressive NHL received R-INO every 4 weeks for up to eight cycles.
Results
In all, 118 patients received one or more cycles of R-INO (median, four cycles). Most common grade 3 to 4 adverse events were thrombocytopenia (31%) and neutropenia (22%). Common low-grade toxicities included hyperbilirubinemia (25%) and increased AST (36%). The MTD of INO in combination with rituximab (375 mg/m2) was confirmed to be the same as that for single-agent INO (1.8 mg/m2). Treatment at the MTD yielded objective response rates of 87%, 74%, and 20% for relapsed FL (n = 39), relapsed DLBCL (n = 42), and refractory aggressive NHL (n = 30), respectively. The 2-year progression-free survival (PFS) rate was 68% (median, not reached) for FL and 42% (median, 17.1 months) for relapsed DLBCL.
Conclusion
R-INO demonstrated high response rates and long PFS in patients with relapsed FL or DLBCL. This and the manageable toxicity profile suggest that R-INO may be a promising option for CD20+/CD22+ B-cell NHL.
INTRODUCTION
Most non-Hodgkin lymphomas (NHLs) are of B-cell origin.1 Because CD22, a B-cell antigen, is expressed in more than 90% of B-cell lymphoid malignancies,2 is not expressed on lymphocyte precursor cells or memory B cells, and is internalized on antibody binding,3 it is an attractive target for treatment of B-cell NHL. Inotuzumab ozogamicin (INO; CMC-544) was designed to take advantage of these properties, combining a humanized immunoglobulin G4 anti-CD22 antibody (G544) with the cytotoxic antibiotic calicheamicin.4–7 Internalization of CD22 allows for the release of calicheamicin to induce apoptosis.3,8,9
A phase I monotherapy study10 established the maximum-tolerated dose (MTD) of INO (1.8 mg/m2 every 4 weeks), reported reversible thrombocytopenia as the main toxicity, and demonstrated preliminary activity in heavily pretreated patients with relapsed/refractory CD22+ NHL. Given these phase I results and data demonstrating synergy between INO and rituximab in animal models,11,12 this study evaluated preliminary safety and efficacy of the combination of rituximab and INO (R-INO) in patients with relapsed/refractory NHL.
PATIENTS AND METHODS
Patients
Patients with CD20+/CD22+ B-cell NHL13 and prior rituximab exposure were enrolled onto dose-escalation (DE) cohorts or were assigned to one of three groups at the MTD: relapsed follicular lymphoma (FL), relapsed diffuse large B-cell lymphoma (DLBCL), or refractory aggressive NHL (eligible subtypes: DLBCL, transformed FL, follicular grade 3b, or mantle cell; Fig 1). Refractory was defined as disease progression less than 6 months from the start of the most recent rituximab-containing treatment. The relapsed groups included patients who had received two or fewer prior therapies and were not refractory to rituximab-containing therapy. The refractory group included patients who had received one or more prior therapies and had no response or were refractory to the most recent rituximab-containing treatment. Testing for CD22 was performed locally. See Appendix (online only) for more eligibility details.
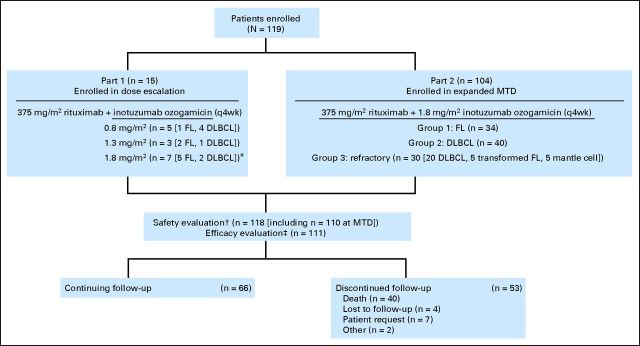
Fig 1.
CONSORT diagram. DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MTD, maximum-tolerated dose; q4wk, every 4 weeks. (*) An additional patient was enrolled over the six planned patients because one patient was unevaluable for safety (increased aminotransferase levels at screening in violation of study eligibility criteria and confirmed alcohol abuse). (†) All patients who received one or more doses of rituximab plus inotuzumab ozogamicin. One patient with DLBCL who was enrolled onto part 2 did not receive study treatment. (‡) All patients enrolled to receive MTD treatment (intent-to-treat): seven in part 1, 104 in part 2. One patient with DLBCL enrolled onto part 2 did not receive study treatment.
The study was approved by each site's institutional review board and conducted in accordance with good clinical practice guidelines. Patients provided signed and dated informed consent before enrollment.
Study Design
This was a multicenter, open-label study. R-INO was administered once every 4 weeks: rituximab on day 1and INO on day 2 of each cycle. Up to eight cycles were planned. The study was performed in two parts: DE to define the MTD (part 1) and an expanded cohort to further evaluate efficacy and safety of the MTD (part 2).
In part 1, INO doses of 0.8 mg/m2, 1.3 mg/m2, and 1.8 mg/m2 were selected. Rituximab was administered at a fixed dose of 375 mg/m2. Three to six patients with relapsed NHL were planned to enroll for each dose (Appendix). DE continued up to 1.8 mg/m2 or until two or more of six patients experienced a dose-limiting toxicity (DLT; Appendix; Table 1). Patients with a DLT were to have subsequent doses reduced by one level; one dose reduction was allowed. In part 2, additional patients were enrolled to further evaluate safety and efficacy of the MTD, including follow-up for progression-free survival (PFS) and overall survival (OS).
Table 1.
Adverse Events in ≥ 15% of Patients Receiving MTD Treatment
| Adverse Event | Relapsed FL (n = 39) |
Relapsed DLBCL (n = 41) |
Refractory NHL (n = 30) |
Total (n = 110) |
||||
|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| Blood and lymphatic system disorders | ||||||||
| Anemia | 7.7 | 0 | 17.1 | 2.4 | 6.7 | 0 | 10.9 | 0.9 |
| Leukopenia | 15.4 | 2.6 | 12.2 | 0 | 6.7 | 3.3 | 11.8 | 1.8 |
| Lymphopenia | 2.6 | 2.6 | 24.4 | 17.1 | 3.3 | 0 | 10.9 | 7.3 |
| Neutropenia | 56.4 | 33.3 | 29.3 | 19.5 | 10.0 | 10.0 | 33.6 | 21.8 |
| Thrombocytopenia | 46.2 | 23.1 | 68.3 | 36.6 | 53.3 | 33.3 | 56.4 | 30.9 |
| Gastrointestinal disorders | ||||||||
| Abdominal pain | 15.4 | 2.6 | 12.2 | 2.4 | 13.3 | 0 | 13.6 | 1.8 |
| Constipation | 23.1 | 0 | 29.3 | 0 | 16.7 | 0 | 23.6 | 0 |
| Diarrhea | 23.1 | 0 | 24.4 | 2.4 | 26.7 | 0 | 24.5 | 0.9 |
| Nausea | 69.2 | 2.6 | 68.3 | 2.4 | 40.0 | 0 | 60.9 | 1.8 |
| Vomiting | 33.3 | 2.6 | 24.4 | 2.4 | 30.0 | 0 | 29.1 | 1.8 |
| General disorders | ||||||||
| Chills | 15.4 | 2.6 | 29.3 | 0 | 6.7 | 0 | 18.2 | 0.9 |
| Fatigue | 48.7 | 7.7 | 61.0 | 2.4 | 46.7 | 3.3 | 52.7 | 4.5 |
| Peripheral edema | 10.3 | 0 | 19.5 | 2.4 | 13.3 | 0 | 14.5 | 0.9 |
| Pyrexia | 38.5 | 2.6 | 24.4 | 0 | 23.3 | 3.3 | 29.1 | 1.8 |
| Hepatobiliary disorders | ||||||||
| Hyperbilirubinemia | 30.8 | 7.7 | 29.3 | 0 | 26.7 | 0 | 29.1 | 2.7 |
| Investigations | ||||||||
| Increased ALT | 23.1 | 2.6 | 26.8 | 4.9 | 23.3 | 6.7 | 24.5 | 4.5 |
| Increased AST | 46.2 | 2.6 | 46.3 | 4.9 | 30.0 | 6.7 | 41.8 | 4.5 |
| Increased AP | 35.9 | 0 | 31.7 | 0 | 23.3 | 3.3 | 30.9 | 0.9 |
| Increased LDH | 30.8 | 2.6 | 34.1 | 2.4 | 20.0 | 6.7 | 29.1 | 3.6 |
| Metabolism and nutrition disorders | ||||||||
| Decreased appetite | 25.6 | 0 | 19.5 | 2.4 | 16.7 | 0 | 20.9 | 0.9 |
| Hypokalemia | 12.8 | 0 | 17.1 | 7.3 | 6.7 | 6.7 | 12.7 | 4.5 |
| Nervous system disorders | ||||||||
| Dizziness | 30.8 | 2.6 | 17.1 | 0 | 3.3 | 0 | 18.2 | 0.9 |
| Headache | 35.9 | 0 | 14.6 | 0 | 13.3 | 3.3 | 21.8 | 0.9 |
| Respiratory, thoracic, and mediastinal disorders | ||||||||
| Cough | 28.2 | 0 | 17.1 | 2.4 | 30.0 | 0 | 24.5 | 0.9 |
| Dyspnea | 23.1 | 2.6 | 17.1 | 0 | 16.7 | 3.3 | 19.1 | 1.8 |
| Epistaxis | 23.1 | 0 | 19.5 | 0 | 13.3 | 0 | 19.1 | 0 |
| Infections and infestations* | 46.2 | 7.7 | 58.5 | 17.1 | 33.3 | 6.7 | 47.3 | 10.9 |
NOTE. Investigator-reported adverse events occurring in ≥ 15% patients (MedDRA preferred term). Includes all patients who received one or more cycles of MTD treatment during the study (n = 110): n = 7 during part 1 (including five patients with FL and two patients with DLBCL), and n = 103 during part 2. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0. MTD, rituximab 375 mg/m2 day 1 and inotuzumab ozogamicin 1.8 mg/m2 day 2 every 4 weeks declared after only one patient had a dose-limiting toxicity at the highest planned inotuzumab ozogamicin dose (1.8 mg/m2). Dose-limiting toxicities were any of the following during the first cycle: febrile neutropenia, grade 4 neutropenia lasting ≥ 7 days, grade 4 thrombocytopenia lasting ≥ 3 days, grade 3 thrombocytopenia associated with hemorrhage requiring platelet transfusion, grade 3 to 4 nonhematologic toxicity (excluding nausea/vomiting not treated with optimal medical therapy and alopecia), and delayed recovery (grade 1/baseline) from a drug-related toxicity that prevented initiation of the next cycle by > 14 days.
Abbreviations: AP, alkaline phosphatase; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LDH, lactate dehydrogenase; MedDRA, Medical Dictionary for Regulatory Activities; MTD, maximum-tolerated dose; NHL, non-Hodgkin lymphoma.
Infections and infestations presented by higher-level MedDRA system class (none ≥ 15% by MedDRA preferred term). The most common infections were urinary tract infections, upper respiratory tract infections, and rhinitis.
Evaluations
All patients receiving one or more cycles of R-INO were evaluated for safety. Efficacy analyses were performed on an intent-to-treat basis for all patients enrolled to receive the MTD. Response to treatment was defined according to the version of the International Working Group (IWG) Response Criteria for NHL available at the time study recruitment began (May 2006)14; PFS was measured from start of treatment until the first date of relapsed disease or progression, initiation of a new anticancer therapy, or death, censored at the last tumor evaluation date. OS was measured from the first dose until date of death, censored at the last date the patient was known to be alive.
Statistical Analysis and Pharmacokinetics/Pharmacodynamics
Details regarding statistical analysis are included in the Appendix. Table 2 and Figures 2A and 2B provide information about pharmacokinetic (PK) and pharmacodynamic parameters.
Table 2.
Summary of Pharmacokinetic Parameters of Inotuzumab Ozogamicin and Calicheamicin in Seruma
| Treatment | Treatment Day | No. of Patients | Cmax(ng/mL) | Tmax (hours)b | AUCT(ng×hour/mL) | AUC(ng×hour/mL) | CL (L/hour) | t1/2 (hours) |
|---|---|---|---|---|---|---|---|---|
| Inotuzumab ozogamicin, mg/m2 | ||||||||
| 0.8 | 2 | 0 | N/C | N/C | N/C | N/C | N/C | N/C |
| 30 | 4 | 250 ± 74.1 | 2.63 | 5,340 ± 4,480 | N/C | N/C | N/C | |
| 58 | 2 | 214 ± 7.78 | 0.97 | 4,890 ± 2,380 | N/C | N/C | N/C | |
| 1.3 | 2 | 0 | N/A | N/A | N/C | N/C | N/C | N/C |
| 30 | 3 | 571 ± 149 | 1.00 | 13,100 ± 4,710 | N/C | N/C | N/C | |
| 58 | 3 | 583 ± 84.0 | 1.00 | 6,650 ± 7,940 | N/C | N/C | N/C | |
| 1.8 | 2 | 107 | 660 ± 707 | 1.00 | 9,760 ± 8,280c | N/C | N/C | N/C |
| 30 | 91 | 704 ± 354 | 1.08 | 24,900 ± 16,500d | 38,200 ± 5,670e | 0.0928 ± 0.0200e | 40.7 ± 5.24e | |
| 58 | 76 | 687 ± 242 | 1.08 | 31,300 ± 47,700f | 43,100 ± 10,600g | 0.0861 ± 0.0240g | 46.9 ± 7.49g | |
| Calicheamicin, mg/m2 | ||||||||
| 0.8 | 2 | 0 | N/C | N/C | N/C | N/C | N/C | N/C |
| 30 | 4 | 37.2 ± 12.0 | 2.63 | 1,740 ± 1,210 | N/C | N/C | N/C | |
| 58 | 2 | 33.5 ± 10.0 | 2.51 | 2,400 ± 2,740 | N/C | N/C | N/C | |
| 1.3 | 2 | 3 | 53.1 ± 23.3 | 1.05 | 1,410 ± 1,140 | 2,460 ± 219c | 1.14 ± 0.0439c | 50.6 ± 2.6c |
| 30 | 3 | 78.0 ± 12.4 | 1.00 | 4,840 ± 3,130 | 7,350 ± 2,300c | 0.404 ± 0.146c | 261.9 ± 91.9c | |
| 58 | 3 | 58.7 ± 3.01 | 1.00 | 7,520 ± 2,790 | 8,380 ± 2,170 | 0.325 ± 0.0795 | 204.8 ± 64.9 | |
| 1.8 | 2 | 107 | 64.1 ± 18.3 | 1.05 | 3,090 ± 2,550e | 6,290 ± 2,900g | 0.654 ± 0.286g | 152.7 ± 105.4g |
| 30 | 92 | 72.3 ± 20.6 | 1.26 | 7,240 ± 3,460d | 9,240 ± 2,950h | 0.409 ± 0.150h | 196.5 ± 93.3h | |
| 58 | 76 | 68.9 ± 22.1 | 1.36 | 8,630 ± 3,760f | 10,200 ± 3,310i | 0.376 ± 0.153i | 197.7 ± 68.8i |
Abbreviations: AUC, area under the curve [extrapolated to infinity for treatment day 2 or the steady-state AUC for treatment days 30 and 58]; AUCT, partial area under the curve [to last measurable time point]; CL, systemic clearance; t1/2, terminal half-life; Cmax, maximum concentration [at time Tmax]; N/A, not applicable; N/C, not calculated; Tmax, time when maximum concentration is reached.
Serum was prepared during cycles 1, 2, and 3 at 0, 1, 4, 72, 168, 216, 336, 504, and 672 hours of treatment. Concentrations of inotuzumab ozogamicin, total calicheamicin (antibody conjugated and unconjugated forms), free (unconjugated) calicheamicin, and the parent G544 antibody were determined in serum by using a validated enzyme-linked immunosorbent assay as previously described.10 Serum concentration-time profiles were constructed for each patient and analyzed by using a noncompartmental method (Jusko WJ: Guidelines for collection and analysis of pharmacokinetic data, in Evans WE, Schentag JJ, Jusko WJ, et al [eds]: Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring [ed 3]. Vancouver, WA, Applied Therapeutics, 1992). Includes 2,336 observations from 110 patients for inotuzumab ozogamicin, 2,356 observations from 112 patients for total calicheamicin, 1,876 observations from 101 patients for unconjugated calicheamicin, and 2,413 observations from 117 patients for immunoglobulin G4 anti-CD22 antibody (G544) following administration of all doses. For inotuzumab ozogamicin, and to some extent for total calicheamicin, estimates of t1/2 and CL were not obtainable because of the inability to characterize the terminal linear concentration phase. Pharmacokinetic profiles and parameters for unconjugated calicheamicin were not derived because most measures were below the limit of quantitation. Concentrations for G544 were qualitatively similar to those for inotuzumab ozogamicin and are not shown.
Median.
n = 2.
n = 91.
n = 106.
n = 73.
n = 48.
n = 72.
n = 65.
Fig 2.
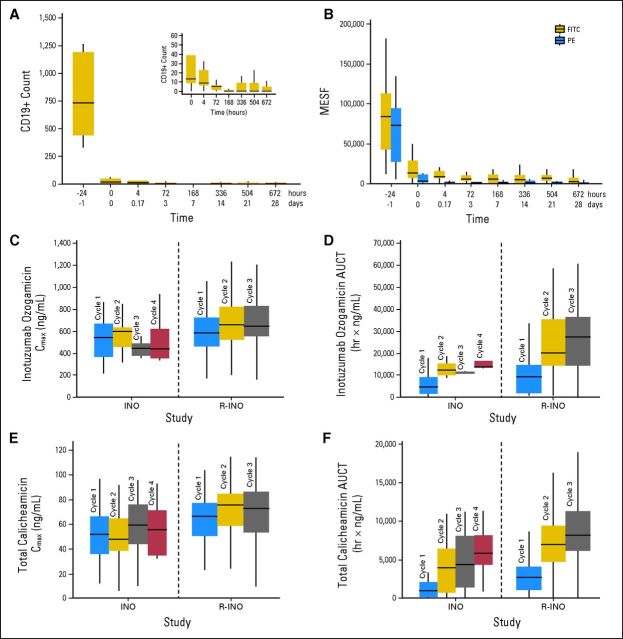
(A-B) CD19+ B-cell count and CD22 expression versus time after rituximab plus inotuzumab ozogamicin (R-INO) treatment (1.8 mg/m2 dose). (A) Total CD19+ B-cell count after treatment with rituximab (time, −24 hours) and INO (time, 0 hours). Inset magnifies the plot area between times 0 and 672 hours. As shown, CD19+ B-cell count declined rapidly after rituximab treatment and further declined after INO treatment, indicating that R-INO targeted the B-cell population. (B) Whole blood was drawn during cycles 1, 2, and 3 for measurement of CD22 saturation in B cells. Analysis was performed by using a differential binding flow cytometry technique as previously described.10 CD22 expression levels were detected with either the fluorescein isothiocyanate (FITC; uninhibited by INO) or phycoerythrin (PE; inhibited by INO) antibody clones. Response is expressed in units of molecular equivalents of soluble fluorescence (MESF) of phycoerythrin CD22+ cells determined from flow cytometry in which samples exhibited at least 300 events that were CD19+ at baseline. Length of box denotes 25th and 75th percentile; horizontal line within box denotes the median of observed data. As shown, CD22 expression declined with R-INO treatment, indicating that the total amount of CD22 receptor available for binding declined with INO treatment. The consistently lower signal of the PE clone indicates the direct binding activity of INO to CD22. (C-F) Serum INO and total calicheamicin exposure by study (this R-INO study, and prior INO monotherapy study10) and treatment cycle after the 1.8 mg/m2 dose. (C) INO maximum concentration (Cmax); (D) INO partial area under the curve (AUCT); (E) total calicheamicin Cmax; (F) total calicheamicin AUCT. Length of box denotes 25th and 75th percentile, and horizontal line within box denotes the median of observed data. These data show some cycle-related increases in exposure with greater increase in AUCT than in C[inf]max[r]. Although potential interstudy variability factors are not specifically accounted for, the data also show that exposures to INO and total calicheamicin tended to be modestly higher for R-INO compared with INO monotherapy. Because INO is thought to undergo target-mediated drug disposition, INO and calicheamicin exposures increase with additional cycles of therapy. Because rituximab therapy has the potential to deplete B cells and reduce the target for INO, we have hypothesized that this clearance of target by rituximab could result in increased INO and calicheamicin exposures.
RESULTS
Patients
Of 119 enrolled patients, 118 received one or more cycles of R-INO (Fig 1). Characteristics of enrolled patients are summarized in Table 3.
Table 3.
Patient Characteristics at Baseline
| Characteristic | Relapsed FL(n = 42)* |
Relapsed DLBCL(n = 47)† |
Refractory NHL(n = 30)‡ |
Total(N = 119) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| Median | 64 | 72 | 63 | 65 | ||||
| Range | 29-82 | 33-85 | 20-79 | 20-85 | ||||
| > 60 | 26 | 62 | 32 | 68 | 16 | 53 | 74 | 62 |
| Sex | ||||||||
| Female | 20 | 48 | 19 | 40 | 9 | 30 | 48 | 40 |
| Male | 22 | 52 | 28 | 60 | 21 | 70 | 71 | 60 |
| Baseline ECOG performance status§ | ||||||||
| 0 | 26 | 62 | 23 | 49 | 13 | 43 | 62 | 54 |
| 1 | 15 | 36 | 15 | 32 | 13 | 43 | 43 | 38 |
| 2 | 0 | 6 | 13 | 3 | 10 | 9 | 8 | |
| Baseline FLIPI/IPI score§ | ||||||||
| 0 | 3 | 7 | 2 | 4 | 1 | 3 | ||
| 1 | 7 | 17 | 8 | 17 | 4 | 13 | ||
| 2 | 15 | 36 | 19 | 40 | 7 | 23 | ||
| 3 | 7 | 17 | 13 | 28 | 9 | 30 | ||
| 4 | 9 | 21 | 3 | 6 | 4 | 13 | ||
| 5 | 1 | 2 | 0 | 1 | 3 | |||
| Disease stage III to IV | 33 | 79 | 32 | 68 | 23 | 77 | 88 | 74 |
| Bulky disease, cm | ||||||||
| > 7.5 | 7 | 17 | 7 | 15 | 9 | 30 | 23 | 19 |
| > 5.0 | 17 | 41 | 16 | 34 | 20 | 67 | 53 | 45 |
| Increased LDH§ | 13 | 31 | 16 | 35 | 18 | 62 | 47 | 40 |
| Bone marrow involvement§ | 13 | 31 | 8 | 17 | 6 | 20 | 27 | 23 |
| Prior rituximab exposure | 42 | 100 | 47 | 100 | 30 | 100 | 119 | 100 |
| No. of prior regimens | ||||||||
| 1 | 20 | 48 | 19 | 40 | 2 | 7 | 41 | 35 |
| 2 | 20 | 48 | 24 | 51 | 9 | 30 | 53 | 45 |
| ≥ 3 | 2 | 5 | 4 | 9 | 19 | 63¶ | 25 | 21 |
| Prior radiotherapy | 6 | 14 | 14 | 30 | 12 | 40 | 32 | 27 |
| Prior stem-cell transplantation | 2 | 5 | 9 | 19 | 3 | 10 | 14 | 12 |
| Response to most recent prior therapy | ||||||||
| Complete response | 24 | 57 | 30 | 64 | 1 | 3 | 55 | 46 |
| Partial response | 13 | 31 | 14 | 30 | 1 | 3 | 28 | 24 |
| Stable disease | 2 | 5 | 2 | 4 | 4 | 13 | 8 | 7 |
| Disease progression | 3 | 7 | 1 | 2 | 24 | 80 | 28 | 24 |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; IPI, International Prognostic Index; LDH, lactate dehydrogenase; NHL, non-Hodgkin lymphoma.
Includes one patient who received 0.8 mg/m2 inotuzumab ozogamicin and two patients who received 1.3 mg/m2 inotuzumab ozogamicin during dose-escalation phase (part 1).
Includes four patients who received 0.8 mg/m2 inotuzumab ozogamicin and one patient who received 1.3 mg/m2 inotuzumab ozogamicin during dose-escalation phase (part 1).
Consists of patients with DLBCL (n = 20), transformed FL (n = 5), and mantle cell lymphoma (n = 5).
Missing ECOG data (FL, n = 1; DLBCL, n = 3; refractory groups, n = 1), FLIPI/IPI data (DLBCL, n = 2; refractory groups, n = 4), LDH data (DLBCL, n = 1; refractory groups, n = 1), and bone marrow involvement data (FL, n = 2; DLBCL, n = 1; refractory groups, n = 1).
Includes nine patients with three prior therapies, seven patients with four prior therapies, and two patients with six prior therapies; one patient with Richter's transformation received nine prior therapies.
Summary of R-INO Treatment
For part 1, 15 patients received R-INO during DE. Five patients received INO 0.8 mg/m2, three received INO 1.3 mg/m2, and seven received INO 1.8 mg/m2 in combination with rituximab at 375 mg/m2 once every 4 weeks. An additional patient was enrolled because one patient was unevaluable for safety (Fig 1). Criteria for stopping DE were not met: no DLTs were observed at the 0.8 mg/m2 or 1.3 mg/m2 dose levels, and only one of seven patients had a DLT (delayed dosing was the result of low neutrophils and platelets) at the highest planned INO dose (1.8 mg/m2). MTD for the regimen was declared to be 375 mg/m2 rituximab day 1 and 1.8 mg/m2 INO day 2 once every 4 weeks.
For part 2, 104 patients were enrolled with 103 dosed in the expanded MTD cohort. Collectively, 110 patients were treated at the declared MTD across study parts 1 and 2 (Fig 1). Median number of cycles at the MTD was five (range, one to eight cycles) for FL, four (range, one to eight cycles) for DLBCL, and two (range, one to eight cycles) for refractory patients. Sixteen patients (14%) received fewer than two cycles of MTD treatment because of disease progression (12 patients) and adverse events (AEs; thrombocytopenia, n = 2; neutropenia, n = 1; and increased liver function tests, n = 1).
Efficacy
Objective response rate.
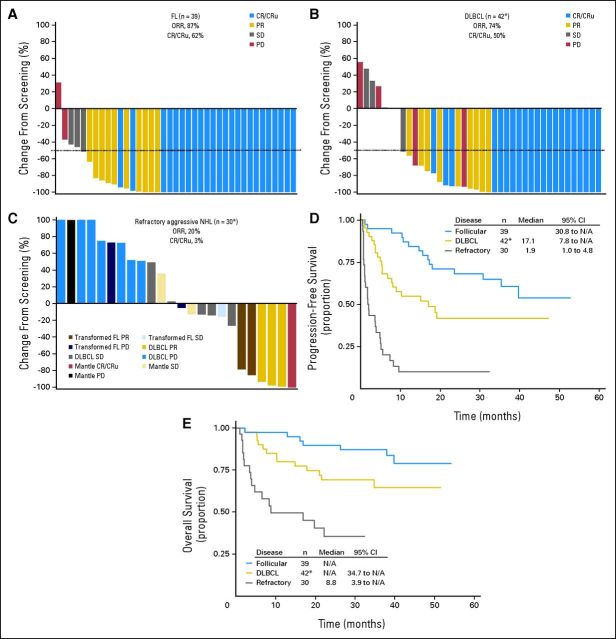
At MTD treatment, objective response rate (ORR) was 87%, 74%, and 20% for patients with FL, DLBCL, and refractory disease, respectively (Figs 3A to 3C). Confirmed complete response (CR) and unconfirmed CR were achieved in 62% of patients with FL and 50% of patients with relapsed DLBCL. Within the refractory aggressive NHL group, responses were observed for each NHL subtype (Fig 3C). Median duration of response was 17.7 months for relapsed DLBCL, 6.1 months for refractory aggressive NHL and, at the time of this report, had not been reached for patients with FL (median follow-up, 40 months).
Fig 3.
In this study, tumor assessments were performed at screening after every two cycles and at end-of-treatment visit. Tumor assessments continued after end-of-treatment visit every 12 weeks until progression, death, or administration of another anticancer therapy, whichever occurred first. After progression or new therapy, patients were observed for survival for up to 5 years. (A-C) Waterfall plots for patients enrolled to receive maximum-tolerated dose (MTD) treatment by best investigator-reported objective response. Nodal lesion sizes were normalized for normal nodal structures (defined as 100 mm2 for nodal lesions with maximum diameter < 15 mm at baseline and 150 mm2 for those with diameters > 15 mm at baseline14). Objective response rate (ORR) is defined as complete response (CR) plus unconfirmed complete response (CRu) plus partial response (PR). (A) ORR and CR/CRu rates and change in lesion size for all relapsed patients with follicular lymphoma (FL) enrolled to receive MTD treatment (n = 39); (B) ORR and CR/CRu rates for all relapsed patients with diffuse large B-cell lymphoma (DLBCL) enrolled to receive MTD treatment (n = 42), with change in lesion size shown for 40 patients. (*) Not shown for two patients: one because of incomplete radiographic tumor assessment at screening, and one, who did not receive inotuzumab ozogamicin [INO], because of no postscreening radiographic tumor assessment); (C) ORR and CR/CRu rates for all patients with refractory aggressive non-Hodgkin lymphoma (NHL) enrolled to receive MTD treatment (n = 30), with change in lesion size shown for 24 patients. (*) Not shown for six patients [five DLBCL, one mantle cell] because there was no or incomplete postscreening radiographic tumor assessment). Kaplan-Meier curves of (D) progression-free survival; (*) includes one patient who did not receive INO. (E) Overall survival for all patients enrolled to receive MTD treatment by NHL type; (*) includes one patient who did not receive INO. N/A, not applicable; PD, progressive disease; SD, stable disease.
PFS and OS.
One- and 2-year PFS rates were 87% and 68%, respectively, for FL (median PFS, not reached) and 55% and 42%, respectively, for relapsed DLBCL (median PFS, 17.1 months; Fig 3D). For patients with refractory disease, median PFS was 1.9 months with a 2-year PFS rate of 10%. One and 2-year OS rates were 97% and 90%, respectively, for relapsed FL and 80% and 69%, respectively, for relapsed DLBCL. In total, 36% of patients with refractory disease were alive at 2 years (median OS, 8.8 months). Median OS had not been reached for either the FL or relapsed DLBCL groups at the time of this report (median follow-up, 40 months for FL, with 82% of patients censored and 30 months for DLBCL with 69% of patients censored; Fig 3E).
Efficacy and Prognostic Factors
Follicular Lymphoma International Prognostic Index (FLIPI) score15 and tumor bulk appeared to affect CR and PFS rates in FL. Activity of R-INO in relapsed/refractory DLBCL appeared to be affected by International Prognostic Index (IPI) score,16 tumor bulk, response to prior therapy, and prior time-to-tumor progression (Table 4).
Table 4.
Response Rate and PFS for Patients With Relapsed FL or Relapsed/Refractory DLBCL Enrolled to Receive MTD Treatment, by Prognostic Factors
| Variable | Response Rate |
PFS |
|||
|---|---|---|---|---|---|
| No. | ORR (%)* | CR/CRu (%) | 1-Year PFS rate (%) | Median (months) | |
| Relapsed FL (n = 39)† | |||||
| Response to prior therapy | |||||
| CR/CRu | 23 | 87 | 65.2 | 87 | N/R |
| PR/SD | 14 | 86 | 50 | 86 | N/R |
| PD | 2 | 100 | 100 | 100 | 30.8 |
| Time to progression with prior therapy, months | |||||
| > 12 | 27 | 85 | 67 | 92 | N/R |
| < 12 | 12 | 92 | 50 | 75 | 30.8 |
| FLIPI score | |||||
| Low risk (< 2) | 10 | 100 | 90 | 100 | N/R |
| Intermediate risk 2 | 12 | 92 | 83 | 100 | N/R |
| High risk (> 2) | 17 | 77 | 29 | 69 | 30.8 |
| Bulky disease, cm | |||||
| < 5.0 | 23 | 87 | 78 | 91 | N/R |
| 5.0-7.5 | 10 | 90 | 50 | 79 | N/R |
| ≥ 7.5 | 6 | 83 | 17 | 83 | N/R |
| Relapsed/refractory DLBCL (n = 67)‡ | |||||
| Response to prior therapy§ | |||||
| CR/CRu | 28 | 82 | 61 | 61 | 18.7 |
| PR/SD | 17 | 47 | 24 | 34 | 7.8 |
| PD | 21 | 19 | 0 | 10 | 1.7 |
| Time to progression with prior therapy, months | |||||
| > 12 | 27 | 74 | 59 | 56 | 17.1 |
| < 12 | 40 | 40 | 15 | 25 | 4.1 |
| IPI§ | |||||
| Low risk (< 2) | 13 | 69 | 54 | 51 | 15.1 |
| Low-intermediate risk 2 | 24 | 58 | 38 | 49 | 9.6 |
| Intermediate-high/high risk (> 2) | 26 | 42 | 15 | 19 | 3.7 |
| Bulky disease, cm | |||||
| < 5.0 | 35 | 69 | 51 | 50 | 10.3 |
| 5.0-7.5 | 18 | 39 | 17 | 28 | 4.0 |
| ≥ 7.5 | 14 | 36 | 7 | 15 | 3.4 |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; CR, complete response; CRu, unconfirmed CR; FL, follicular lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; IPI, International Prognostic Index; MTD, maximum-tolerated dose; N/R, not reached; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
ORR includes CR, CRu, and PR.
All patients with FL enrolled to receive MTD treatment (five enrolled in part 1; 34 enrolled in part 2).
All patients with relapsed or refractory DLBCL who were enrolled to receive MTD treatment (42 relapsed DLBCL [two patients enrolled in part 1; 40 enrolled in part 2, group 2], 20 refractory DLBCL [part 2, group 3], and five refractory transformed FL [part 2, group 3]). One patient enrolled in part 2, group 2 did not receive study treatment.
Prior response not reported for one patient; IPI score not reported for four patients.
Safety
For all patients who received R-INO (n = 118), the most common AEs included thrombocytopenia (56%), nausea (57%), fatigue (53%), increased AST (41%), neutropenia (33%), increased alkaline phosphatase (30%), and vomiting (28%).
At MTD treatment (n = 110; Table 1), thrombocytopenia and neutropenia were the most frequent grade 3 to 4 events (9% of patients had grade 4 thrombocytopenia; 8% of patients had grade 4 neutropenia). Increases in AST, ALT, bilirubin, alkaline phosphatase, and lactate dehydrogenase were also observed, but grade 3 to 4 reports were rare (each ≤ 4.5%). There were no grade 4 occurrences of increases in aminotransferase or bilirubin. Grade 3 increases in AST and ALT were generally reversible; in total, five (83%) of six patients with grade 3 AST or ALT had levels return to grade ≤ 1 for continued dosing (without cycle delay, or within the 21-day delay period), and only one patient discontinued treatment due to persisting increases. For this patient, grade 3 ALT and AST increases continued to persist approximately 1 month after the last dose of R-INO. For the three patients with grade 3 hyperbilirubinemia (each starting after ≥ four cycles of R-INO), none recovered in time for redosing. Each of these three patients continued to have grade 2 to 3 bilirubin persisting approximately 1 month after the last dose of R-INO, with levels ultimately returning to grade ≤ 1 during long-term follow-up.
Of the patients treated with R-INO, 30% experienced serious AEs. Serious AEs for more than one patient included pneumonia (n = 5; 4%); sepsis (n = 4; 3.4%); thrombocytopenia, vomiting, or nausea (n = 3 [for each AE]; 2.5% each); and nodular regenerative hyperplasia, peripheral edema, duodenal ulcer, infection, chest pain, or dizziness (n = 2 [for each AE]; 1.7% each). In addition to the two patients with nodular regenerative hyperplasia, there were three other serious hepatic events: grade 3 aminotransferase increase (reported as cytolytic hepatitis), hepatic fibrosis, and hepatic failure (fatal) in one patient each. For the two patients with nodular regenerative hyperplasia, one patient's condition (which resolved) was confirmed by liver biopsy approximately 1 year after five cycles of R-INO; the other patient (whose condition still had not resolved at last report) presented with ascites, increased bilirubin, and mild splenomegaly approximately 3 months after six cycles of R-INO. The patient with cytolytic hepatitis had increased liver function tests (AST/ALT) that resolved within 7 days. One patient had hepatic fibrosis that occurred 10 months after study treatment and was confounded by alcohol abuse. One patient with hepatic failure had previously been treated with with CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], then with R-DHAP [rituximab plus dexamethasone, cisplatin, cytarabine], and then was consolidated with BEAM [carmustine, etoposide, cytarabine, melphalan] and autologous stem-cell transplantation. The patient received five cycles of R-INO, had disease progression, and was then treated with R-CHOP [rituximab plus CHOP], which was complicated by febrile neutropenia, sepsis, and hepatic failure. The relationship between R-INO and this event is difficult to discern because the subsequent R-CHOP regimen could be a significant factor as well.
Forty patients (34%) have died. The most common reasons for death were disease progression (n = 22), pneumonia (n = 3), and multiorgan failure (n = 3, including two associated with sepsis). Three deaths occurred at 30 days of treatment or before: two as a result of disease progression and one as a result of perforated bowel with bulky intestinal mass resulting in sepsis and death.
During MTD treatment, 47 patients (43%) had toxicities requiring dose delays, and 10 patients (9%) required dose reductions. Thrombocytopenia and neutropenia were the most common reasons for these dosing modifications. Fifty-four patients (49%) discontinued MTD treatment because of toxicities. Grade ≥ 2 thrombocytopenia or hyperbilirubinemia lasting more than 3 weeks after the scheduled day of dosing were the most common reasons that patients discontinued MTD treatment. Twenty patients (18%) discontinued treatment because of thrombocytopenia: of these, 13 patients (65%) recovered to grade ≤ 1 (approximately 2 to 10 months after last dose); five (25%) with limited follow-up laboratory data did not recover by approximately 2 months after last dose; and one (5%) did not recover by 8 months after the last dose, although new anticancer therapy was also initiated during this period (no data were available for one patient). Of note, 22% of patients treated at MTD had thrombocytopenia at baseline before dosing (study eligibility required platelets ≥ 75,000/μL). Nineteen patients (17%) discontinued treatment because of hyperbilirubinemia, and most (79%) had grade 1 to 2 increases. In total, 65% of all MTD discontinuations due to thrombocytopenia occurred within three or fewer cycles of R-INO, whereas 90% of all MTD discontinuations due to hyperbilirubinemia occurred after cycle 4 or greater.
Pharmacokinetics and Pharmacodynamics
Summaries of pharmacokinetic parameters are shown in Table 2. Data indicate that exposure to INO and associated products increased with dose and cycle. See Appendix for more details.
B-cell response to R-INO is shown and discussed in Figures 2A and 2B. Exposures (maximum concentration and partial area under the curve) of INO and total calicheamicin after R-INO (1.8 mg/m2) dosing were compared with respective values previously reported for INO monotherapy (Figs 2C to 2F).10
DISCUSSION
R-INO presents a novel treatment for NHL with INO and rituximab targeting the CD22 and CD20 antigens, respectively. On the basis of cycle 1 DLTs, this combination of INO 1.8 mg/m2 and a standard dose of rituximab 375 mg/m2 (once every 4 weeks) was determined to be at or below the MTD. With repeated dosing, patients with relapsed DLBCL or FL were able to tolerate a median of four to five cycles. At this dose and schedule, many patients with prior chemotherapy treatment may not be able to tolerate more than six cycles without dose delays or reductions. For R-INO, 2-year PFS rates of 68% (median not reached) in patients with relapsed FL and 42% (median, 17.1 months) in patients with relapsed DLBCL were achieved compared with single-agent INO, which yielded a median PFS of 10.4 months and 1.6 months for the analogous NHL subtypes in a completed phase I study.10 The different patient populations partly account for the increased efficacy observed for R-INO. Patients in the monotherapy trial (61% had four or more prior therapies and included those with refractory disease) more closely resemble the heavily pretreated refractory group in this study (63% with three or more prior therapies) who had an ORR of 20% and median PFS of 1.9 months. Still, nonclinical data with Ramos B-lymphoma xenograft models support at least additive effects between INO and rituximab,11 and the increased activity seemingly conferred by the addition of rituximab to INO was expected, given that its addition has increased the efficacy of other chemotherapies.17–20
As with MTD treatment with single-agent INO,10 the main grade 3 to 4 toxicities observed with MTD treatment with R-INO were thrombocytopenia and neutropenia. The frequency of these hematologic toxicities for R-INO was lower than that observed with INO monotherapy (31% and 22%, respectively, for R-INO; 63% and 35%, respectively, for monotherapy),10 and likely related to differences in patient populations, with patients in the monotherapy study being more heavily pretreated (Discussion, first paragraph) than those in the this study (79% with two or fewer prior therapies). Thrombocytopenia was the primary reason for discontinuation of treatment with R-INO.
Hepatotoxicity has also been observed with INO. In the monotherapy study, one patient developed veno-occlusive disease (VOD), although a medical history significant for VOD-like syndrome with prior therapy was noted.10 No VOD occurred in this study, but other notable serious hepatotoxicity included two occurrences of nodular regenerative hyperplasia, one of hepatic fibrosis, and one of hepatic failure, although other factors including other chemotherapies or alcohol abuse may have contributed. Increased liver function tests and hyperbilirubinemia were also observed in this study but were primarily lower grade (Table 1). Onset of hyperbilirubinemia was most likely to occur after multiple cycles of R-INO (90% of MTD discontinuations because of hyperbilirubinemia occurred after cycle four or greater). Thirteen patients (11%) had serious infections, with pneumonia being most common (n = 5). Only one patient had febrile neutropenia.
Despite the targeted design of R-INO and studies showing that INO directly binds to CD22 receptors and targets B cells (Figs 2A and 2B), some toxicities indicate that nontarget effects still occur. Risk for some of these toxicities may be linked with increased cumulative INO exposure following multiple cycles resulting from decreased CD22 antigen sink. Increased exposure with repeated dosing may have contributed to discontinuation of treatment because of AEs following cycle 1. Dose reductions after cycle 1 may allow more cycles in some patients. Although preliminary analyses suggest exposure (AUClast) of total calicheamicin was positively correlated with an increase in AST in cycle 1, the effect was not evident at subsequent cycles. Further analyses may be helpful to better understand potential associations between exposure and nontarget effects.
The nonlinear PK of INO found in this study, marked by disproportionate increases in partial area under the curve and decreases in systemic clearance with dose and cycle, is characteristic of therapeutic antibodies that undergo target-mediated drug disposition. Internalization and disposition of the drug-target complex from the cell surface further contributes to the overall systemic clearance, with the effect of target-mediated drug disposition on the PK of an antibody typically more prominent at lower doses than higher doses because of target saturation in the latter case.
In the context of current treatments, activity of R-INO is noteworthy. For relapsed/refractory FL, commonly used multiagent chemotherapies as well as the combination of rituximab and bendamustine yield high response rates (85% to 94%) and long PFS (median, 33 months) in patient populations inclusive of those not previously treated with rituximab.17,20,21 Single-agent rituximab is expected to have ORR rates of approximately 50% in patients with relapsed/refractory FL.22,23 Thus, R-INO appears to be promising therapy for patients with relapsed FL, including those with prior rituximab exposure, potentially being more efficacious than single-agent rituximab and a particularly good option for those not suited for intensive multiagent therapies.
For more aggressive NHLs, salvage therapy with autologous stem-cell transplantation is generally regarded as the best option for those who are qualified. One-year PFS rates of approximately 38% to 42% and a 3-year PFS rate of 30% have been reported for patients with relapsed/refractory DLBCL who have had prior rituximab exposure.24,25 In these studies, most patients had only one prior therapy and were approximately age 55 years. Although the different patient populations make direct comparison difficult, R-INO efficacy in relapsed DLBCL appears notable, given the advanced age of patients with relapsed DLBCL in this trial (median age, 72 years).
R-INO treatment of patients with refractory aggressive NHL was challenging. Chemosensitivity of aggressive NHL appears to be a primary determinant of response to R-INO. Other baseline characteristics, including heavy pretreatment (Table 3), could also be relevant. Still, within this refractory population, responses were observed for each NHL subtype: one patient with mantle cell NHL had complete radiographic reduction of lesions, and three patients with DLBCL and two patients with transformed FL each had ≥ 75% lesion reduction (Fig 3C).
In addition to prior rituximab exposure, other factors have been previously identified to adversely affect efficacy.24,26,27 Response to prior therapy, time-to-progression with prior therapy, IPI score, and tumor bulk appear to affect R-INO outcomes in patients with relapsed/refractory DLBCL (Table 4). Complete responders with prior treatment had the highest response rates and longest PFS. For relapsed FL, high-risk FLIPI scores and tumor bulk appeared to have an adverse impact on CR rates and 1-year PFS rates, although response to prior therapy and prior time-to-tumor progression appeared less important (Table 4).
In conclusion, R-INO yielded high response rates and long PFS for relapsed FL and DLBCL. R-INO activity in the context of prior rituximab exposure is significant, given the current prevalence of first-line rituximab treatment. As in reports for other chemotherapies, possible adverse prognostic factors included high FLIPI/IPI scores, poor response or early relapse to prior treatment, and greater tumor bulk. Toxicity of R-INO is characterized primarily by manageable thrombocytopenia and neutropenia. Increases in liver function tests and hyperbilirubinemia (primarily grade 1 to 2 in this study) will continue to be evaluated in ongoing and future trials, along with PK studies to evaluate potential correlations between exposure and toxicity. R-INO may offer the reduction of certain toxicities such as febrile neutropenia compared with other multiagent chemotherapies. Results for relapsed/refractory DLBCL are promising, given that the patient population (risk factors include advanced age, heavy pretreatment, and poor response to prior therapy) represents a group with limited treatment options. Future studies comparing R-INO to other chemotherapies will help define the role for R-INO in treatment of CD22+ malignancies.
Acknowledgment
Presented in part at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008; the 14th Congress of the European Hematology Association, Berlin, Germany, June 4-7, 2009; the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009; and the 15th Congress of the European Hematology Association, Barcelona, Spain, June 10-13, 2010.
We thank the patients, their families, clinical personnel, and additional principal investigators for their help and participation; Mark Shapiro and Hemant Patel for their roles with study design and execution; Ying Jiang and Hui Zhang for programming and statistical support; and Sharon Sullivan for assistance with manuscript preparation. Additional editorial support was provided by Kimberly Brooks, PhD, at SciFluent Communications.
Appendix
Study Eligibility
Main eligibility criteria included age ≥ 18 years; Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; life expectancy ≥ 12 weeks; absolute neutrophil count ≥ 1,500/μL; platelets ≥ 75,000/μL; serum creatinine and total bilirubin ≤ 1.5× upper limit of normal; AST and ALT ≤ 2.5× upper limit of normal; one or more disease lesion ≥ 1.5 × 1.5 cm; and no prior allogeneic hematopoietic stem-cell transplantation, radioimmunotherapy, or anti-CD22 antibody treatment. Persons deemed candidates for potentially curative therapies were not eligible for the study.
Study Design and Dose Escalation
Per protocol, three to six patients were planned to enroll for each dose-escalation cohort. Planned doses of inotuzumab ozogamicin (INO) were 0.8 mg/m2, 1.3 mg/m2, and 1.8 mg/m2. The highest planned dose of 1.8 mg/m2 was based on results from a monotherapy study.10 Criteria for cohort enrollment and dose escalation were as follows:
If none of three to six evaluable patients experiences a dose-limiting toxicity (DLT) by day 28 of the first dose, then dose escalation will occur, and three to six patients will be treated at the next higher dose level.
If one patient at a dose level experiences a DLT by day 28 of the first dose, up to a total of six evaluable patients will be treated at the same dose level. The dose will be escalated only if none of the additional patients presents with a DLT.
If two or greater of three to six patients at a higher dose level experience a DLT by day 28 of the first dose, dose escalation will stop and the prior dose level will be considered the maximum-tolerated dose (MTD).
Statistics
Summary statistics were performed for continuous variables; patient percentages were provided for categorical variables. Efficacy end points included objective response rate, duration of response, progression-free survival (PFS), and overall survival. Objective response rate was estimated by using an exact confidence interval approach; duration of response, PFS, and overall survival were analyzed by using Kaplan-Meier methods. All patients treated with rituximab plus INO were included in safety analyses. Efficacy analyses were performed on all patients enrolled to receive MTD treatment. Prognostic factors were not prespecified for this study, and the statistical significance of prognostic factors on efficacy was not evaluated. For part 1, with cohort sizes of three to six patients, if the true underlying rates of DLTs were 0.1, 0.2, 0.3, 0.4, or 0.5, there would be a 91%, 71%, 49%, 31%, or 17% chance, respectively, of escalating to the next full dose. For part 2, the probabilities of detecting one or more adverse event of grade ≥ 3 with 30 patients receiving drug at the MTD were 0.260, 0.785, 0.958, 0.992, or 0.999 when the true rates were 1%, 5%, 10%, 15%, or 20%, respectively. Furthermore, by using historical data available at the time the study protocol was developed, a sample size of 30 was judged to provide meaningful efficacy assessment as follows:
Thirty patients with follicular lymphoma in the MTD cohort will provide 82% power to detect a difference of 90% 6-month PFS (study data) versus 70% 6-month PFS (estimated from historical data; this 6-month PFS was the expected PFS for patients treated with rituximab.23), assuming a type I error rate of 10%.
Thirty patients with diffuse large B-cell lymphoma in the MTD cohort will provide 90% power to detect a difference of 84% 6-month PFS (study data) versus 59% 6-month PFS (estimated from historical data; this 6-month PFS was the expected PFS for patients treated with standard chemotherapy. PFS in this patient population varies but was expected to be in the 50% to 60% range similar to that reported in Philip et al: N Engl J Med 333:1540-1545, 1995), assuming a type I error rate of 10%.
DLTs
DLTs were any of the following during the first cycle: febrile neutropenia, grade 4 neutropenia lasting ≥ 7 days, grade 4 thrombocytopenia lasting ≥ 3 days, grade 3 thrombocytopenia associated with hemorrhage requiring platelet transfusion, grade 3 to 4 nonhematologic toxicity (excluding nausea/vomiting not treated with optimal medical therapy and alopecia), and delayed recovery (grade 1 or baseline) from a drug-related toxicity that prevented initiation of the next cycle by more than 14 days.
Permitted Concomitant Treatment
Secondary prophylactic use of growth factors (eg, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor) was allowed for grade 4 neutropenia ≥ 7 days, grade 4 febrile neutropenia, or grade 4 febrile neutropenia/infection after cycle 1 or greater.
Pharmacokinetic Results
Summaries of pharmacokinetic parameters are shown for INO and for total calicheamicin by dose in Table 2. Data indicate that exposure to INO and associated products increased with dose and number of doses. Specifically, increases in maximum concentration and partial area under the curve were observed with dose, and increases in partial area under the curve with cycle were apparent (cycle effects on maximum concentration were not seen). For INO, mean terminal half-life after multiple cycles ranged from 40.7 to 46.9 hours (1.8 mg/m2 dosing). For total calicheamicin, systemic clearance tended to decrease with cycle, ranging from 0.654 L/h for cycle 1 to 0.376 L/h for cycle 3 (1.8 mg/m2 dosing). Half-life of total calicheamicin tended to increase with cycle number.
Formation of antibodies to INO was rare during the study (observed in three patients who exhibited a low response at baseline and/or post-treatment). No antirituximab antibodies were observed.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00299494.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Michelle Kneissl, Pfizer (C); Kenneth T. Luu, Pfizer (C); Steven Y. Hua, Pfizer (C); Joseph Boni, Pfizer (C); Erik Vandendries, Pfizer (C) Consultant or Advisory Role: Peter Johnson, Pfizer (C); Jonathan L. Kaufman, Celgene (C), Millennium Pharmaceuticals (C), Novartis (C), Onyx Pharmaceuticals (C); Anjali Advani, Pfizer (C); Georg Hess, Pfizer (C); Myron Czuczman, Genentech (C) Stock Ownership: Michelle Kneissl, Pfizer; Kenneth T. Luu, Pfizer; Steven Y. Hua, Pfizer; Joseph Boni, Pfizer; Erik Vandendries, Pfizer Honoraria: Peter Johnson, Pfizer; Anjali Advani, Pfizer; Georg Hess, Pfizer; Nam H. Dang, Pfizer Research Funding: Mitchell R. Smith, Genentech, Pfizer; Jonathan L. Kaufman, Celgene, Merck; Anjali Advani, Pfizer; James Foran, Pfizer; Georg Hess, Pfizer; Simon Durrant, Pfizer; Nam H. Dang, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Peter Johnson, Myron Czuczman, Simon Durrant, Michelle Kneissl, Joseph Boni
Provision of study materials or patients: Peter Johnson, Ama Rohatiner, Anjali Advani, Bertrand Coiffier, Myron Czuczman, Eva Giné
Collection and assembly of data: Fritz Offner, Mitchell R. Smith, Jonathan L. Kaufman, Ama Rohatiner, Anjali Advani, James Foran, Georg Hess, Myron Czuczman, Simon Durrant, Michelle Kneissl, Steven Y. Hua, Nam H. Dang
Data analysis and interpretation: Luis Fayad, Gregor Verhoef, Peter Johnson, Jonathan L. Kaufman, Ama Rohatiner, Anjali Advani, James Foran, Georg Hess, Bertrand Coiffier, Eva Giné, Simon Durrant, Michelle Kneissl, Kenneth T. Luu, Steven Y. Hua, Joseph Boni, Erik Vandendries
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by Pfizer (formerly Wyeth Research). Inotuzumab ozogamicin was co-developed with UCB, Brussels, Belgium. Editing support was funded by Pfizer.
REFERENCES
- 1.American Cancer Society. Lymphoma, non-Hodgkin type. http://www.cancer.org/cancer/non-hodgkinlymphoma/detailedguide/non-hodgkin-lymphoma-types-of-non-hodgkin-lymphoma.
- 2.Cesano A, Gayko U, Brannan C. Differential expression of CD22 in indolent and aggressive non-Hodgkin's lymphoma (NHL): Implications for targeted immunotherapy. Blood. 2002;100:350a. abstr 1358. [Google Scholar]
- 3.Vaickus L, Ball ED, Foon KA. Immune markers in hematologic malignancies. Crit Rev Oncol Hematol. 1991;11:267–297. doi: 10.1016/1040-8428(91)90029-c. [DOI] [PubMed] [Google Scholar]
- 4.Hinman LM, Hamann PR, Wallace R, et al. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: A novel and potent family of antitumor antibiotics. Cancer Res. 1993;53:3336–3342. [PubMed] [Google Scholar]
- 5.Lee MD, Dunne TS, Chang CC, et al. Calicheamicins, a novel family of antitumor antibiotics. 4. structural elucidations of calicheamicins beta 1Br, gamma 1Br, alpha 2I, alpha 3I, beta 1I, gamma 1I, and delta 1I. J Am Chem Soc. 1992;114:985–997. [Google Scholar]
- 6.Thorson JS, Sievers EL, Ahlert J, et al. Understanding and exploiting nature's chemical arsenal: The past, present and future of calicheamicin research. Curr Pharm Des. 2000;6:1841–1879. doi: 10.2174/1381612003398564. [DOI] [PubMed] [Google Scholar]
- 7.Zein N, Sinha AM, McGahren WJ, et al. Calicheamicin gamma 1I: An antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988;240:1198–1201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]
- 8.DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody-targeted chemotherapy with CMC-544: A CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103:1807–1814. doi: 10.1182/blood-2003-07-2466. [DOI] [PubMed] [Google Scholar]
- 9.Hamann PR, Hinman LM, Hollander I, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13:47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 10.Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: Results of a phase I study. J Clin Oncol. 2010;28:2085–2093. doi: 10.1200/JCO.2009.25.1900. [DOI] [PubMed] [Google Scholar]
- 11.DiJoseph JF, Dougher MM, Kalyandrug LB, et al. Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin's B-cell lymphoma. Clin Cancer Res. 2006;12:242–249. doi: 10.1158/1078-0432.CCR-05-1905. [DOI] [PubMed] [Google Scholar]
- 12.DiJoseph JF, Goad ME, Dougher MM, et al. Potent and specific antitumor efficacy of CMC-544, a CD22-targeted immunoconjugate of calicheamicin, against systemically disseminated B-cell lymphoma. Clin Cancer Res. 2004;10:8620–8629. doi: 10.1158/1078-0432.CCR-04-1134. [DOI] [PubMed] [Google Scholar]
- 13.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting—Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 15.Solal-Céligny P, Roy P, Colombat P, et al. Follicular Lymphoma International Prognostic Index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 16.Shipp MA, Harrington DP, Anderson JR, et al. A predictive model for aggressive non-Hodgkin's lymphoma: The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 17.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 18.Hiddemann W, Dreyling M, Unterhalt M. Rituximab plus chemotherapy in follicular and mantle cell lymphomas. Semin Oncol. 2003;30:16–20. doi: 10.1053/sonc.2003.50024. [DOI] [PubMed] [Google Scholar]
- 19.Jermann M, Jost LM, Taverna Ch, et al. Rituximab-EPOCH, an effective salvage therapy for relapsed, refractory or transformed B-cell lymphomas: Results of a phase II study. Ann Oncol. 2004;15:511–516. doi: 10.1093/annonc/mdh093. [DOI] [PubMed] [Google Scholar]
- 20.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: Results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 21.Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4473–4479. doi: 10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- 22.Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 23.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 24.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martín A, Conde E, Arnan M, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: The influence of prior exposure to rituximab on outcome—A GEL/TAMO study. Haematologica. 2008;93:1829–1836. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- 26.López A, Gutiérrez A, Palacios A, et al. GEMOX-R regimen is a highly effective salvage regimen in patients with refractory/relapsing diffuse large-cell lymphoma: A phase II study. Eur J Haematol. 2008;80:127–132. doi: 10.1111/j.1600-0609.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 27.Pfreundschuh M, Ho AD, Cavallin-Stahl E, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: An exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008;9:435–444. doi: 10.1016/S1470-2045(08)70078-0. [DOI] [PubMed] [Google Scholar]