Abstract
Purpose
In phase I/II trials, the cytotoxic T lymphocyte–associated antigen-4–blocking monoclonal antibody tremelimumab induced durable responses in a subset of patients with advanced melanoma. This phase III study evaluated overall survival (OS) and other safety and efficacy end points in patients with advanced melanoma treated with tremelimumab or standard-of-care chemotherapy.
Patients and Methods
Patients with treatment-naive, unresectable stage IIIc or IV melanoma were randomly assigned at a ratio of one to one to tremelimumab (15 mg/kg once every 90 days) or physician's choice of standard-of-care chemotherapy (temozolomide or dacarbazine).
Results
In all, 655 patients were enrolled and randomly assigned. The test statistic crossed the prespecified futility boundary at second interim analysis after 340 deaths, but survival follow-up continued. At final analysis with 534 events, median OS by intent to treat was 12.6 months (95% CI, 10.8 to 14.3) for tremelimumab and 10.7 months (95% CI, 9.36 to 11.96) for chemotherapy (hazard ratio, 0.88; P = .127). Objective response rates were similar in the two arms: 10.7% in the tremelimumab arm and 9.8% in the chemotherapy arm. However, response duration (measured from date of random assignment) was significantly longer after tremelimumab (35.8 v 13.7 months; P = .0011). Diarrhea, pruritus, and rash were the most common treatment-related adverse events in the tremelimumab arm; 7.4% had endocrine toxicities. Seven deaths in the tremelimumab arm and one in the chemotherapy arm were considered treatment related by either investigators or sponsor.
Conclusion
This study failed to demonstrate a statistically significant survival advantage of treatment with tremelimumab over standard-of-care chemotherapy in first-line treatment of patients with metastatic melanoma.
INTRODUCTION
Tremelimumab (CP-675206) is an immunoglobulin (Ig) G2 cytotoxic T lymphocyte–associated antigen-4 (CTLA4) –blocking monoclonal antibody that has been tested in clinical trials in patients with cancer. Antibodies that block CTLA4 antagonize the effects of this coinhibitory receptor on immune responses to tumor and self-antigens, leading to immune stimulation. In early clinical trials, a subset of patients achieved objective tumor responses, many of which proved to be extremely durable, with follow-up for up to 10 years from the start of phase I testing.1 The most common toxicities were skin rash and diarrhea, with a small percentage of patients experiencing endocrine abnormalities such as thyroiditis and hypophysitis.1,2
The tremelimumab dosing regimen of 15 mg/kg once every 90 days used in the current study was chosen based on prior preclinical and clinical data. Preclinical studies have reported observation of biologic activity (enhancement of in vitro interleukin-2 production by peripheral blood mononuclear cells) at 10- to 30-μg/mL concentrations. Pharmacokinetic data from phase I and II trials have shown that tremelimumab has a long plasma half-life of 22 days,1 and concentrations > 10 to 30 μg/mL can be sustained for 2 to 3 months after a single dose of 15 mg/kg.3,4 A phase II study was conducted to test the regimens of 10 mg/kg once per month and 15 mg/kg once every 3 months. There was no apparent difference between these dosing regimens in terms of response rate or survival, whereas there was a trend toward increased toxicity with the regimen of 10 mg/kg once per month, leading to the selection of 15 mg/kg once every 3 months as the pivotal trial dosing regimen.2
Subsequently, a single-arm pivotal phase II clinical trial with central radiologic review was conducted in patients with previously treated metastatic melanoma (n = 251). The response rate was 9.1% per investigator and 6.6% per independent radiologic review; the study failed to reject the null hypothesis that the response rate would not exceed 10%.5
At the time of design of our study, dacarbazine (DTIC) was the standard reference therapy for patients with metastatic melanoma. Oral temozolomide and intravenous (IV) DTIC are both prodrugs for the same active antitumor metabolite. Although temozolomide is not approved for patients with melanoma, it was commonly used for this indication in some countries. In a randomized phase III study comparing temozolomide with DTIC, no statistically significant difference in overall survival (OS) or response rate was found.6 Therefore, temozolomide was included as a treatment option for investigators who used this drug in their standard practice for melanoma.
PATIENTS AND METHODS
Study Population
Patients age ≥ 18 years with stage IIIc or IV melanoma considered to be surgically incurable were eligible if they had measurable or evaluable disease as defined by RECIST guidelines.7 Eligible patients had an Eastern Cooperative Oncology Group performance status of ≤ 1; serum lactate dehydrogenase (LDH) ≤ 2× the upper limit of normal (ULN) at screening; and adequate bone marrow, hepatic, and renal function. Patients with a history of brain metastases were excluded based on baseline computed tomography or magnetic resonance imaging. Patients with uveal melanoma were also excluded. This study was conducted according to the Declaration of Helsinki and its amendments and relevant International Conference on Harmonisation Good Clinical Practice guidelines and with preliminary approval by the local institutional review board, independent ethics committee, or research ethics board of all participating study sites. All study participants provided written informed consent before participating in the trial. This clinical trial was registered with clinicaltrials.gov.
Study Design
In this phase III open-label randomized comparative study, patients were randomly assigned at a ratio of one to one to one of two treatment arms (tremelimumab v chemotherapy). The primary end point was OS. Secondary end points included progression-free survival (PFS), best overall response, duration of response, PFS at 6 months after random assignment, and safety. Random assignment was stratified by disease stage (stage IIIC v IV; M1a, M1b v M1c) and presence or absence of measurable lesions. Tremelimumab at 15 mg/kg was administered by IV infusion once every 90 days for up to four cycles. Tremelimumab mechanism of action involves stimulation of an immune response, and there is an expected lag period before an effective immune response is initiated. Therefore, patients with evidence of disease progression at the first tumor assessment were allowed to continue to receive tremelimumab if they did not have clinical signs or symptoms of progression. No dose reductions were permitted; however, dose delays were permitted to allow recovery from potential treatment-related toxicity. Patients randomly assigned to the standard-of-care arm received either single-agent DTIC (1,000 mg/m2) IV on day 1 of a 21-day cycle or single-agent temozolomide (200 mg/m2) orally on days 1 to 5 of a 28-day cycle. Choice of chemotherapeutic agent was at the discretion of the investigator. Chemotherapy was administered for up to 12 cycles or until disease progression, unacceptable toxicity, or withdrawal of consent. Dose reductions or delays were permitted in this cohort. Crossover to the tremelimumab cohort was not allowed for patients who progressed after treatment with DTIC or temozolomide.
Study Assessments
Tumor responses were assessed every 90 days (one cycle) in patients treated with tremelimumab, every 42 days (two cycles) in patients treated with DTIC, and every 56 days (two cycles) in patients treated with temozolomide. In both study arms, there was a planned assessment of tumor response at 6 months to determine PFS rate at this time point. Tumor data assessed by investigators were reviewed by the sponsor to ensure compliance with RECIST criteria. Patients were evaluated for toxicity at every scheduled visit, and any toxicities were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.7
Statistical Analysis
A total of 537 events (deaths) was required to provide 90% power for a two-sided log-rank test to reject the null hypothesis of no difference in survival at a .045 significance level when true hazard ratio (HR) was 0.75 in favor of tremelimumab. A total of 630 patients was required for enrollment to achieve 537 events. Two equally spaced interim analyses based on Lan-DeMets alpha spending function using O'Brien-Fleming–type boundaries for efficacy or futility were planned when approximately one third and two thirds of events had been observed. OS results for patients in the two arms were compared using an unstratified log-rank test.
RESULTS
Patients
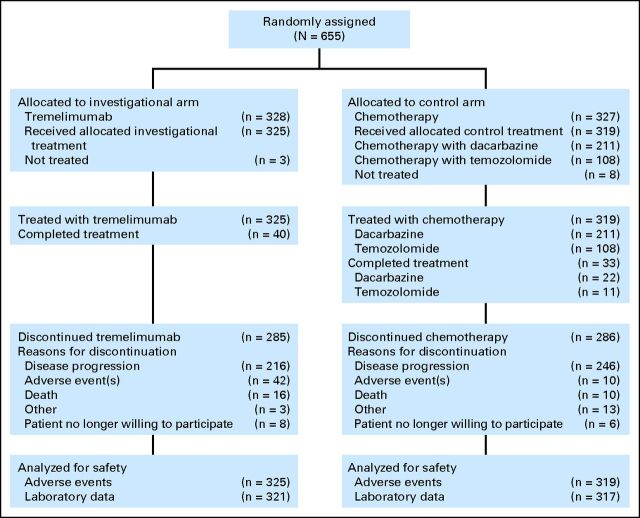
Between March 2006 and July 2007, 655 patients at 114 sites in 24 countries were randomly assigned to treatment with tremelimumab (n = 328) or chemotherapy (n = 327; Fig 1). Demographics and baseline characteristics were similar between treatment arms (Table 1). In the tremelimumab and chemotherapy arms, mean ages were 57 and 56 years, respectively, and 93% of patients were white. Most patients (95%) had stage IV disease; 58% had stage M1c. Serum LDH was elevated in 30% of patients and was > 2× ULN in 5% of patients at the start of the study, despite the requirement for LDH to be < 2× ULN at screening. Only 6% of patients had nonmeasurable disease.
Fig 1.
CONSORT diagram. Randomly assigned patients were stratified by measurability of disease and disease stage.
Table 1.
Baseline Patient Demographic and Clinical Characteristics
| Characteristic | Tremelimumab (n = 328) |
Chemotherapy (n = 327) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Male | 190 | 58 | 182 | 56 |
| Female | 138 | 42 | 145 | 44 |
| Race | ||||
| White | 304 | 93 | 304 | 93 |
| Black | 0 | 0 | 0 | 0 |
| Asian | 0 | 0 | 0 | 0 |
| Other | 8 | 2 | 4 | 1 |
| Not reported | 16 | 5 | 19 | 6 |
| Age, years | ||||
| Mean | 57 | 56 | ||
| Range | 22-90 | 22-90 | ||
| ≥ 65 | 110 | 34 | 90 | 28 |
| ECOG PS | ||||
| 0 | 222 | 68 | 227 | 69 |
| 1 | 101 | 31 | 90 | 28 |
| Disease stage | ||||
| IIIC | 19 | 6 | 14 | 4 |
| IV M1a | 46 | 14 | 50 | 15 |
| IV M1b | 75 | 23 | 69 | 21 |
| IV M1c | 188 | 57 | 194 | 59 |
| LDH | ||||
| 1 to 2× ULN | 84 | 26 | 87 | 27 |
| > 2× ULN | 15 | 5 | 19 | 6 |
| Nonmeasurable disease | 20 | 6 | 17 | 5 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Median duration of treatment was 3.0 months (range, 0.1 to 13.5 months) with tremelimumab and 2.2 months (range, 0.3 to 12.7 months) with chemotherapy. Subsequent therapy was reported in 200 patients (61%) in the tremelimumab arm and in 217 patients (66%) in the chemotherapy arm; 46 of the patients in the control arm (14%) reported receiving ipilimumab. Five additional patients reported enrolling onto a blinded trial that randomly assigned patients at a ratio of three to one to ipilimumab-containing treatment arms. However, this is likely to be an underestimate of the number of patients who actually received ipilimumab, because some patients received ipilimumab at sites other than the investigational site, which may have been underreported.
Efficacy
At the planned second interim analysis, after 340 deaths had occurred (March 28, 2008, 8 months after the last patient had entered the study), median OS was 11.8 months (95% CI, 10.3 to 13.9 months) in the tremelimumab arm and 10.7 months (95% CI, 9.3 to 11.9 months) in the chemotherapy arm (HR, 0.96; P = .73). The data safety monitoring board determined that the test statistic had crossed the prespecified futility boundary. However, follow-up for survival was continued.
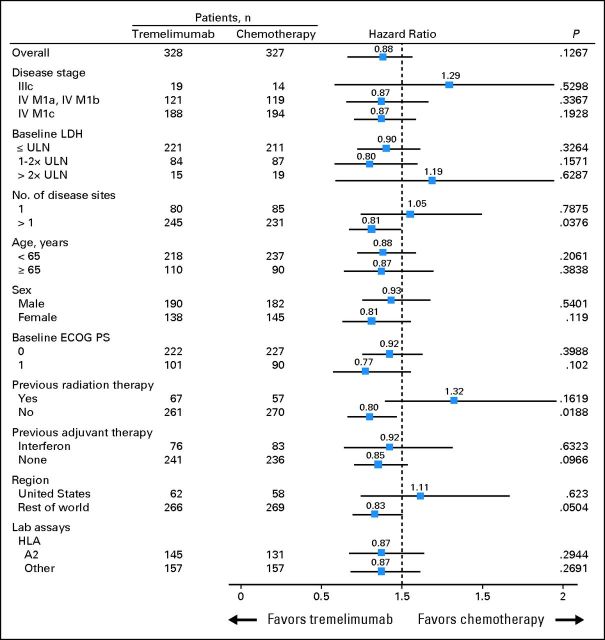
The final study analysis was performed in October 2010, when 534 events (82%) had occurred. Median OS by intent to treat was 12.6 months (95% CI, 10.8 to 14.3 months) in the tremelimumab arm and 10.7 months (95% CI, 9.4 to 12.0 months) in the chemotherapy arm (HR, 0.88; P = .127; Fig 2A). Survival at 2 and 3 years was 26.4% (95% CI, 22.0% to 31.7%) and 20.7% (95% CI, 16.7% to 25.6%), respectively, in patients treated with tremelimumab and 22.7% (95% CI, 18.5% to 27.8%) and 17.0% (95% CI, 13.3% to 21.7%), respectively, in patients in the chemotherapy arm. Subset analysis of survival is presented in Figure 3. There was no apparent association of baseline characteristics such as age, HLA A2, or disease substage with treatment effect of tremelimumab compared with chemotherapy. There was a trend toward a more favorable treatment effect of tremelimumab compared with chemotherapy in patients with the following markers of advanced disease: Eastern Cooperative Oncology Group performance status 1 versus 0, baseline LDH 1 to 2× ULN compared with ≤ ULN, and > one disease site compared with one disease site.
Fig 2.
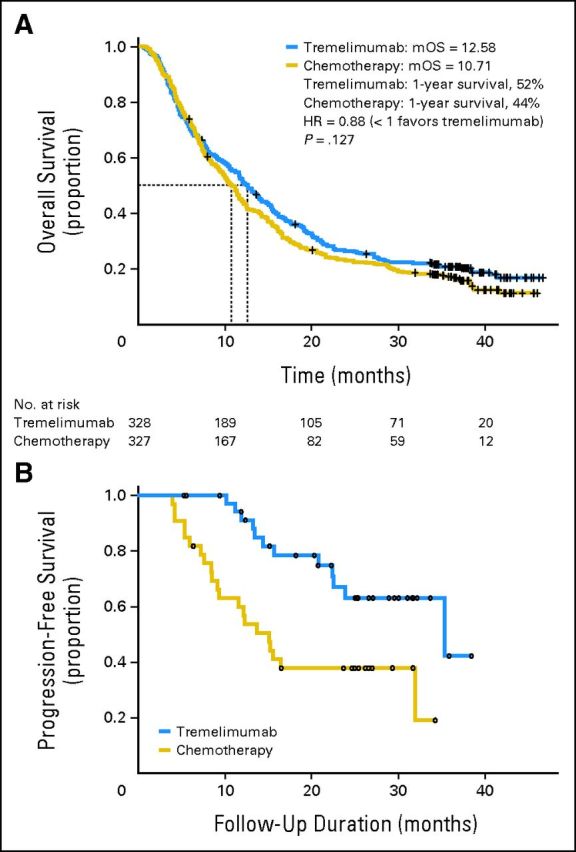
Kaplan-Meier estimate of (A) overall survival (primary end point) and (B) duration of objective responses from date of random assignment (secondary end point). HR, hazard ratio; mOS, months overall survival.
Fig 3.
Exploratory analysis of factors associated with overall survival; forest plot of final data. One additional planned analysis category (ie, measurable disease) was removed because the vast majority of patients had measurable disease. ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; ULN, upper limit of normal.
The objective response rates based on investigator assessment were similar in both study arms: 10.7% in the tremelimumab arm and 9.8% in the chemotherapy arm (Table 2). There were no significant differences between study arms in rate of complete or partial response. However, a majority of responses to tremelimumab were durable. Median response duration (defined as time from random assignment to progression or death resulting from disease progression for the objective responders) was significantly longer among tremelimumab responders compared with chemotherapy responders: 35.8 months (range, 5.6 to 44.3 months) versus 13.7 months (range, 4.0 to 44.3 months; P = .0011; Fig 2B). The probability of PFS at 6 months was similar in the two arms: 20.3% (95% CI, 15.9% to 24.6%) in the tremelimumab arm and 18.1% (95% CI, 13.9% to 22.3%) in the chemotherapy arm.
Table 2.
Responses to Therapy and PFS
| Response/PFS | Tremelimumab (n = 328) |
Chemotherapy (n = 327) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Response to therapy | |||||
| CR | 11 | 3 | 8 | 2 | .489 |
| PR | 25 | 8 | 24 | 7 | |
| Objective response (CR plus PR) | 36 | 11 | 32 | 10 | .618 |
| 95% CI | 7.8 to 14.9 | 6.8 to 13.5 | |||
| Kaplan-Meier estimate of PFS at 6 months | |||||
| Patients with events | 308 | 94 | 309 | 95 | |
| 6-month PFS | 20.3 | 18.1 | .477 | ||
Abbreviations: CR, complete response; PFS, progression-free survival; PR, partial response.
Safety
The most common adverse events (AEs) related to tremelimumab were GI events, dermatologic events, and fatigue; common AEs related to chemotherapy included GI events, hematologic events, and fatigue (Table 3). Among AEs related to treatment, the only grade ≥ 3 events reported in ≥ 10% of patients were diarrhea (14%) in the tremelimumab arm and neutropenia (10%) in the chemotherapy arm. In the tremelimumab arm, the median time for onset of diarrhea was 23 days. The incidence of rash was also higher among patients treated with tremelimumab; median onset of rash was at day 15.
Table 3.
Treatment-Emergent AEs of All Causality
| AE | Tremelimumab (n = 325)* |
Chemotherapy (n = 319)* |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Any AE | 312 | 96 | 292 | 92 |
| Any grade 3 or 4 AE | 170 | 52 | 119 | 37 |
| Any serious AE | 121 | 37 | 50 | 16 |
| Any grade 5 AE | 22 | 7 | 13 | 4 |
| Discontinued treatment because of AEs | 43 | 13 | 10 | 3 |
| AE | Tremelimumab (n = 325)* |
Chemotherapy (n = 319)* |
||||||
|---|---|---|---|---|---|---|---|---|
| Any Grade |
Grade≥ 3 |
Any Grade |
Grade≥ 3 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Treatment-emergent AE of any causality in ≥ 10% of patients | ||||||||
| Diarrhea/colitis | 166 | 51 | 60 | 18 | 56 | 18 | 6 | 2 |
| Nausea | 109 | 34 | 14 | 4 | 158 | 50 | 10 | 3 |
| Fatigue | 106 | 33 | 19 | 6 | 118 | 37 | 5 | 2 |
| Rash | 106 | 33 | 7 | 2 | 17 | 5 | 1 | < 1 |
| Pruritus | 100 | 31 | 3 | 1 | 16 | 5 | 0 | 0 |
| Vomiting | 74 | 23 | 14 | 4 | 92 | 29 | 9 | 3 |
| Decreased appetite | 67 | 21 | 14 | 4 | 40 | 13 | 1 | < 1 |
| Thrombocytopenia | 5 | 2 | 1 | < 1 | 63 | 20 | 26 | 8 |
| Pyrexia | 53 | 16 | 4 | 1 | 27 | 9 | 0 | 0 |
| Abdominal pain | 68 | 21 | 12 | 4 | 35 | 11 | 3 | 1 |
| Neutropenia | 2 | 1 | 1 | < 1 | 50 | 16 | 34 | 11 |
| Constipation | 48 | 15 | 2 | 1 | 102 | 32 | 2 | 1 |
| Cough | 48 | 15 | 1 | < 1 | 28 | 9 | 0 | 0 |
| Dyspnea | 43 | 13 | 8 | 3 | 26 | 8 | 2 | 1 |
| Headache | 37 | 11 | 2 | 1 | 42 | 13 | 1 | < 1 |
| Weight decrease | 36 | 11 | 1 | < 1 | 10 | 3 | 1 | < 1 |
| Asthenia | 0 | 0 | 0 | 0 | 34 | 11 | 5 | 2 |
| Peripheral edema | 32 | 10 | 5 | 2 | 18 | 6 | 1 | < 1 |
| Treatment-emergent AEs of special interest | ||||||||
| Thyroid disorders | 17 | 5 | 2 | 1 | 2 | 1 | 0 | 0 |
| Hypothalamus and pituitary disorders | 6 | 2 | 4 | 1 | 0 | 0 | 0 | 0 |
| Adrenal insufficiency | 4 | 1 | 3 | 1 | 0 | 0 | 0 | 0 |
| Ocular infections, irritations, or inflammation | 13 | 4 | 0 | 0 | 3 | 1 | 0 | 0 |
| Hepatitis | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 |
| Pancreatitis | 3 | 1 | 3 | 1 | 0 | 0 | 0 | 0 |
Abbreviation: AE, adverse event.
Patients who were evaluable for AEs.
Twenty patients in the tremelimumab arm and 21 in the chemotherapy arm reported grade 4 AEs attributed to study treatment. The only grade 4 AEs that were reported in more than one patient included colitis (n = 2) in the tremelimumab arm and pancytopenia (n = 3), thrombocytopenia (n = 8), and neutropenia (n = 11) in the chemotherapy arm.
Because of the presumed mechanism of action for tremelimumab, the incidence of certain infrequent AEs of special significance was scrutinized. In the tremelimumab arm, 24 patients (7%) had endocrine events, including 17 patients (5%) with thyroid disorders, six (2%) with hypothalamic or pituitary gland disorders, and four (1%) with adrenal insufficiency (Table 3). In the chemotherapy arm, the only reported endocrine disorders were in two patients with goiter. Hepatitis and pancreatitis were reported by < 1% of patients in the tremelimumab arm and not reported in the chemotherapy arm.
There were seven deaths (2%) considered by either study investigators or sponsor to be related to tremelimumab treatment; causes of these deaths (n = 1 each) were cardiac arrest (day 159), pneumonia (day 71), septic shock (day 110), electrolyte imbalance (day 38), pulmonary embolism (day 69), hemorrhage (day 48), and large intestine perforation (day 24). One patient (< 1%) in the chemotherapy arm died as a result of treatment-related pneumonia on day 39.
Forty-three patients discontinued tremelimumab and 10 patients discontinued chemotherapy because of AEs. Of these, 39 and eight patients, respectively, discontinued treatment because of treatment-related AEs. A majority of AEs leading to discontinuation in the tremelimumab arm were GI related.
DISCUSSION
The final survival results of this phase III study comparing single-agent tremelimumab with chemotherapy in patients with metastatic melanoma naive to previous systemic therapy failed to demonstrate a statistically significant survival advantage with tremelimumab over chemotherapy. The rate of objective tumor response was also similar in both arms, but duration of response was significantly longer after tremelimumab. The durable responses seen in this trial confirm that a subset of patients may derive benefit from treatment with tremelimumab. Prolonged responses in a minority of patients were consistent with the effect of other types of immunotherapy, such as high-dose interleukin-2.8 Subset analysis by predefined baseline demographic and disease factors did not identify a factor that selects for benefit from tremelimumab compared with chemotherapy.
Since the trial described in this report was initiated, the standard of care for melanoma has changed. Ipilimumab (Yervoy [previously MDX-010]; Bristol-Myers Squibb, New York, NY), a CTLA4-blocking IgG1 monoclonal antibody, and the BRAF inhibitor vemurafenib (Zelboraf [previously PLX4032]; Genentech, South San Francisco, CA) have recently been approved in several countries, including the United States, the European Union, and Australia. A pivotal single-arm phase II study of ipilimumab at 10 mg/kg once every 3 weeks in previously treated patients with metastatic melanoma showed a response rate similar to that of the tremelimumab pivotal phase II trial (5.8% per independent review, 8% per investigator) and also failed to reject the null hypothesis of a 10% response rate.9 However, in a landmark study, ipilimumab (single agent or combined with an investigational gp100 peptide vaccine) administered at a lower dose of 3 mg/kg once per month demonstrated improvement in OS of patients with previously treated metastatic melanoma compared with the gp100 peptide vaccine.10–12 The objective response rates in the two ipilimumab-containing arms were 5.7% and 10.9%. A second phase III clinical trial of ipilimumab, in this case combined with DTIC and compared with single-agent DTIC, in first-line treatment of patients with metastatic melanoma also demonstrated a survival advantage in the ipilimumab combination therapy arm.13
An important difference in the pivotal trials of ipilimumab and tremelimumab was that the tremelimumab clinical trials excluded patients with LDH > 2× ULN, whereas baseline LDH was not an exclusion criterion for the ipilimumab pivotal trials. It is possible that this patient selection criterion may have resulted in an enrichment of tumor responses and improved outcome in the control arm of our study and thus decreased the survival difference between the two study arms. This phenomenon can be noted in the analysis of the forest plot in Figure 3, wherein there is a trend toward better HR for patients with markers of more advanced disease, including LDH 1 to 2× ULN compared with ≤ ULN.
As an open-label study, ours was vulnerable to unintended crossover of patients in the control chemotherapy arm to ipilimumab. Because survival was the primary end point, crossover to tremelimumab was not allowed within the protocol for this study. However, during the conduct of this trial, ipilimumab became widely available to patients in the comparator group, both in clinical trials (NCT00094652) and through a worldwide expanded-access program (NCT00495066). In contrast, patients in the control groups of ipilimumab randomized phase III studies were excluded from all tremelimumab (Data Supplement) and ipilimumab trials and from the ipilimumab expanded-access program.12,13 Use of CTLA4 blockade in both arms of this study could have decreased the power of the study to demonstrate a statistically significant difference in survival and biased the estimates of survival in the control arm. Centralized monitored data collection for this clinical trial captured an approximate 16% use of ipilimumab in the control arm, but this is likely an underestimate. An indirect readout of the impact of this crossover to the other anti-CTLA4 antibody is the strong trend toward better outcome for the control arm in patients treated in the United States (Fig 3), whereas there is an opposite trend in favor of tremelimumab when analyzing patients from the rest of the world (HR, 0.83; P = .504), where there were many more sites at which the ipilimumab programs were not available.
In conclusion, tremelimumab induces a low-frequency but reproducible durable response rate in patients with metastatic melanoma. However, this study did not demonstrate a statistically significant improvement in OS over first-line treatment with standard-of-care chemotherapy. Patient selection, dosing regimen, and use of another CTLA4-blocking agent (ipilimumab) as salvage therapy for patients in the comparator arm may explain the differences between the results of this phase III trial and those of two positive phase III trials with ipilimumab.
Supplementary Material
Acknowledgment
We thank all of the investigators and staff at the participating sites in this study as well as the patients and their families and Tamara Fink, PhD, Accuverus, a division of ProEd Communications, for editorial/medical writing support.
Footnotes
Supported by Pfizer.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008, and 25th Annual Meeting of the International Society for Biological Therapy of Cancer, Washington, DC, October 2-4, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00257205.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Margaret A. Marshall, Pfizer (C); Jesus Gomez-Navarro, Pfizer (C); Bo Huang, Pfizer (C); Dmitri Pavlov, Pfizer (C) Consultant or Advisory Role: Antoni Ribas, Amgen (C), Bristol-Myers Squibb (C), Genentech (C), GlaxoSmithKline (C), Millennium Pharmaceuticals (C), Novartis(C), Pfizer (C); Richard Kefford, Pfizer (C); John B. Haanen, Pfizer (C); Claus Garbe, Amgen (C), Bristol-Myers Squibb (C), Genta (C), GlaxoSmithKline (C), MSD (C), Philogen (C), Roche (C), SOBI (C); Helen Gogas, Bristol-Myers Squibb (C), Merck (C); Paul Lorigan, Pfizer (C); Uwe Trefzer, Bristol-Myers Squibb (C), GlaxoSmithKline (C), MSD (C), Roche (C); Michael Smylie, Bristol-Myers Squibb (C), Roche (C); Grant A. McArthur, Bristol-Myers Squibb (U), GlaxoSmithKline (U), Plexikkon (U), Roche (U); Brigitte Dreno, Pfizer (C); John M. Kirkwood, GlaxoSmithKline (C), Merck (C), Novartis (C); Axel Hauschild, Bristol-Myers Squibb (C), Celgene (C), Eisai (C), GlaxoSmithKline (C), MSD (C), Novartis (C), Pfizer (C), Roche (C) Stock Ownership: Margaret A. Marshall, Pfizer; Bo Huang, Pfizer Honoraria: Claus Garbe, Amgen, Bristol-Myers Squibb, Genta, GlaxoSmithKline, MSD, Philogen, Roche, SOBI; Gerald Linette, Bristol-Myers Squibb, Genentech, GlaxoSmithKline; Uwe Trefzer, Bristol-Myers Squibb, MSD; Michael Smylie, Bristol-Myers Squibb, Pfizer, Roche; Axel Hauschild, Bristol-Myers Squibb, Celgene, Eisai, MSD, Pfizer Research Funding: Antoni Ribas, Pfizer; John B. Haanen, Pfizer; Claus Garbe, Bristol-Myers Squibb, Genta, GlaxoSmithKline, MSD, SOBI; Kari L. Kendra, Genentech, GlaxoSmithKline, Pfizer, Synta Pharmaceuticals; Grant A. McArthur, Pfizer; Axel Hauschild, Bristol-Myers Squibb, Celgene, Eisai, GlaxoSmithKline, MSD, Novartis, Pfizer, Roche Expert Testimony: None Other Remuneration: Paul D. Nathan, Pfizer
AUTHOR CONTRIBUTIONS
Conception and design: Antoni Ribas, Richard Kefford, Margaret A. Marshall, Jesus Gomez-Navarro, Dmitri Pavlov, Axel Hauschild
Provision of study materials or patients: Antoni Ribas, Richard Kefford, Cornelis J.A. Punt, John B. Haanen, Maribel Marmol, Claus Garbe, Helen Gogas, Jacob Schachter, Gerald Linette, Paul Lorigan, Kari L. Kendra, Michele Maio, Uwe Trefzer, Michael Smylie, Grant A. McArthur, Brigitte Dreno, Paul D. Nathan, Jacek Mackiewicz, John M. Kirkwood, Axel Hauschild
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: Antoni Ribas, Margaret A. Marshall, Richard Kefford, Axel Hauschild
Final approval of manuscript: All authors
REFERENCES
- 1.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 2.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 3.Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12:873–883. doi: 10.1634/theoncologist.12-7-873. [DOI] [PubMed] [Google Scholar]
- 4.Millham R, Pavlov D, Canniff P, et al. Ex vivo blood stimulation assay as a translational research tool in the development of the ticilimumab (CP-675206) J Clin Oncol. 2006;24(suppl):110s. abstr 2542. [Google Scholar]
- 5.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 6.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 9.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 10.O'Day S, Hodi FS, McDermott DF, et al. A phase III, randomized, double-blind, multicenter study comparing monotherapy with ipilimumab or gp100 peptide vaccine and the combination in patients with previously treated, unresectable stage III or IV melanoma. J Clin Oncol. 2010;28(suppl):6s. abstr 4. [Google Scholar]
- 11.Hodi FS, O'Day S, McDermott DF, et al. Re-induction with ipilimumab, gp100 peptide vaccine, or a combination of both from a phase III, randomized, double-blind, multicenter study of previously treated patients with unresectable stage III or IV melanoma. J Clin Oncol. 2010;28(suppl):613s. abstr 8509. [Google Scholar]
- 12.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.