Abstract
Purpose
Lenalidomide is an immunomodulatory drug active as salvage therapy for chronic lymphocytic leukemia (CLL). We combined lenalidomide with rituximab to improve response rates in patients with relapsed or refractory CLL.
Patients and Methods
Fifty-nine adult patients (age 42 to 82 years) with relapsed or refractory CLL were enrolled onto a phase II study of lenalidomide and rituximab. Patients had received prior fludarabine-based therapy or chemoimmunotherapy. Rituximab (375 mg/m2 intravenously) was administered weekly during cycle one and on day 1 of cycles three to 12. Lenalidomide was started on day 9 of cycle one at 10 mg orally and administered daily continuously. Each cycle was 28 days. Rituximab was administered for 12 cycles; lenalidomide could continue indefinitely if patients benefitted clinically.
Results
The overall response rate was 66%, including 12% complete responses and 12% nodular partial remissions. Time to treatment failure was 17.4 months. Median overall survival has not been reached; estimated survival at 36 months is 71%. The most common grade 3 or 4 toxicity was neutropenia (73% of patients). Fourteen patients (24%) experienced a grade 3 to 4 infection or febrile episode. There was one episode of grade 3 tumor lysis; one patient experienced renal failure during the first cycle of therapy, and one venous thromboembolic event occurred during the study.
Conclusion
The combination of lenalidomide and rituximab is active in patients with recurrent CLL and warrants further investigation.
INTRODUCTION
The introduction of fludarabine-based chemoimmunotherapy as first-line therapy for chronic lymphocytic leukemia (CLL) has led to significant improvement in response duration and overall survival (OS).1,2 Chemotherapy combinations including fludarabine, cyclophosphamide, and rituximab (FCR) or bendamustine are effective in relapsed patients3–7; however, patients who have repeated relapses or short response duration after standard chemoimmunotherapy have limited salvage therapy options.8
Although monoclonal antibodies such as alemtuzumab and ofatumumab are effective and approved for patients with relapsed and refractory CLL, as monotherapy, these agents are associated with median progression-free survival (PFS) of generally less than 1 year.9–12 Development of therapies with novel mechanisms of action may increase the options available to this therapeutically challenging population.
Lenalidomide is an immunomodulatory drug with activity in multiple myeloma. Lenalidomide enhances T-cell and natural killer (NK) –cell cytolytic activity in vitro and in vivo.13 In CLL, lenalidomide alters the tumor microenvironment by modulating cytokine production by dendritic cells as well as modifying expression of costimulatory molecules by T cells, potentially repairing defective humoral immunity14–18 and defective T-cell to B-cell synapse formation characteristic of CLL.19,20
Phase II clinical trials have shown that lenalidomide is active in relapsed and refractory CLL. Using a starting dose of 25 mg on a schedule of 21 of 28 days, lenalidomide induced responses in patients with CLL but was associated with tumor lysis syndrome (TLS) and tumor flare reaction (TFR).21 After reducing the starting dose to 5 mg with dose escalation, Chanan-Khan et al21 demonstrated this regimen to be safe and effective. Severe tumor lysis or other toxicity has also been noted by other groups with higher starting doses and rapid dose escalation of lenalidomide.22,23 Our group confirmed the activity and safety of lenalidomide monotherapy administered continuously at a starting dose of 10 mg escalated up to 25 mg per day in heavily pretreated patients with CLL.24
There is synergistic activity between rituximab and lenalidomide against CLL and non-Hodgkin lymphoma cells in vitro. Lenalidomide enhances rituximab-mediated antibody-dependent NK- and T-cell cytotoxicity via improved B-cell synapse formation and upregulation of costimulatory molecules.16 In non-Hodgkin lymphoma, lenalidomide was shown to enhance NK- and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ cells.13 We hypothesized that rituximab administered before lenalidomide could also act as a debulking agent and reduce the rate and severity of TFR. We therefore investigated the activity of the combination of lenalidomide and rituximab in patients with relapsed or refractory CLL.
PATIENTS AND METHODS
This study protocol was approved by The University of Texas MD Anderson Cancer Center institutional review board and registered at clinicaltrials.gov (NCT00759603). Informed consent was obtained in accordance with institutional guidelines and the Declaration of Helsinki.
Patients with relapsed or refractory CLL were enrolled onto a phase II study of lenalidomide and rituximab at the MD Anderson Cancer Center between September 2008 and September 2009. All patients were at least 18 years of age. Patients had a diagnosis of CLL and active disease with indication for therapy in accordance with the 1996 National Cancer Institute working group criteria.25,26 All patients had received prior purine analog-based therapy, except one patient (age 82 years), who had received R-CVP (rituximab plus cyclophosphamide, vincristine, and prednisone). Patients were required to have Eastern Cooperative Oncology Group/WHO performance status ≤ 2 and adequate renal (serum creatinine ≤ 2 mg/dL) and hepatic (serum bilirubin ≤ 2 mg/dL) function. Patients with another malignancy within 3 years of study entry were excluded, with the exception of patients with localized skin, breast, or prostate cancer likely to be cured. Patients with active hepatitis B or C virus, HIV positivity, or a history of tuberculosis and patients with a history of deep venous thrombosis or pulmonary embolism within 6 months of study entry were excluded.
Pretreatment Evaluation
Before initiation of therapy, all patients were assessed by history, physical examination, and peripheral blood studies, including blood count, serum chemistry, thyroid-stimulating hormone, and β2-microglobulin (B2M). Bone marrow aspiration and biopsy were performed before therapy for immunophenotype by flow cytometry for clonality, CD38 and ZAP-70 expression by flow, and immunoglobulin heavy chain variable region (IGHV) gene mutation analysis.27 Genomic abnormalities were detected by fluorescent in situ hybridization (FISH) using standard CLL probes on bone marrow samples (Vysis CLL FISH Probe Kit; Abbott Molecular, Des Plaines, IL). Fludarabine refractoriness was defined as no response or progression within 6 months of the latest fludarabine-containing regimen.
Drug Administration
Rituximab (375 mg/m2) was administered intravenously on days 1, 8, 15, and 22 during cycle one and once every 4 weeks on day 1 for cycles three to 12. Lenalidomide was started on day 9 of cycle one at 10 mg per day and administered continuously. Each cycle of treatment was 28 days. Treatment duration was planned for 12 cycles, although patients could continue lenalidomide indefinitely beyond 12 cycles if there was a significant clinical benefit, such as ongoing partial (PR) or complete response (CR). Allopurinol was administered for the first 14 days of cycle one. Growth factor support was permissible at the investigator's discretion as per American Society of Clinical Oncology guidelines.28 No antibacterial, antiviral, deep venous thrombosis, or tumor flare prophylaxis was mandated.
Dose Adjustment
Lenalidomide dosing could be adjusted for sustained (≥ 7 days) grade 3 to 4 neutropenia or thrombocytopenia. Dose reductions were recommended for grade 3 rash, allergic reaction, or neuropathy. Lenalidomide was discontinued for grade 4 nonhematologic toxicity. Lenalidomide was held for serious (grade ≥ 3) nonhematologic toxicity and could be reinitiated without time limitation at a lower dose level after resolution of toxicity to grade ≤ 2. Dose reduction steps are summarized in Appendix Table A1 (online only).
Response Evaluation
Response was evaluated after three, six, and 12 cycles and every six cycles thereafter. Clinical response was defined as best response obtained with therapy, assessed according to 1996 National Cancer Institute working group25 criteria, including bone marrow aspirate and biopsy evaluation with three-color flow cytometry performed at each assessment. Computed tomography (CT) scans were not required for response assessment but were used if clinically indicated.
Study End Points and Statistical Analysis
The primary end point of this study was overall response rate (ORR) assessed on an intent-to-treat basis. The effects of pretreatment characteristics were compared using Fisher's exact test (two tailed). Differences were considered significant if P < .05. Additional study objectives included time to treatment failure (TTF), defined as time from the start of therapy to death, progression of disease, or initiation of next therapy, and OS. TTF and OS were calculated using Kaplan-Meier estimates including all patients in the study, and survival estimates were compared among subgroups of patients using the log-rank test. All analyses were performed using SPSS (version 19.0; SPSS, Chicago, IL). Treatment-related toxicity was assessed using Common Terminology Criteria for Adverse Events (version 3.0).
RESULTS
Patient Characteristics
The median age of 59 patients enrolled onto this study was 62 years (range, 42 to 82 years); 47% had Rai stage III to IV CLL. The median number of prior treatments received was two (range, one to nine). Other pretreatment characteristics are listed in Table 1. Fifty-eight patients (98%) had received prior rituximab, 58 patients (98%) had received prior fludarabine, including 55 patients (93%) who had received at least one prior purine analog-based chemoimmunotherapy combination (Appendix Table A2, online only). Twelve patients (20%) were refractory to their last fludarabine- containing therapy.
Table 1.
Patient Pretreatment Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 62 | |
| Range | 42-83 | |
| Hemoglobin, g/dL | ||
| Median | 12.2 | |
| Range | 8.8-15.8 | |
| Platelets, ×109/L | ||
| Median | 129 | |
| Range | 22-338 | |
| WBC, ×109/L | ||
| Median | 40.7 | |
| Range | 2.5-190 | |
| Lymphocytes, ×109/L | ||
| Median | 34.4 | |
| Range | 0.4-188 | |
| ANC, ×109/L | ||
| Median | 3.0 | |
| Range | 0.1-28.2 | |
| < 1.5 | 20 | 34 |
| ≥ 1.5 | 39 | 66 |
| β2-microglobulin, mg/L | ||
| Median | 3.5 | |
| Range | 1.5-9.0 | |
| No. of prior treatments | ||
| Median | 2 | |
| Range | 1-9 | |
| 1 | 22 | 37 |
| 2 | 13 | 22 |
| ≥ 3 | 24 | 41 |
| Rai stage | ||
| 0 | 7 | 12 |
| I-II | 24 | 41 |
| III-IV | 28 | 47 |
| Sex | ||
| Female | 13 | 22 |
| Male | 46 | 78 |
| CD38 flow, % (ND = 2)* | ||
| ≥ 30 | 31 | 53 |
| < 30 | 26 | 44 |
| IGHV mutation status (ND = 2) | ||
| Mutated | 14 | 24 |
| Unmutated | 43 | 73 |
| FISH bone marrow (ND = 1) | ||
| 13q deletion | 9 | 15 |
| Negative | 11 | 19 |
| Trisomy 12 | 7 | 12 |
| 11q deletion | 16 | 27 |
| 17p deletion† | 15 | 25 |
| Fludarabine | ||
| Not refractory | 47 | 80 |
| Refractory | 12 | 20 |
Abbreviations: ANC, absolute neutrophil count; FISH, fluorescent in situ hybridization; IGHV, immunoglobulin variable heavy chain; ND, not done.
CD38 expression by flow cytometry on CD19-positive lymphocytes in bone marrow aspirate.
Twelve of 15 patients had > 20% p53-deleted cells (range, 10.5% to 93%).
Efficacy
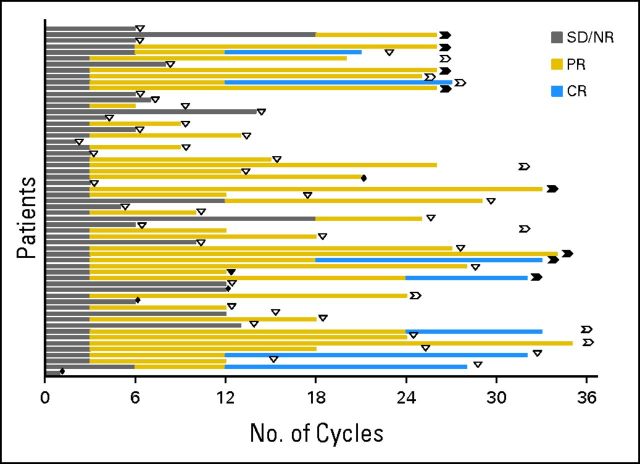
The ORR was 66%, including seven CRs (12%), seven nodular PRs (12%), and 25 PRs (42%). Two patients (3%) had flow cytometry–negative CRs. Response assessment was confirmed by CT in 12 patients (20%). A majority of patients had achieved an objective response (PR or better) by three cycles of therapy (Table 2), whereas all CRs were observed to occur after 12 or more cycles of therapy (Appendix Fig A1, online only).
Table 2.
Response at Each Assessment Point and Best Response to Lenalidomide and Rituximab by Intent to Treat (n = 59)
| NCI-WG Response | Three Cycles |
Six Cycles |
12 Cycles |
Best Response* |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| CR | 0 | 0 | 0 | 0 | 4 | 7 | 7 | 12 |
| Nodular PR | 5 | 8 | 8 | 14 | 8 | 14 | 7 | 12 |
| PR | 27 | 46 | 27 | 46 | 22 | 37 | 25 | 42 |
| ORR | 32 | 54 | 35 | 59 | 34 | 58 | 39 | 66 |
Abbreviations: CR, complete response; NCI-WG, National Cancer Institute Working Group criteria for response; ORR, overall response rate; PR, partial response.
Best response could occur beyond 12 cycles of therapy.
The ORR was 53% for patients with chromosome 17p deletion and did not differ significantly from patients without 17p deletion (ORR, 70%; P = .35). Response to treatment was correlated with prior response to fludarabine, with an ORR of 70% among patients not refractory to their last fludarabine-containing regimen compared with 33% among patients refractory to fludarabine (P = .041). We also noted a nonsignificant trend toward lower ORR in patients with bulky adenopathy (≥ 5 cm) compared with patients without bulky adenopathy (ORR, 40% v 67%, respectively; P = .07). There were no other statistically significant associations between biologic pretreatment characteristics and ORR (Table 3).
Table 3.
ORR According to Pretreatment Characteristics
| Characteristic | No. | ORR (%) | P |
|---|---|---|---|
| All patients | 59 | 66 | |
| Age, years | 1.00 | ||
| < 65 | 34 | 65 | |
| ≥ 65 | 25 | 68 | |
| Rai stage | .18 | ||
| I-II | 31 | 71 | |
| III-IV | 28 | 54 | |
| Maximum lymph node size, cm | .07 | ||
| < 5 | 49 | 67 | |
| ≥ 5 | 10 | 40 | |
| β2-microglobulin, mg/L | .16 | ||
| < 4.0 | 34 | 74 | |
| ≥ 4.0 | 24 | 54 | |
| FISH hierarchy (n = 58) | |||
| 13q deletion | 9 | 67 | |
| Negative | 11 | 55 | |
| Trisomy 12 | 7 | 71 | |
| 11q deletion | 16 | 69 | |
| 17p deletion | 15 | 53 | .35* |
| CD38, % (n = 57) | .57 | ||
| < 30 | 19 | 73 | |
| ≥ 30 | 20 | 65 | |
| IGHV mutation status (n = 57) | .34 | ||
| Mutated | 14 | 79 | |
| Unmutated | 43 | 60 | |
| No. of prior treatments | .16 | ||
| 1-2 | 35 | 74 | |
| ≥ 3 | 24 | 54 | |
| Fludarabine | .041 | ||
| Not refractory | 47 | 70 | |
| Refractory | 12 | 33 |
Abbreviations: FISH, fluorescent in situ hybridization; IGHV, immunoglobulin variable heavy chain; ORR, overall response rate.
17p deletion versus no 17p deletion.
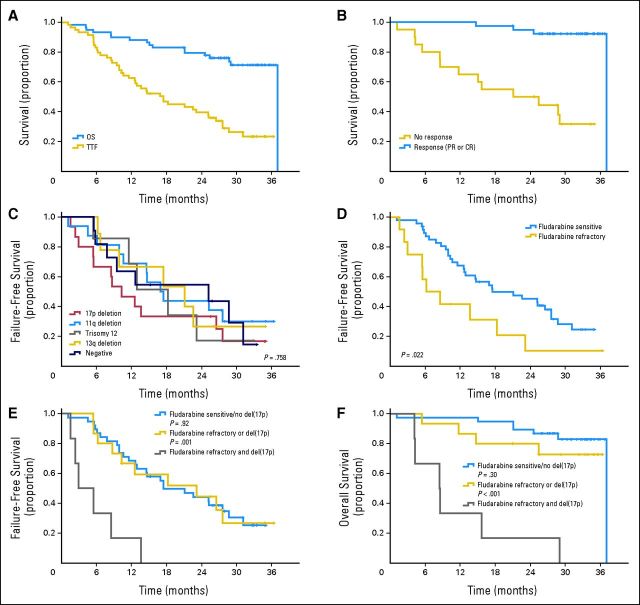
Forty-five patients (76%) have discontinued therapy (Appendix Table A3, online only); 42 patients (71%) had disease progression, required another CLL therapy, or died. The median TTF for all patients was 17.4 months (95% CI, 11.9 to 23.0 months; Fig 1A). The median time to progression for responding patients was 27.6 months (95% CI, 24.6 to 30.6 months). Four (10%) of 39 patients who achieved a response to treatment have died (Fig 1B), three patients after progression of disease and one patient 15 months after the development of del(5q) myelodysplastic syndrome.
Fig 1.
Kaplan-Meier overall (OS) and failure-free survival curves for all study patients. (A) OS and time to treatment failure (TTF) for all patients receiving lenalidomide and rituximab (n = 59), with median 33 months of follow-up. (B) OS for responding patients (complete [CR] or partial response [PR], n = 39) compared with nonresponders (stable or progressive disease, n = 20). (C) TTF according to pretreatment fluorescent in situ hybridization by Döhner cytogenetic hierarchy. (D) TTF by refractoriness to fludarabine. (E) TTF by cytogenetic risk group and prior fludarabine response; patients with 17p deletion and refractory to last fludarabine therapy (n = 6) are compared with patients with only one of these high-risk features (ie, fludarabine refractory or del(17p), n = 15) and patients without these high-risk features (n = 38). (F) OS by cytogenetic risk group and prior fludarabine response comparing the same patient subgroups as in (E); patients who were fludarabine refractory but had no 17p deletion and patients who had 17p deletions but were not refractory to fludarabine had TTF and OS similar to those in patients with neither of these high-risk features; patients who had 17p deletions and were also refractory to fludarabine had significantly shorter TTF and OS compared with all other patients.
We analyzed the impact of biologic prognostic markers on TTF. Elderly patients or patients with advanced Rai stage, bulky lymphadenopathy, high B2M, or unmutated IGHV genes did not experience shorter TTF (data not shown). Although limited by small subgroup size, patients with 17p deletion by FISH did not seem to have significantly shorter TTF than patients in other cytogenetic risk groups (Fig 1C). Response to prior therapy correlated with TTF. Patients who were refractory to their last fludarabine-containing regimen had significantly shorter TTF than those sensitive to fludarabine (Fig 1D). In particular, the combination of fludarabine refractoriness and del(17p) was associated with significantly shorter TTF and OS compared with all other patients, whereas patients with only one of these high-risk characteristics had similar TTF or OS compared with patients without these high-risk characteristics (Figs 1E, 1F).
OS
There have been 17 deaths after a median follow-up for surviving patients of 32.8 months (range, 21.0 to 36.8 months). The median OS for all patients has not been reached (Fig 1A), and the estimated proportion of patients alive at 36 months is 71% (95% CI, 59% to 83%). Four deaths occurred during treatment, including one death after complications of an ischemic stroke, one death after an exacerbation of chronic obstructive pulmonary disease, one death in a patient with Richter's transformation, and one death resulting from an unknown cause. Another two patients died within 6 months of therapy discontinuation, including one patient after subsequent therapy for Richter's transformation and one patient with Pneumocystis jiroveci pneumonia while receiving immunosuppressive therapy for autoimmune hemolytic anemia. The remaining patients died after progression of disease (10 patients) or myelodysplastic syndrome (one patient) a median of 15 months (range, 7 to 24 months) after cessation of treatment with lenalidomide and rituximab.
Toxicity
A total of 1,054 cycles were administered, with a median of 15 cycles of treatment per patient. Hematologic toxicity is summarized in Table 4. Neutropenia was the most common grade 3 or 4 hematologic toxicity experienced at least once by 73% of patients and during 32% of treatment cycles. Twenty-five patients (42%) required granulocyte colony-stimulating factor for neutropenia, and 19 patients (32%) required interruption of therapy lasting a median of 10 days (range, 4 to 31 days). Grade 3 or 4 anemia and thrombocytopenia were less common, occurring during 10% and 3% of treatment cycles, respectively.
Table 4.
Hematologic and Infectious Toxicity
| Toxicity | Grade 3-4 |
Grade 4 Only |
|||||
|---|---|---|---|---|---|---|---|
| Patients |
No. of Episodes | % of Cycles | Patients |
% of Cycles | |||
| No. | % | No. | % | ||||
| Hematologic | |||||||
| Neutropenia | 43 | 73 | 17 | 30 | 51 | 9.0 | |
| Thrombocytopenia | 20 | 34 | 7.4 | 9 | 15 | 2.1 | |
| Anemia | 9 | 15 | 3.0 | 1 | 1.7 | 0.2 | |
| Infection* | |||||||
| Pneumonia/bronchitis | 6 | 10 | 6 | ||||
| Urinary tract | 1 | 2 | 1 | ||||
| Other infection† | 2 | 3 | 2 | ||||
| Any infectious event‡ | 9 | 15 | 9 | ||||
| Fever | |||||||
| Neutropenic fever | 6 | 10 | 6 | ||||
| Febrile, non-neutropenic | 2 | 3 | 3 | ||||
| Any febrile or infectious event§ | 14 | 24 | 18 | ||||
All infectious toxicity was grade ≤ 3.
Other infection: orodental (n = 1), wound (n = 1).
Total of pneumonia/bronchitis, urinary tract, and other infections.
Total of neutropenic and non-neutropenic febrile events and infectious events.
Nine patients (15%) experienced a grade 3 or 4 infectious episode, and eight patients experienced a febrile episode, mostly neutropenic fever (Table 4). Most episodes were lower respiratory tract infections, including pneumonia or bronchitis (six episodes), febrile neutropenia without a documented source (six episodes), or fever without a documented source (three episodes).
Other grade 3 or 4 nonhematologic toxicity was uncommon (Table 5), with the exception of grade 3 fatigue reported by eight patients (14%). One patient experienced grade 3 TLS at the start of therapy; another developed a TFR complicated by grade 4 hypercalcemia, acute renal failure, and myocardial ischemia during the first cycle of therapy and discontinued treatment. Other toxicities (grade 3) included one thromboembolic event and one patient with cardiac arrhythmia. Other events observed in one patient only are listed in Table 5. Grade 1 or 2 TFR was observed in 27% of patients. Other grade 1 or 2 toxicities included fatigue, diarrhea, sensory neuropathy, and rash, as previously described with lenalidomide monotherapy.21,23,24,29
Table 5.
Other Nonhematologic/Noninfectious Toxicity
| Toxicity* | Grade 1-2 |
Grade 3-4 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Renal failure† | 0 | 0 | 1 | 1.7 |
| Acute myocardial infarction† | 0 | 0 | 1 | 1.7 |
| Tumor lysis syndrome | 0 | 0 | 1 | 1.7 |
| Pain (abdomen) | 0 | 0 | 1 | 1.7 |
| Venous thrombosis | 0 | 0 | 1 | 1.7 |
| Weakness | 0 | 0 | 1 | 1.7 |
| Fatigue | 24 | 41 | 8 | 14 |
| Diarrhea | 21 | 36 | 1 | 1.7 |
| Tumor flare | 16 | 27 | 0 | 0 |
| Sensory neuropathy | 14 | 24 | 0 | 0 |
| Rash | 13 | 22 | 0 | 0 |
| Constipation | 11 | 20 | 1 | 1.7 |
| Nausea | 10 | 19 | 0 | 0 |
| Neurologic (other) | 10 | 17 | 0 | 0 |
| Arthralgia | 9 | 17 | 1 | 1.7 |
| Anorexia | 9 | 15 | 0 | 0 |
| Metabolic or laboratory | 9 | 15 | 0 | 0 |
| Hyperglycemia | 8 | 15 | 0 | 0 |
| Pruritus | 8 | 14 | 0 | 0 |
| Elevated serum creatinine | 7 | 14 | 0 | 0 |
| GI pain | 7 | 12 | 0 | 0 |
| Hypomagnesemia | 7 | 12 | 0 | 0 |
| Hyperbilirubinemia | 6 | 12 | 1 | 1.7 |
| Dyspnea | 6 | 10 | 0 | 0 |
| Peripheral edema | 6 | 10 | 0 | 0 |
| Heartburn | 6 | 10 | 0 | 0 |
| Headache | 6 | 10 | 0 | 0 |
Toxicity listed if any grade 3 or 4, or grade 1 or 2 in ≥ 10% of patients.
One patient developed grade 4 acute renal failure after tumor flare reaction complicated by acute myocardial infarction; all other nonhematologic toxicities were grade ≤ 3.
There were five patients with Richter's transformation, either during or after completion of therapy, a median of 8 months (range, 2 to 13 months) after initiation of study. Three of these patients were heavily pretreated and had 17p deletion by FISH at the start of study, one patient was enrolled with bulky adenopathy after four prior regimens including an allogeneic stem-cell transplantation, and one patient had trisomy 12 by FISH with a complex karyotype. We noted four second malignancies during the study: one melanoma in situ, one squamous cell carcinoma of the skin, one patient with recurrence of a head and neck cancer, and one patient with del(5q) myelodysplastic syndrome.
DISCUSSION
This phase II study is the first to our knowledge to demonstrate the effectiveness of lenalidomide combined with rituximab in patients with relapsed or refractory CLL. The majority of responders remained on lenalidomide therapy until failure of therapy or lack of tolerance. Studies of lenalidomide monotherapy in more heavily pretreated patients with CLL have reported ORRs of 32% to 47%.21,24 Although we cannot directly compare outcomes with the monotherapy studies, the ORR of 66% and sustained responses observed suggest a benefit with the addition of rituximab.
The outcomes in this study are comparable to those of commonly used salvage therapies. In a phase II study of FCR as salvage therapy, patients who had received prior fludarabine and alkylating-agent therapy experienced 73% ORR, with median PFS of 19 months.3 The combination of bendamustine and rituximab (BR) is also effective in relapsed and refractory CLL.4,5,7 The ORR after BR was 59%, the CR rate was 9%, and median PFS was 14.7 months.7 Most of our patients had received prior FCR-like therapy; therefore, the combination of lenalidomide and rituximab could be considered in patients for whom standard initial and salvage chemoimmunotherapy has failed.
Patients in this study are not comparable to high-risk fludarabine-refractory or bulky-refractory patients enrolled onto studies of alemtuzumab9,11 or ofatumumab10; however, a significant proportion of patients demonstrated high-risk characteristics such as B2M > 4 mg/L, unmutated IGHV genes, high-risk cytogenetic abnormalitites, and prior exposure to chemoimunotherapy or allogeneic stem-cell transplantation. Adequate responses and response duration were noted in these patients.
Patients with relapsed CLL with 17p deletions represent a particularly high-risk group of patients with limited response to chemotherapy. We noted responses in eight of the 15 patients with del(17p) treated with lenalidomide and rituximab, although del(17p) patients who were also refractory to fludarabine had poor responses to therapy. Two objective responses to lenalidomide monotherapy have been reported among 14 patients (15%) in two studies of patients with relapsed CLL with del(17p).24,30 After BR, only one of 14 patients with del(17p) had an objective response.7 Monoclonal antibody therapy including alemtuzumab or ofatumumab are effective in refractory patients and patients with del(17p) but have generally been associated with median PFS < 12 months in high-risk patients.9–11,31–35 Subgroup analysis was not a primary objective of this study, and although interesting, our results should be interpreted with caution and require confirmation by larger clinical trials with a focus on high-risk CLL populations.
In comparison with the estimated survival of 71% at 3 years in this study, patients treated with FCR as salvage therapy in our center had an approximate survival of 62% at 3 years.3 The estimated median survival was 33.9 months for patients treated with BR. In the current study, the majority of patients had received prior FCR-like therapy, and treatment with lenalidomide and rituximab offered an effective alternative for patients who had relapsed after fludarabine-containing chemoimmunotherapy.36 The survival rate in this study is encouraging and supports further prospective comparison of this combination with commonly used salvage regimens.
Early lenalidomide monotherapy studies in relapsed and refractory CLL demonstrated episodes of severe toxicity, including TLS and TFR.21,22,37 These complications were associated with high lenalidomide starting doses (25 mg) on a schedule of 21 of 28 days. Although the optimal starting dose of lenalidomide has not been clearly established, we attempted to reduce the incidence of TLS and TFR by starting therapy at the low dose of 10 mg on a continuous schedule because we previously established this dose could be safely administered in patients with CLL.24 In addition, we administered rituximab before lenalidomide, aiming to reduce the severity of TFR. With this schedule, we observed one episode of grade 3 TLS, and one patient experienced a grade 4 episode of hypercalcemia and renal failure after a TFR at initiation of lenalidomide therapy. Although our dosing schedule seems safe for administration in this setting, appropriate precautions should be taken against TLS, including antihyperuricemic medication and adequate hydration as per standard recommendations.38 On the basis of phase I data demonstrating safe escalation of lenalidomide to 20 mg,39 a phase III study to establish the optimal starting doses of lenalidomide (5, 10, or 15 mg) is currently ongoing.40
Although clinical responses to therapy occurred early in this treatment regimen, patients who continued to receive lenalidomide therapy demonstrated improvement in quality of response. All complete remissions in this study occurred at or beyond 12 cycles of therapy. The majority of patients with continued responses remained on lenalidomide therapy indefinitely. One of the challenges with continued lenalidomide therapy is persistence of grade 1 to 2 toxicities, leading to patient discontinuation of treatment. These include GI symptoms, fatigue, sensory neuropathy, and neutropenia. Therefore, approaches including patient education and supportive care are important to improve tolerance of long-term treatment with lenalidomide.41
We monitored the occurrence of other malignancies in this study in view of the recent reports of other malignancies after lenalidomide administration in patients with multiple myeloma.42 Of the four patients who developed nonhematologic malignancies, two had a history of malignancy. Increased rates of other malignancies have been well described in patients with CLL.43,44 Because of the size of our study, we are not able to make conclusive statements about the role of lenalidomide in second malignancies. The potential association between lenalidomide and other malignancies should be explored further in larger studies using this therapy.
In our experience, the combination of lenalidomide and rituximab has a role in the treatment of patients with relapsed CLL. With an ORR of 66%, the combination is comparable to currently employed chemotherapy combinations. At a starting dose of 10 mg daily administered continuously, we noted few grade 3 or 4 nonhematologic adverse events. Continued therapy led to durable and some complete remissions. Given these results, we plan to further evaluate the activity of this combination as initial therapy for patients unfit for chemoimmunotherapy and as a partner for novel agents.
Acknowledgment
We thank Susan Lerner, Susan Smith, Manolo Pasia, and Dawn Urbanowsky for their continued contributions to this study and support in data analysis.
Appendix
Fluorescent In Situ Hybridization Analysis Methods
Genomic abnormalities were detected by fluorescent in situ hybridization using standard chronic lymphocytic leukemia probes on bone marrow samples (Vysis CLL FISH Probe Kit; Abbott Molecular, Des Plaines, IL). Patients were defined as having a 17p deletion using the fluorescent in situ hybridization threshold for our probe set (ie, > 6.6%). In this cohort, the percentage of 17p-deleted cells ranged from 10.5% to 93%, and 12 of 15 patients had > 20% 17p-deleted cells.
Response Evaluation
The study was designed before the introduction of the 2008 International Workshop on Chronic Lymphocytic Leukemia criteria for response. We therefore used the 1996 National Cancer Institute–sponsored working group for response assessment, in which the category complete response with cytopenia did not exist. Instead, these patients were considered to have a partial response until evidence of resolution of cytopenia. Bone marrow examination including flow cytometry was performed at each assessment time point and incorporated into response assessment. Computed tomography scans were not required for response assessment but were used if clinically indicated.
Statistical Design
A sample size of 60 patients was chosen to differentiate between a good response rate of 40% and poor response rate of 20%, with 90% power at a significance level of .1. One patient who consented to another study was erroneously included in the database for this study through administrative error and subsequently removed.
Results
Twelve patients (20%) were older than age 70 years. The overall response rate was 67% in patients age ≥ 70 years, which was not significantly different from the overall response rate of 66% in patients age < 70 years.
Duration of Response to Last Treatment
We compared duration of response with lenalidomide and rituximab with the last treatment received. Because 15 patients received their last treatment from an outside institution, the intervals between visits and frequency of restaging tests were highly variable in these patients. On the basis of a retrospective review, the median duration of response following last treatment was 17.5 months (range, 1 to 100 months). Twenty-six of the responding patients (n = 39) had a longer duration of response when compared with their last treatment, and another six patients without evidence of progression had not yet reached the time to progression of the last treatment (Appendix Table A4).
Table A1.
Lenalidomide Dose Reduction Schedule
| Step | Lenalidomide Dose Reduction |
|---|---|
| 0 | 10 mg daily |
| −1 | 5 mg daily |
| −2 | 5 mg every other day or 5 mg for 21 days of 28-day schedule |
| −3 | 2.5 mg daily |
| −4 | 2.5 mg for 21 days of 28-day schedule |
Table A2.
Prior Therapies Received by Patients
| Prior Treatment* | No. | % |
|---|---|---|
| Fludarabine based | ||
| FCR | 46 | 78 |
| CFAR | 5 | 8 |
| PCR | 2 | 3 |
| OFAR | 5 | 8 |
| Fludarabine plus rituximab | 5 | 8 |
| FC | 2 | 3 |
| PC | 1 | 2 |
| Fludarabine monotherapy | 12 | 20 |
| Alkylator based | ||
| Chlorambucil | 7 | 12 |
| Cyclophosphamide ± prednisone | 3 | 5 |
| R-CVP | 1 | 2 |
| R-CHOP | 2 | 3 |
| R-HyperCVAD | 4 | 7 |
| Bendamustine plus rituximab | 1 | 2 |
| R-ICE | 1 | 2 |
| Immunotherapy only | ||
| Rituximab | 14 | 24 |
| Alemtuzumab ± rituximab | 8 | 14 |
| Ofatumumab | 1 | 2 |
| Other | ||
| Stem-cell transplantation | 2 | 3 |
| Experimental | 12 | 20 |
Abbreviations: CFAR, alemtuzumab plus fludarabine, cyclophosphamide, and rituximab; FC, fludarabine and cyclophosphamide; FCR, fludarabine, cyclophosphamide, and rituximab; OFAR, oxaliplatin, fludarabine, cytarabine, and rituximab; PC, pentostatin and cyclophosphamide; PCR, pentostatin, cyclophosphamide, and rituximab; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab plus cyclophosphamide, vincristine, and prednisone; R-HyperCVAD, rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with cytarabine and methotrexate; R-ICE, rituximab plus ifosfamide, carboplatin, and etoposide.
Each patient may have received more than one therapy, and some therapies were administered more than once per patient (counted once).
Table A3.
Discontinuation of Therapy
| Reason for Discontinuation | No. | % |
|---|---|---|
| No response or progression of disease | 19 | 32 |
| Intolerance or adverse event | 18 | 31 |
| Recurrent diarrhea | 4 | 7 |
| Peripheral neuropathy | 4 | 7 |
| URTI/sinus congestion | 3 | 5 |
| Neutropenia | 1 | 2 |
| Cramps | 1 | 2 |
| Syncope | 1 | 2 |
| Shortness of breath | 1 | 2 |
| Seizures | 1 | 2 |
| Infection | 1 | 2 |
| Death during study | 4 | 7 |
| Withdrawal/insurance | 2 | 3 |
| AIHA | 1 | 2 |
| MDS/AML | 1 | 2 |
Abbreviations: AIHA, autoimmune hemolytic anemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; URTI, upper respiratory tract infection.
Table A4.
DOR With Lenalidomide and Rituximab Compared With Patients' Previous Treatment
| Response | No. | % |
|---|---|---|
| Total responders | 39 | 66 |
| DOR longer after lenalidomide and rituximab | 26 | 44 |
| DOR shorter after lenalidomide and rituximab | 7 | 12 |
| Ongoing response | 6 | 10 |
Abbreviation: DOR, duration of response.
Fig A1.
Time to first response and response duration with lenalidomide and rituximab. Individual patients are graphed on ordinate, and time measured in cycles of therapy on abscissa. Gray bars demonstrate time without objective response (stable disease [SD]/nonresponse [NR]); gold bars indicate partial response (PR) or nodular PR; blue bars indicate complete response (CR). Open triangles represent loss of response off therapy; solid triangles represent loss of response on therapy. Open chevrons demonstrate continued response off therapy; solid chevrons demonstrate continued response on therapy. Solid diamonds demonstrate treatment cessation because of death.
Footnotes
Presented in part at the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, December 10-13, 2011, and the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 4-7, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00759603.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Michael J. Keating, Celgene (C); William G. Wierda, Celgene (C), Genentech (C); Susan M. O'Brien, Celgene (C) Stock Ownership: None Honoraria: None Research Funding: Alessandra Ferrajoli, Celgene, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Michael J. Keating, Alessandra Ferrajoli
Provision of study materials or patients: Michael J. Keating, William G. Wierda, Susan M. O′Brien, Stefan Faderl, Jan A. Burger, Alessandra Ferrajoli
Collection and assembly of data: Stefan Faderl, Alessandra Ferrajoli
Data analysis and interpretation: Xavier C. Badoux, Michael J. Keating, Sijin Wen, William G. Wierda, Susan M. O'Brien, Rachel Sargent, Jan A. Burger, Alessandra Ferrajoli
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann MA, Goebeler ME, Herold M, et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase I/II study of the German CLL Study Group. Haematologica. 2005;90:1357–1364. [PubMed] [Google Scholar]
- 5.Iannitto E, Morabito F, Mancuso S, et al. Bendamustine with or without rituximab in the treatment of relapsed chronic lymphocytic leukaemia: An Italian retrospective study. Br J Haematol. 2011;153:351–357. doi: 10.1111/j.1365-2141.2011.08597.x. [DOI] [PubMed] [Google Scholar]
- 6.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 7.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: A multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 8.Tsimberidou AM, Keating MJ. Treatment of patients with fludarabine-refractory chronic lymphocytic leukemia: Need for new treatment options. Leuk Lymphoma. 2010;51:1188–1199. doi: 10.3109/10428194.2010.486089. [DOI] [PubMed] [Google Scholar]
- 9.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 10.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stilgenbauer S, Zenz T, Winkler D, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: Clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27:3994–4001. doi: 10.1200/JCO.2008.21.1128. [DOI] [PubMed] [Google Scholar]
- 12.Keating M, Coutré S, Rai K, et al. Management guidelines for use of alemtuzumab in B-cell chronic lymphocytic leukemia. Clin Lymphoma. 2004;4:220–227. doi: 10.3816/clm.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 14.Aue G, Njuguna N, Tian X, et al. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009;94:1266–1273. doi: 10.3324/haematol.2009.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 16.Lapalombella R, Andritsos L, Liu Q, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115:2619–2629. doi: 10.1182/blood-2009-09-242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BN, Gao H, Cohen EN, et al. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117:3999–4008. doi: 10.1002/cncr.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica. 2009;94:1198–1202. doi: 10.3324/haematol.2009.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 22.Andritsos LA, Johnson AJ, Lozanski G, et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008;26:2519–2525. doi: 10.1200/JCO.2007.13.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CI, Bergsagel PL, Paul H, et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011;29:1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 26.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute working group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 28.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 29.Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489–3498. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sher T, Miller KC, Lawrence D, et al. Efficacy of lenalidomide in patients with chronic lymphocytic leukemia with high-risk cytogenetics. Leuk Lymphoma. 2010;51:85–88. doi: 10.3109/10428190903406806. [DOI] [PubMed] [Google Scholar]
- 31.Knauf W, Rieger K, Blau W, et al. Remission induction using alemtuzumab can permit chemotherapy-refractory chronic lymphocytic leukemia (CLL) patients to undergo allogeneic stem cell transplantation. Leuk Lymphoma. 2004;45:2455–2458. doi: 10.1080/10428190400005346. [DOI] [PubMed] [Google Scholar]
- 32.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 34.Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma. 2007;48:2412–2417. doi: 10.1080/10428190701724801. [DOI] [PubMed] [Google Scholar]
- 35.Castro JE, Sandoval-Sus JD, Bole J, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia. 2008;22:2048–2053. doi: 10.1038/leu.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keating MJ, Wierda WG, Tam CS, et al. Long-term outcome following treatment failure of FCR chemoimmunotherapy as initial therapy for chronic lymphocytic leukemia. Blood. 2009:114. abstr 2381. [Google Scholar]
- 37.Moutouh-de Parseval LA, Weiss L, DeLap RJ, et al. Tumor lysis syndrome/tumor flare reaction in lenalidomide-treated chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5047. doi: 10.1200/JCO.2007.14.2141. [DOI] [PubMed] [Google Scholar]
- 38.Coiffier B, Altman A, Pui CH, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: An evidence-based review. J Clin Oncol. 2008;26:2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 39.Wendtner C, Hillmen P, Mahadevan D, et al. Final results of the phase I study of lenalidomide in patients with relapsed/refractory chronic lymphocytic leukemia (CLL-001 study) Blood. 2010:116. doi: 10.3109/10428194.2011.618232. abstr 1376. [DOI] [PubMed] [Google Scholar]
- 40.Wendtner C, Fraser G, Goldberg SL, et al. Interim results for the safety and efficacy of different lenalidomide starting dose regimens in subjects with relapsed or refractory chronic lymphocytic leukemia (CC-5013-CLL-009 study) Blood. 2011:118. abstr 2859. [Google Scholar]
- 41.Miller KC, Musial L, Whitworth A, et al. Management of patients with chronic lymphocytic leukemia treated with lenalidomide. Clin J Oncol Nurs. 2010;14:491–499. doi: 10.1188/10.CJON.491-499. [DOI] [PubMed] [Google Scholar]
- 42.Dimopoulos MA, Richardson PG, Brandenburg N, et al. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood. 2012;119:2764–2767. doi: 10.1182/blood-2011-08-373514. [DOI] [PubMed] [Google Scholar]
- 43.Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: Differences by lymphoma subtype. J Clin Oncol. 2010;28:4935–4944. doi: 10.1200/JCO.2010.29.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]