Abstract
Purpose
Increased hepatocyte growth factor/MET signaling is associated with poor prognosis and acquired resistance to epidermal growth factor receptor (EGFR) –targeted drugs in patients with non–small-cell lung cancer (NSCLC). We investigated whether dual inhibition of MET/EGFR results in clinical benefit in patients with NSCLC.
Patients and Methods
Patients with recurrent NSCLC were randomly assigned at a ratio of one to one to receive onartuzumab plus erlotinib or placebo plus erlotinib; crossover was allowed at progression. Tumor tissue was required to assess MET status by immunohistochemistry (IHC). Coprimary end points were progression-free survival (PFS) in the intent-to-treat (ITT) and MET-positive (MET IHC diagnostic positive) populations; additional end points included overall survival (OS), objective response rate, and safety.
Results
There was no improvement in PFS or OS in the ITT population (n = 137; PFS hazard ratio [HR], 1.09; P = .69; OS HR, 0.80; P = .34). MET-positive patients (n = 66) treated with erlotinib plus onartuzumab showed improvement in both PFS (HR, .53; P = .04) and OS (HR, .37; P = .002). Conversely, clinical outcomes were worse in MET-negative patients treated with onartuzumab plus erlotinib (n = 62; PFS HR, 1.82; P = .05; OS HR, 1.78; P = .16). MET-positive control patients had worse outcomes versus MET-negative control patients (n = 62; PFS HR, 1.71; P = .06; OS HR, 2.61; P = .004). Incidence of peripheral edema was increased in onartuzumab-treated patients.
Conclusion
Onartuzumab plus erlotinib was associated with improved PFS and OS in the MET-positive population. These results combined with the worse outcomes observed in MET-negative patients treated with onartuzumab highlight the importance of diagnostic testing in drug development.
INTRODUCTION
Non–small-cell lung cancer (NSCLC) represents 85% of all lung cancer cases; once metastatic, median survival is 10 to 12 months.1 Treatment with erlotinib, a small-molecule inhibitor of the epidermal growth factor receptor (EGFR), improves survival in patients with recurrent NSCLC and progression-free survival (PFS) in patients with untreated NSCLC with EGFR-activating mutations.2–4 Unfortunately, resistance eventually occurs; thus, understanding mechanisms of resistance has been the focus of much research.
MET, a transmembrane tyrosine kinase receptor, is involved in cell proliferation, survival, motility, and invasion in normal and tumor cells.5 MET is frequently dysregulated in tumor cells via multiple mechanisms, particularly elevated expression, with or without gene amplification.5,6 Elevated MET expression, observed commonly in NSCLC tumor tissues (61%),7 has been associated with worse prognosis.5,8,9
MET activation increases the expression of some EGFR ligands,10 and coactivation of EGFR and MET is described in a distinct subset of NSCLCs.11 Genetic amplification/overexpression of MET has been implicated as a mechanism of erlotinib resistance in tumors with EGFR-activating mutations,12 and resistance to erlotinib has been observed in an NSCLC wild-type cell line (H596) on MET activation.13 Thus, EGFR and MET may cooperate in driving tumorigenesis.
MET is activated on binding by hepatocyte growth factor (HGF; also known as scatter factor), the only known ligand for the MET receptor.5 Onartuzumab (MetMAb; Genentech, South San Francisco, CA) is a humanized monovalent (one-armed) monoclonal antibody that binds the extracellular domain of MET to block HGF binding and activation.14,15 The unique one-armed design of onartuzumab circumvents agonist activity observed with bivalent anti-MET antibodies.16 We conducted a global, randomized, placebo-controlled, phase II study in patients with recurrent NSCLC evaluating onartuzumab plus erlotinib versus placebo plus erlotinib.
PATIENTS AND METHODS
Patients
Eligible patients had to be age ≥ 18 years; have received one or two systemic regimens (including platinum-based chemotherapy) for advanced (stage IIIB/IV) NSCLC; have an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2; have measurable disease; consent to providing sufficient representative tumor tissue; and have adequate hematologic, renal, and liver function. Key exclusion criteria included ≥ 30 days of exposure to an EGFR-targeted therapy, untreated CNS metastasis, or any major medical condition that could interfere with participation.
Study Design
The study was sponsored and study drugs provided by Genentech. The protocol was approved by the institutional review board of each participating center and conducted according to the Declaration of Helsinki. All patients provided written informed consent. The clinical investigators collected the data, which were stored by the sponsor.
OAM4558g was a phase II, double-blind, placebo-controlled, randomized (ratio of one to one) global trial designed to evaluate the activity and safety of onartuzumab plus erlotinib versus placebo plus erlotinib in patients with recurrent NSCLC. Dynamic hierarchic random assignment was performed through an interactive voice response system and stratified according to smoking status (never or < 100 cigarettes lifetime v current smoker or ≥ 100 cigarettes), ECOG PS (0/1 v 2), and histology (squamous cell carcinoma [SCC] v non-SCC). After disease progression, patients randomly assigned to placebo plus erlotinib could receive onartuzumab added to erlotinib, provided they met specific eligibility criteria (ECOG PS ≤ 2, no new CNS metastasis).
Study Treatments
Onartuzumab (15 mg/kg diluted in 0.9% normal saline solution to total volume of 250 cm3) or placebo (250 cm3 0.9% normal saline solution provided by investigative site) was administered by intravenous infusion every 3 weeks. Erlotinib was administered orally at 150 mg daily; dose reductions (to no less than 50 mg daily) or interruptions (≤ 7 consecutive days) for related toxicities were permitted. No dose reductions of onartuzumab were allowed. Blinded treatment continued until disease progression, unacceptable toxicity, or death.
Assessments
During the study, tumor measurement and survival status were collected for evaluation of PFS, overall survival (OS), and objective response rate (ORR). Computed tomography (CT) scans were obtained at baseline and every two cycles (6 weeks) for the first six cycles and then every three cycles thereafter. Disease status per RECIST was assessed by the investigator. Patients were also monitored for adverse events (AEs), changes in laboratory values, and physical examination findings.
Tumor Tissue Assessments
Tumor tissue, archival permitted, was collected for confirmation of NSCLC and evaluation of MET expression by immunohistochemistry (IHC) using the CONFIRM SP44 anti-MET monoclonal antibody (Ventana Medical Systems, Tucson, AZ; cat No. 790-4430). A MET IHC scoring system was used to evaluate both staining intensity (negative, weak, moderate, or strong) and prevalence of these intensities in tumor cells.17 The four MET diagnostic subgroups were defined as: 3+ (≥ 50% of tumor cells staining with strong intensity); 2+ (≥ 50% of tumor cells with moderate or higher staining but < 50% with strong intensity); 1+ (≥ 50% of tumor cells with weak or higher staining but < 50% with moderate or higher intensity); or 0 (no staining or < 50% of tumor cells with any intensity). MET positivity was defined as a score of 2+ or 3+. MET status was determined centrally after random assignment and before unblinding.
Statistical Analysis
The coprimary end points were PFS in the intent-to-treat (ITT) and MET-positive populations, defined as the time from random assignment to the first occurrence of disease progression (according to RECIST 1.0) or death resulting from any cause within 30 days of the last treatment or the latest CT assessment (censored). It was anticipated that 50% of enrolled patients would have MET-positive tumors. The study was to accrue 120 patients to provide 84 PFS events overall, with 42 in the MET-positive population. For patients with MET-positive tumors, the median PFS in the control arm was expected to be 3.3 months, and the desired median PFS in the onartuzumab plus erlotinib arm was 5.5 months, corresponding to a hazard ratio (HR) of 0.6. The type I (two-sided) and II error rates were set as 10% and 50%, respectively. Additional objectives included assessment of OS, ORR, safety, and tolerability. All outcomes were assessed in the ITT and MET diagnostic groups. Median PFS and OS were estimated from Kaplan-Meier curves. Stratified log-rank test was used to test the difference in PFS and OS between treatment arms. Estimated HRs and 95% CIs were determined using a stratified Cox regression model. All authors reviewed the data and manuscript and vouch for the accuracy, completeness, and fidelity of this report to the study protocol.
RESULTS
Patients
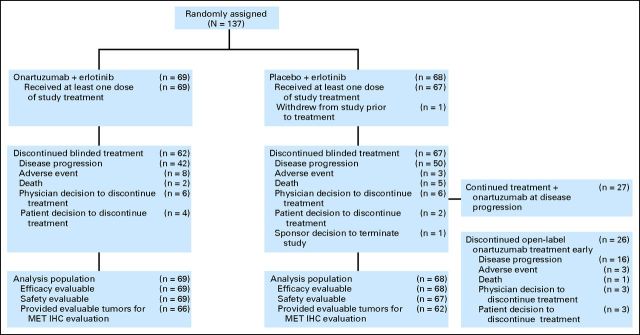
From March 2009 to August 2010, 137 patients were randomly assigned, 69 to onartuzumab plus erlotinib and 68 to placebo plus erlotinib. One hundred thirty-six patients received at least one dose of study treatment (one patient assigned to placebo was removed for pain before receiving any study drug; Fig 1). Median patient follow-up was 10.4 months (range, .1 to 18.4 months).
Fig 1.
CONSORT diagram. IHC, immunohistochemistry.
Baseline characteristics were well balanced between the treatment groups in the ITT population and within the MET diagnostic subgroups, with the exception of EGFR mutation status (Table 1). Of note, SCC was more prevalent in MET-negative versus MET-positive patients (42% v 15%, respectively), whereas never-smokers were less prevalent in MET-negative versus MET-positive patients (5% v 20%, respectively).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | ITT |
MET Negative |
MET Positive |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Plus Erlotinib (n = 68) |
Onartuzumab Plus Erlotinib (n = 69) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n = 31) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n = 35) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||||
| Median | 63 | 64 | 61 | 63 | 64 | 66 | ||||||
| Range | 42-83 | 30-83 | 42-83 | 45-82 | 44-82 | 30-83 | ||||||
| Sex* | ||||||||||||
| Male | 42 | 62 | 40 | 58 | 17 | 55 | 20 | 65 | 20 | 65 | 18 | 51 |
| Female | 26 | 38 | 29 | 42 | 14 | 45 | 11 | 35 | 11 | 35 | 17 | 49 |
| Race* | ||||||||||||
| White | 61 | 90 | 61 | 88 | 28 | 90 | 27 | 87 | 28 | 90 | 32 | 91 |
| Black or African American | 5 | 7 | 4 | 6 | 3 | 10 | 3 | 10 | 1 | 3 | 1 | 3 |
| Asian | 1 | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 3 |
| Other | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| Not available | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 3 | 1 | 3 | 0 | 0 |
| ECOG PS* | ||||||||||||
| 0 | 21 | 31 | 22 | 32 | 9 | 29 | 8 | 26 | 10 | 32 | 13 | 37 |
| 1 | 45 | 66 | 43 | 62 | 22 | 71 | 20 | 65 | 19 | 61 | 21 | 60 |
| 2 | 2 | 3 | 4 | 6 | 0 | 0 | 3 | 10 | 2 | 6 | 1 | 3 |
| Histology* | ||||||||||||
| Adenocarcinoma | 41 | 60 | 40 | 58 | 17 | 55 | 12 | 39 | 21 | 68 | 26 | 74 |
| Squamous cell | 20 | 29 | 20 | 29 | 12 | 39 | 14 | 45 | 5 | 16 | 5 | 14 |
| Large cell | 3 | 4 | 6 | 9 | 1 | 3 | 4 | 13 | 2 | 6 | 2 | 6 |
| Bronchioloalveolar | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 |
| Other | 3 | 4 | 3 | 4 | 1 | 3 | 1 | 3 | 2 | 6 | 2 | 6 |
| Smoking history* | ||||||||||||
| Current/former | 60 | 88 | 59 | 86 | 30 | 97 | 29 | 93 | 25 | 81 | 28 | 80 |
| Never-smoker† | 8 | 12 | 10 | 14 | 1 | 3 | 2 | 7 | 6 | 19 | 7 | 20 |
| Line of therapy* | ||||||||||||
| Second | 46 | 68 | 46 | 67 | 20 | 65 | 21 | 68 | 22 | 71 | 22 | 63 |
| Third | 22 | 32 | 23 | 33 | 11 | 35 | 10 | 32 | 9 | 29 | 13 | 37 |
| MET IHC status* | ||||||||||||
| Positive | 31 | 46 | 35 | 51 | 0 | 0 | 0 | 0 | 31 | 100 | 35 | 100 |
| Negative | 31 | 46 | 31 | 45 | 31 | 100 | 31 | 100 | 0 | 0 | 0 | 0 |
| Unknown | 6 | 9 | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KRAS mutation* | ||||||||||||
| Wild type | 43 | 63 | 43 | 62 | 21 | 68 | 19 | 61 | 20 | 65 | 24 | 69 |
| Mutant | 13 | 19 | 13 | 19 | 7 | 23 | 6 | 19 | 6 | 19 | 7 | 20 |
| Unknown | 12 | 18 | 13 | 19 | 3 | 10 | 6 | 19 | 5 | 16 | 4 | 11 |
| EGFR mutation* | ||||||||||||
| Wild type | 50 | 74 | 49 | 71 | 24 | 77 | 25 | 81 | 24 | 77 | 24 | 69 |
| Mutant | 6 | 9 | 7 | 10 | 4 | 13 | 0 | 0 | 2 | 7 | 7 | 20 |
| Unknown | 12 | 18 | 13 | 19 | 3 | 10 | 6 | 19 | 5 | 16 | 4 | 11 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IHC, immunohistochemistry; ITT, intent to treat.
Because of rounding, some percentages do not sum to 100.
Never-smokers defined as those who had never smoked or smoked < 100 cigarettes in lifetime.
All patients enrolled had tissue submitted for analysis. MET status was determined in 128 patients (93%; Fig 1); 66 (52%) were MET positive. Mutation testing was performed in 112 patients (88%): 26 (23%) harbored a KRAS mutation, and 13 (12%) had a nonoverlapping EGFR mutation.
Twenty-seven patients (12 MET positive, 13 MET negative, and two MET with status unevaluable) randomly assigned to placebo had onartuzumab added to continued treatment with erlotinib at the time of disease progression (Fig 1).
Efficacy
PFS.
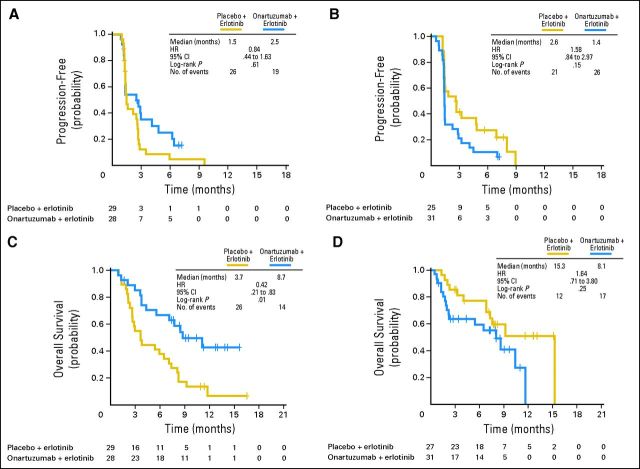
PFS did not differ between treatment arms (median, 2.6 months for placebo plus erlotinib v 2.2 months for onartuzumab plus erlotinib; HR, 1.09; P = .69; Fig 2A) in the ITT population. However, the addition of onartuzumab treatment resulted in a 47% reduction in the risk of disease progression in the MET-positive subgroup, which was statistically significant (median, 1.5 v 2.9 months; HR: 0.53; P = .04; Fig 2B). MET-negative patients experienced progression earlier with onartuzumab versus placebo (median, 2.7 v 1.4 months; HR, 1.82; P = .05; Fig 2C).
Fig 2.
Kaplan-Meier estimates of (A, B, C) progression-free and (D, E, F) overall survival outcomes in (A, D) intent-to-treat, (B, E) MET-positive, and (C, F) MET-negative populations. HR, hazard ratio.
OS.
OS did not differ significantly between treatment arms (median, 7.4 months for placebo plus erlotinib v 8.9 months for onartuzumab plus erlotinib; HR, 0.80; P = .34; Fig 2D) in the ITT population. However, the addition of onartuzumab nearly tripled survival compared with placebo in the MET-positive population (median, 3.8 v 12.6 months, HR, 0.37; P = .002; Fig 2E). In the MET-negative population, those randomly assigned to onartuzumab had shorter survival versus those receiving placebo (median, 15.3 v 8.1 months; HR, 1.78; P = .16; Fig 2F).
ORRs.
The ORRs were not significantly different between the two treatment arms in all three specified populations (ITT: 4.4% for placebo plus erlotinib v 5.8% for onartuzumab plus erlotinib; MET positive: 3.2% v 8.6%; MET negative: 6.5% v 3.2%; Appendix Table A1, online only).
Exploratory and sensitivity analyses.
The impact of demographic and baseline characteristics, including histology, smoking history, sex, baseline ECOG PS, line of therapy, age, MET IHC score (0, 1+, 2+, or 3+), EGFR mutation status, and KRAS mutation status, on PFS and OS was examined using exploratory analyses. Analysis of the MET-positive subgroup for both PFS (data not shown) and OS (Fig 3A) showed that the treatment effect of onartuzumab plus erlotinib was similar across most of the subgroups. The statistical significance of the treatment effect on OS was maintained in the MET-positive subgroup after adjusting for sex in the Cox regression model (OS: HR, 0.35; P = .0013). Similarly, results for most subgroups of the ITT population were consistent with the OS primary analysis (Fig 3B).
Fig 3.
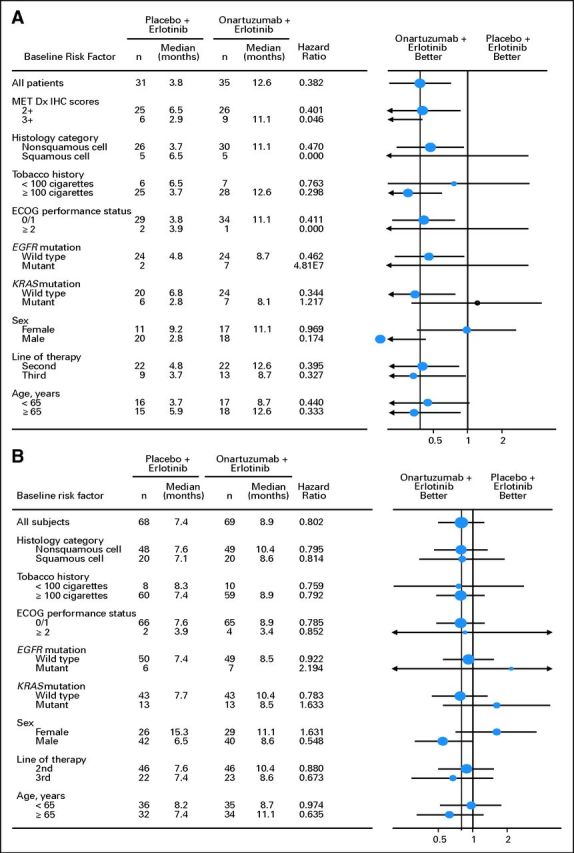
Forest plots of overall survival outcomes in (A) MET-positive and (B) intent-to-treat populations. Dx, diagnosis; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IHC, immunohistochemistry.
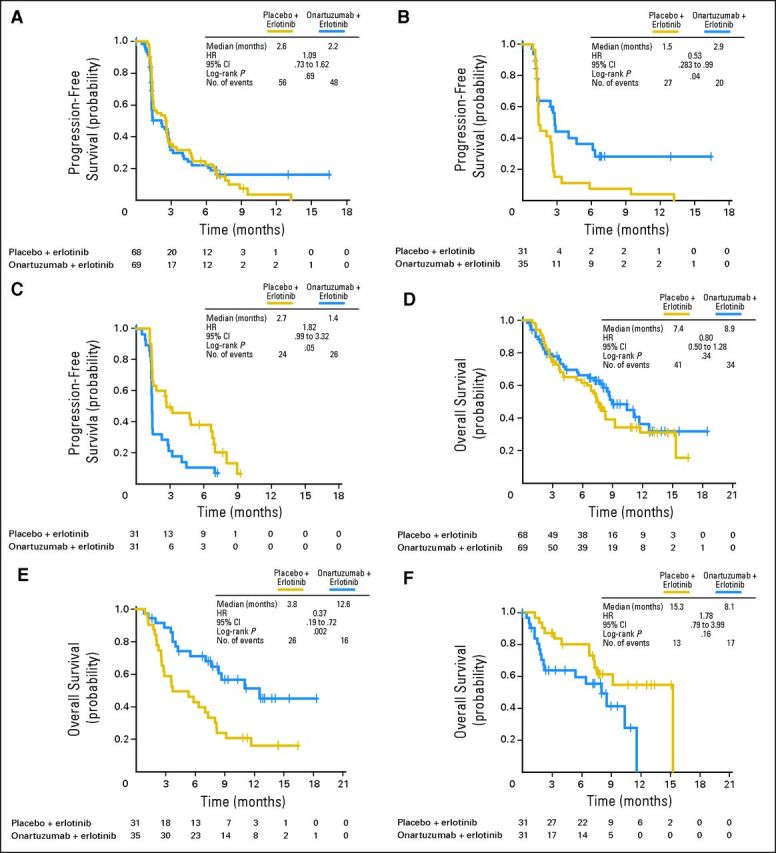
PFS and OS analyses, removing known EGFR mutation–positive patients, were performed to address the imbalance in both MET-positive and MET-negative populations. The overall interpretation of the results was unchanged (Fig 4).
Fig 4.
Kaplan-Meier estimates of (A, B) progression-free and (C, D) overall survival in (A, C) MET-positive and (B, D) MET-negative populations, excluding known EGFR mutation–positive patients. HR, hazard ratio.
Prognosis, assessed in patients assigned to placebo plus erlotinib, was worse in MET-positive versus MET-negative patients for both PFS (HR, 1.71; P = .06) and OS (HR, 2.61; P = .004; Appendix Fig A1, online only).
For patients originally randomly assigned to receive placebo who chose to receive onartuzumab added to continued erlotinib treatment, the majority experienced events at or before the next subsequent tumor assessment. There was no difference in PFS for these patients based on their tumor MET status (median, 1.3 months for MET positive v 1.5 months for MET negative).
IHC evaluation.
To determine the appropriateness of the IHC cut point of ≥ 50% of tumor cells staining moderately or strongly, outcome analyses were performed in populations with ≥ 10% or ≥ 90% of tumor cells staining at moderate to strong intensity. Compared with the 50% cutoff, treatment benefit in both PFS and OS was diminished using the less stringent cutoff of ≥ 10% (PFS: HR, 0.78; P = .317; OS: HR, 0.52; P = .023) and was similar using the more stringent cutoff of ≥ 90% (PFS: HR, 0.47; P = .028; OS: HR, 0.3; P = .001). To assess the adequacy of the intensity cut point, PFS and OS were assessed by each of the four MET IHC scores (3+, 2+, 1+, 0). The benefit of adding onartuzumab was maintained in both 2+ and 3+, and the detriment was observed in both 0 and 1+ (Appendix Fig A2, online only).
Safety
During the blinded stage, 129 patients (94.2%) discontinued onartuzumab/placebo treatment: 62 patients (89.9%) in the onartuzumab plus erlotinib arm and 67 patients (98.5%) in the placebo plus erlotinib arm. The rate of discontinuation because of AEs was slightly higher in the onartuzumab plus erlotinib arm (11.6%) compared with the placebo plus erlotinib arm (4.4%). Within the onartuzumab plus erlotinib arm, this rate was higher in MET-positive patients (22.9%) than in MET-negative patients (6.5%). The most common reason for treatment discontinuation was disease progression (onartuzumab plus erlotinib, 42 patients [60.9%]; placebo plus erlotinib, 50 patients [73.5%]; Fig 1).
The most common AEs (all grades) in the safety-evaluable population (n = 136) were rash (60.9% v 61.2%), diarrhea (40.6% v 52.2%), fatigue (31.9% v 35.8%), and nausea (31.9% v 31.3%) for erlotinib plus onartuzumab versus erlotinib plus placebo, respectively (Table 2). AEs more frequently observed with onartuzumab were peripheral edema, pyrexia, asthenia, insomnia, and pneumonia. Most were grade 1 or 2 in severity. For patients originally randomly assigned to receive placebo who chose to receive onartuzumab added to continued erlotinib, the subsequent AE profile was similar.
Table 2.
Most Commonly Reported AEs
| AE | ITT |
MET Negative |
MET Positive |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Plus Erlotinib (n = 67) |
Onartuzumab Plus Erlotinib (n = 69) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n = 31) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n = 35) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Rash | ||||||||||||
| All grades | 41 | 61.2 | 42 | 60.9 | 18 | 58.1 | 18 | 58.1 | 19 | 61.3 | 22 | 62.9 |
| Grade 3 | 2 | 3.0 | 6 | 8.7 | 1 | 3.2 | 2 | 6.5 | 1 | 3.2 | 4 | 11.4 |
| Grade 4 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.9 |
| Diarrhea | ||||||||||||
| All grades | 35 | 52.2 | 28 | 40.6 | 17 | 54.8 | 8 | 25.8 | 13 | 41.9 | 18 | 51.4 |
| Grade 3 | 3 | 4.5 | 4 | 5.8 | 1 | 3.2 | 1 | 3.2 | 2 | 6.5 | 3 | 8.6 |
| Grade 4 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.9 |
| Fatigue* | ||||||||||||
| All grades | 24 | 35.8 | 22 | 31.9 | 11 | 35.5 | 6 | 19.4 | 12 | 38.7 | 16 | 45.7 |
| Grade 3 | 2 | 3.0 | 6 | 8.7 | 1 | 3.2 | 2 | 6.5 | 1 | 3.2 | 4 | 11.4 |
| Nausea* | ||||||||||||
| All grades | 21 | 31.3 | 22 | 31.9 | 10 | 32.3 | 9 | 29.0 | 9 | 29.0 | 13 | 37.1 |
| Grade 3 | 2 | 3.0 | 0 | 0.0 | 1 | 3.2 | 0 | 0.0 | 1 | 3.2 | 0 | 0.0 |
| Decreased appetite* | ||||||||||||
| All grades | 16 | 23.9 | 14 | 20.3 | 5 | 16.1 | 4 | 12.9 | 10 | 32.3 | 10 | 28.6 |
| Grade 3 | 1 | 1.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Dyspnea* | ||||||||||||
| All grades | 16 | 23.9 | 13 | 18.8 | 6 | 19.4 | 4 | 12.9 | 8 | 25.8 | 8 | 22.9 |
| Grade 3 | 3 | 4.5 | 3 | 4.3 | 1 | 3.2 | 1 | 3.2 | 2 | 6.5 | 1 | 2.9 |
| Cough† | ||||||||||||
| All grades | 13 | 19.4 | 13 | 18.8 | 7 | 22.6 | 4 | 12.9 | 6 | 19.4 | 7 | 20.0 |
| Vomiting† | ||||||||||||
| All grades | 13 | 19.4 | 4 | 5.8 | 7 | 22.6 | 2 | 6.5 | 5 | 16.1 | 2 | 5.7 |
| Anemia* | ||||||||||||
| All grades | 10 | 14.9 | 10 | 14.5 | 3 | 9.7 | 4 | 12.9 | 6 | 19.4 | 6 | 17.1 |
| Grade 3 | 1 | 1.5 | 0 | 0.0 | 1 | 3.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Dermatitis acneiform† | ||||||||||||
| All grades | 10 | 14.9 | 10 | 14.5 | 6 | 19.4 | 5 | 16.1 | 3 | 9.7 | 5 | 14.3 |
| Dry skin† | ||||||||||||
| All grades | 10 | 14.9 | 8 | 11.6 | 5 | 16.1 | 2 | 6.5 | 5 | 16.1 | 5 | 14.3 |
| Pain* | ||||||||||||
| All grades | 8 | 11.9 | 4 | 5.8 | 6 | 19.4 | 0 | 0.0 | 1 | 3.2 | 4 | 11.4 |
| Grade 3 | 3 | 4.5 | 1 | 1.4 | 3 | 9.7 | 0 | 0.0 | 0 | 0.0 | 1 | 2.9 |
| Pruritus† | ||||||||||||
| All grades | 8 | 11.9 | 4 | 5.8 | 3 | 9.7 | 3 | 9.7 | 5 | 16.1 | 0 | 0.0 |
| Anxiety | ||||||||||||
| All grades | 7 | 10.4 | 6 | 8.7 | 2 | 6.5 | 2 | 6.5 | 4 | 12.9 | 3 | 8.6 |
| Back pain* | ||||||||||||
| All grades | 7 | 10.4 | 7 | 10.1 | 2 | 6.5 | 5 | 16.1 | 5 | 16.1 | 2 | 5.7 |
| Grade 3 | 2 | 3.0 | 1 | 1.4 | 1 | 3.2 | 1 | 3.2 | 1 | 3.2 | 0 | 0.0 |
| Chest pain† | ||||||||||||
| All grades | 7 | 10.4 | 2 | 2.9 | 5 | 16.1 | 1 | 3.2 | 2 | 6.5 | 1 | 2.9 |
| Pyrexia* | ||||||||||||
| All grades | 6 | 9.0 | 10 | 14.5 | 2 | 6.5 | 5 | 16.1 | 3 | 9.7 | 5 | 14.3 |
| Grade 3 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.9 |
| Asthenia* | ||||||||||||
| All grades | 6 | 9.0 | 9 | 13.0 | 3 | 9.7 | 4 | 12.9 | 2 | 6.5 | 5 | 14.3 |
| Grade 3 | 0 | 0.0 | 3 | 4.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 8.6 |
| Urinary tract infection† | ||||||||||||
| All grades | 6 | 9.0 | 4 | 5.8 | 4 | 12.9 | 2 | 6.5 | 2 | 6.5 | 2 | 5.7 |
| Insomnia* | ||||||||||||
| All grades | 5 | 7.5 | 8 | 11.6 | 2 | 6.5 | 3 | 9.7 | 3 | 9.7 | 5 | 14.3 |
| Grade 3 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.9 |
| Peripheral edema* | ||||||||||||
| All grades | 5 | 7.5 | 16 | 23.2 | 3 | 9.7 | 7 | 22.6 | 2 | 6.5 | 8 | 22.9 |
| Grade 3 | 0 | 0.0 | 2 | 2.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.7 |
| Pneumonia‡ | ||||||||||||
| All grades | 3 | 4.5 | 5 | 7.2 | 2 | 6.5 | 4 | 12.9 | 1 | 3.2 | 1 | 2.9 |
| Grade 3 | 2 | 3.0 | 4 | 5.8 | 1 | 3.2 | 3 | 9.7 | 1 | 3.2 | 1 | 2.9 |
| Grade 5 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 1 | 3.2 | 0 | 0.0 | 0 | 0.0 |
NOTE. Table shows AEs occurring at frequency of ≥ 10%, ordered by frequency in placebo plus erlotinib (ITT) group.
Abbreviations: AE, adverse event; ITT, intent to treat.
No grade 4 or 5 AEs.
No grade 3 to 5 AEs.
No grade 4 AEs.
Grade ≥ 3 AEs were more frequent in patients receiving onartuzumab, regardless of MET status; however, no specific pattern was identified (Table 2). In the ITT population, serious AEs were reported in 42.0% of patients randomly assigned to onartuzumab and in 32.8% of patients randomly assigned to placebo. A majority of serious AEs were grade 3 in severity and indistinguishable from the underlying disease (pneumonia, dyspnea, hemoptysis, pulmonary embolism, respiratory distress) or from a potential erlotinib effect (interstitial lung disease and rash). There were four AEs, primarily NSCLC associated, that resulted in death in each treatment arm (Appendix Table A2, online only).
DISCUSSION
Patients with MET-positive NSCLC seemed to benefit from the combination of onartuzumab and erlotinib. Patients randomly assigned to placebo plus erlotinib performed similarly to historic controls.18,19 Consistent with previous reports, MET expression was associated with worse prognosis. The addition of onartuzumab in MET-positive patients resulted in median PFS and median OS results similar to those observed in MET-negative patients receiving erlotinib alone. This suggests that the addition of onartuzumab to erlotinib in MET-positive patients abrogated the negative prognostic effect of MET expression. Clinical benefit from the addition of onartuzumab to erlotinib was observed in nearly all analyzed MET-positive subgroups. Furthermore, the degree of benefit seemed to be proportional to the relative intensity of MET expression, supporting the criteria used to define MET-positive disease.
In the MET-positive population, the ORRs were low (confined mostly to those harboring EGFR mutations), and there was no difference between treatment groups. Interestingly, the magnitude of gain in median OS in the onartuzumab versus placebo treatment arms was much greater than that in median PFS (median OS gain of 8.8 months v 1.4 months for mPFS).
Low response rates, coupled with a disproportionate PFS to OS improvement, suggest that blockade of MET signaling in MET-positive disease may be acting through a mechanism distinctly different from other receptor tyrosine kinase inhibitors. MET has been implicated in the spread of metastases, and therefore, the therapeutic benefit of onartuzumab may derive from inhibition of cancer-cell migration and invasion rather than direct inhibition of existing tumor growth.
Analyses of pharmacokinetics, safety, AEs, patterns of disease progression, and cause of death did not explain the worse outcome observed in the MET-negative population treated with onartuzumab plus erlotinib. The pharmacokinetics of both agents was not altered (data not shown), nor were there obvious safety signals to explain the findings. MET, like other proteins, has been postulated to possess both tumor-suppressor and oncogenic properties.20 If such were the case in NSCLC, MET might be acting as a tumor suppressor in MET-negative disease and as an oncogene in MET-positive disease. Alternatively, dual inhibition of EGFR and MET may have different consequences in tumors with lower versus higher MET expression, suggesting this phenomenon may only be seen against a background of EGFR inhibition. The worse outcomes observed in the onartuzumab-treated MET-negative NSCLC population may be an effect unique to patients with NSCLC. Other cancers for which MET has been described as a negative prognostic factor, such as gastric cancer,21 exhibit lower levels of MET expression as measured by IHC using the SP44 antibody (unpublished data). The definition of MET positivity used to predict for benefit from onartuzumab may differ for cancers other than NSCLC. Future studies evaluating outcomes in patients with MET-negative tumors treated with onartuzumab will require close observation for similar results.
Despite the supporting sensitivity analyses regarding the efficacy outcomes and diagnostic cut points, there are limitations to this study, including small sample size, which could have been affected by both known and unknown confounders, and no prospective stratification on MET status (definition of MET positivity was determined before unblinding but after random assignment). Nonetheless, the results are encouraging given the magnitude of benefit observed in more than one half of the study patients with NSCLC. Without a diagnostic hypothesis, the results observed in the ITT population would have likely led to a decision to discontinue onartuzumab development. Combined with the observations in the MET-negative subgroup, these results highlight the importance of diagnostic development in clinical oncology studies. The activity observed in this study, combined with the prevalence of MET and its association with poor prognosis in other indications, provides a rationale for further clinical evaluation in NSCLC and other indications. A randomized phase III study evaluating erlotinib with or without onartuzumab in patients with MET-positive NSCLC is under way.
Supplementary Material
Acknowledgment
We thank the patients and families who participated in this study; John Bothos, Anshu Vashistha, and Lukas Amler; all clinical trial investigators (listed in Appendix, online only); and the following individuals for their contributions to histologic or molecular correlative assessments: Xiaoling Xia, Linda Rangell, Rajiv Raja, Rajesh Patel, Ling Fu, An Do, and Rupal Desai.
Appendix
Full List of Trial Investigators
North America:
Dr David R. Spigel (Sarah Cannon Research Institute [SCRI], Tennessee Oncology, Nashville, TN); Dr Thomas Ervin (SCRI, Florida Cancer Specialists, Fort Myers, FL); Dr Davey Daniel (SCRI, Chattanooga Oncology and Hematology Associates, Chattanooga, TN); Dr Jerome Goldschmidt (Blue Ridge Cancer Care, Salem, VA); Dr George Blumenschein (MD Anderson Cancer Center, Houston, TX); Dr Ramaswamy Govindan (Washington University, St Louis, MO); Dr Taral Patel (Mid Ohio Oncology Hematology, Columbus, OH); Dr Michael Wertheim (Hematology Onc Treasure Coast, Port St Lucie, FL); Dr Elke Friedman (SCRI, Virginia Cancer Institute, Richmond, VA); Dr Fred Kudrik (SCRI, South Carolina Oncology Associates, Columbia, SC); and Dr Ian Anderson (Redwood Regional Med Group, Santa Rosa, CA).
Europe and Australia:
Dr Rodryg Ramlau (Centrum Pulmonologii i Torakochirurgii, Poznan, Poland); Professor Maciej J. Krzakowski (Centrum Onkologii, Warsaw, Poland); Dr Gilles Robinet (University Hospital Morvan, Brest, France); Professor Yves Martinet (Hôpital de Brabois, Nancy, France); Professor Fabrice Barlesi (Assistance Publique Hopitaux de Marseille [APHM], Université Méditerranée, Marseille, France); Professor Sergey Orlov (Gosudarstvennogo Obrazovatelnogo Uchrezhdeniya Vysshego Professionalnogo Obrazovaniya [GOU VPO], St Petersburg, Russia); Professor Christina Brambilla (Centre Hospitalier Universitaire [CHU] de Grenoble, France); Professor Eric Dansin (Centre Oscar Lambret, Lille, France); Professor Anne Madroszyk (Institut Paoli-Calmettes, Marseille, France); Professor Alain Vergnenegre (Hopital du Cluzeau, Limoges, France); Dr Michael Boyer (Royal Prince Alfred Hospital, Camperdown, New South Wales, Australia); Dr Philip Clingan (Southern Medical Day Care, Wollongong, New South Wales, Australia); Dr Elisabeth Quoix (Nouvel Hopital Civil, Strasbourg, France); and Dr Piotr Sawrycki (Szpital Zespolony w Toruniu, Torun, Poland).
Table A1.
ORRs in Randomly Assigned Patients
| ORR | ITT (n = 137) |
MET Positive (n = 66) |
MET Negative (n = 62) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Plus Erlotinib (n = 68) |
Onartuzumab Plus Erlotinib (n = 69) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n =35) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n = 31) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of patients experiencing OR | 3 | 4.4* | 4 | 5.8* | 1 | 3.2 | 3 | 8.6 | 2 | 6.5 | 1 | 3.2 |
| 95% CI | 1.2 to 11.7 | 2.0 to 13.9 | 0.2 to 16.1 | 2.4 to 21.5 | 1.2 to 20.0 | 0.2 to 16.1 | ||||||
| Difference in ORR | 1.4 | 5.3 | −3.2 | |||||||||
| 95% CI | −6.0 to 8.7 | −5.8 to 16.5 | −13.9 to 7.4 | |||||||||
| Stratified P | .7101 | .3671 | .6726 | |||||||||
Abbreviations: ITT, intent to treat; OR, objective response; ORR, objective response rate.
Six of these patients had known EGFR mutation–positive tumors.
Table A2.
Summary of Safety Data
| AE | All Patients |
MET Negative |
MET Positive |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Plus Erlotinib (n = 67) |
Onartuzumab Plus Erlotinib (n = 69) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n = 31) |
Placebo Plus Erlotinib (n = 31) |
Onartuzumab Plus Erlotinib (n = 35) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Any adverse event | 67 | 100.0 | 68 | 98.6 | 31 | 100.0 | 31 | 100.0 | 31 | 100.0 | 35 | 100.0 |
| Grade ≥ 3 | 32 | 47.8 | 38 | 55.1 | 13 | 41.9 | 17 | 54.8 | 17 | 54.8 | 20 | 57.1 |
| Grade 3 | 25 | 37.3 | 26 | 37.7 | 12 | 38.7 | 10 | 32.3 | 11 | 35.5 | 15 | 42.9 |
| Grade 4 | 4 | 4.5 | 8 | 11.6 | 1 | 3.2 | 4 | 12.9 | 2 | 6.5 | 4 | 11.4 |
| Serious AE | 22 | 32.8 | 29 | 42.0 | 9 | 29.0 | 13 | 41.9 | 11 | 35.5 | 15 | 42.9 |
| AE leading to onartuzumab/placebo discontinuation | 2 | 3.0 | 10 | 14.5 | 0 | 0.0 | 2 | 6.5 | 2 | 6.5 | 8 | 22.9 |
| AE leading to death | 4 | 6.0 | 4 | 5.8 | 0 | 0.0 | 3* | 9.7 | 4† | 12.9 | 1‡ | 2.9 |
Abbreviation: AE, adverse event.
AEs reported: hemoptysis, pulmonary embolism, and pneumonia.
AEs reported: interstitial lung disease, respiratory distress (n = 2), and unspecified cause (n = 1).
AE reported: aspiration pneumonia.
Fig A1.
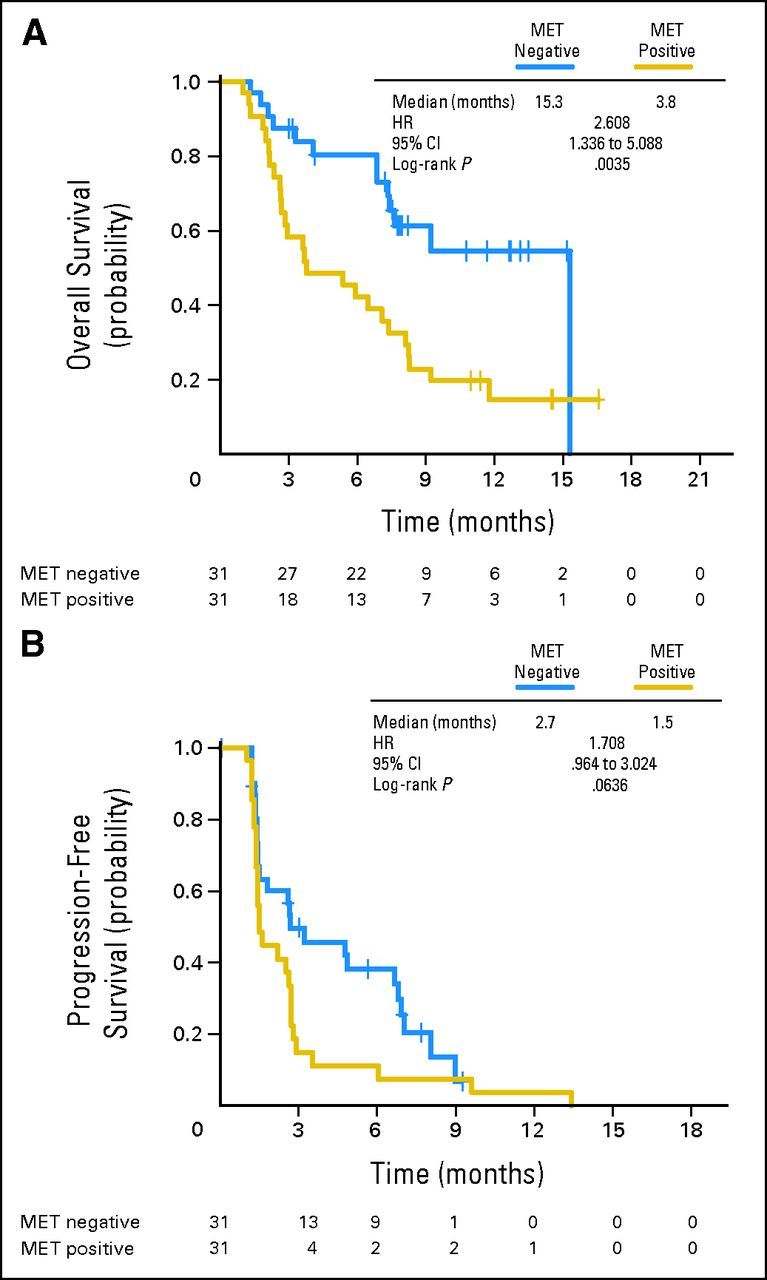
(A) Overall and (B) progression-free survival by MET status in patients receiving erlotinib plus placebo. HR, hazard ratio.
Fig A2.
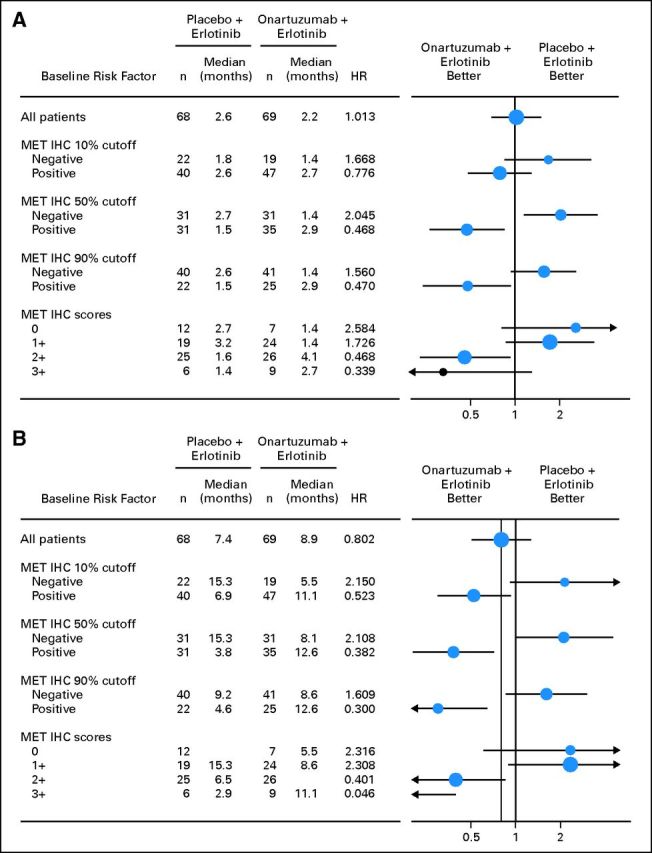
Forest plots of (A) progression-free and (B) overall survival by MET immunohistochemistry (IHC) cutoff (≥ 10%, ≥ 50%, and ≥ 90%) and MET IHC score (0, 1+, 2+, and 3+). HR, hazard ratio.
Footnotes
See accompanying article on page 4148
Supported by Genentech; support for third-party writing assistance was provided by F. Hoffmann-La Roche and Genentech.
Presented at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011, and 14th World Conference on Lung Cancer, Amsterdam, the Netherlands, July 3-7, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00854308.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Wei Yu, Genentech (C); Jiping Zha, Crown Bioscience (C); Robert L. Yauch, Genentech (C); Premal H. Patel, Genentech (C); See-Chun Phan, Genentech (C); Amy C. Peterson, Genentech (C) Consultant or Advisory Role: David R. Spigel, Genentech (U); George R. Blumenschein Jr, Genentech (C); Fabrice Barlesi, Roche (C), Genentech (C); Ramaswamy Govindan, Abbott Laboratories (C), Boehringer Ingelheim (C), Bristol-Myers Squibb (C), Covidien (C), Merck (C), Pfizer (C), Roche (C), Genentech (C); Taral Patel, Genentech (C) Stock Ownership: Wei Yu, Roche; Robert L. Yauch, Roche; Premal H. Patel, Genentech, Roche; See-Chun Phan, Roche; Amy C. Peterson, Genetech, Roche Honoraria: Rodryg A. Ramlau, Roche; Benoit Godbert, Roche; Ramaswamy Govindan, Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Covidien, Merck, Pfizer, Roche, Genentech Research Funding: George R. Blumenschein Jr, Genentech; Fabrice Barlesi, Genentech Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: David R. Spigel, Ramaswamy Govindan, Wei Yu, Robert L. Yauch, Amy C. Peterson
Provision of study materials or patients: David R. Spigel, Thomas J. Ervin, Rodryg A. Ramlau, Davey B. Daniel, Maciej J. Krzakowski, Fabrice Barlesi, Sergey V. Orlov, Michael S. Wertheim
Collection and assembly of data: David R. Spigel, Rodryg A. Ramlau, Davey B. Daniel, Jerome H. Goldschmidt Jr, George R. Blumenschein Jr, Maciej J. Krzakowski, Gilles Robinet, Benoit Godbert, Fabrice Barlesi, Ramaswamy Govindan, Taral Patel, Sergey V. Orlov, Michael S. Wertheim, Wei Yu, Jiping Zha, Robert L. Yauch, Premal H. Patel, Amy C. Peterson
Data analysis and interpretation: David R. Spigel, Thomas J. Ervin, George R. Blumenschein Jr, Fabrice Barlesi, Ramaswamy Govindan, Wei Yu, Robert L. Yauch, Premal H. Patel, See-Chun Phan, Amy C. Peterson
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA. Current paradigms in first-line treatment of non-small-cell lung cancer. Oncology (Williston Park) 2004;18(suppl 5):13–20. [PubMed] [Google Scholar]
- 3.Patel JD. Epidermal growth factor receptor pathway targeted therapy in patients with aerodigestive malignancies. Curr Opin Oncol. 2006;18:609–614. doi: 10.1097/01.cco.0000245308.67144.d2. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 6.Trusolino L, Bertotti A, Comoglio PM. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 7.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: Progress and challenges. Trends Mol Med. 2010;16:37–45. doi: 10.1016/j.molmed.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non–small-cell lung cancer patients. J Clin Oncol. 2009;27:1667–1674. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo A, Villén J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsubara D, Ishikawa S, Sachiko O, et al. Co-activation of epidermal growth factor receptor and c-MET defines a distinct subset of lung adenocarcinomas. Am J Pathol. 2010;177:2191–2204. doi: 10.2353/ajpath.2010.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 13.Spigel D, Ervin T, Ramlau R, et al. Randomized multicenter double-blind placebo-controlled Phase II study evaluating MetMAb, an antibody to Met receptor, in combination with erlotinib, in patients with advanced non-small-cell lung cancer. Ann Oncol. 2010;21(suppl) abstr LBA15. [Google Scholar]
- 14.Martens T, Schmidt NO, Eckerich C, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res. 2006;12:6144–6152. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 15.Jin H, Yang R, Zheng Z, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360–4368. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- 16.Prat M, Crepaldi T, Pennacchietti S, et al. Agonistic monoclonal antibodies against the Met receptor dissect the biological responses to HGF. J Cell Sci. 1998;111:237–247. doi: 10.1242/jcs.111.2.237. [DOI] [PubMed] [Google Scholar]
- 17.Koeppen H, Januario T, Filvaroff E, et al. Characterization and clinical validation of an immunohistochemical assay for Met in non-small cell lung cancer. Presented at the 101st Annual Meeting of the United States and Canadian Academy of Pathology; March 17-23, 2012; Vancouver, British Columbia, Canada. (abstr 2001) [Google Scholar]
- 18.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 20.Tulasne D, Foveau B. The shadow of death on the MET tyrosine kinase receptor. Cell Death Differ. 2008;15:427–434. doi: 10.1038/sj.cdd.4402229. [DOI] [PubMed] [Google Scholar]
- 21.Graziano F, Gallucio N, Lorenzini P, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29:4789–4795. doi: 10.1200/JCO.2011.36.7706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.