Abstract
Background:
Recent studies have suggested an association between elevated pelvic incidence (PI) and the development of lumbar spondylolysis. However, there is still lack of investigation for Han Chinese people concerning the normal range of spinopelvic parameters and relationship between abnormal sagittal parameters and lumbar diseases. The objective of the study was to investigate sagittal lumbosacral parameters of adult lumbar spondylolysis patients in Han Chinese population.
Methods:
A total of 52 adult patients with symptomatic lumbar spondylolysis treated in the General Hospital of Armed Police Force (Beijing, China) were identified as the spondylolysis group. All the 52 patients were divided into two subgroups, Subgroup A: 36 patients with simple lumbar spondylolysis, and Subgroup B: 16 patients with lumbar spondylolysis accompanying with mild lumbar spondylolisthesis (slip percentage <30%). Altogether 207 healthy adults were chosen as the control group. All patients and the control group took lumbosacral lateral radiographs. Seven sagittal lumbosacral parameters, including PI, pelvic tilt (PT), sacral slope (SS), lumbar lordosis (LL), L5 incidence, L5 slope, and sacral table angle (STA), were measured in the lateral radiographs. All the parameters aforementioned were compared between the two subgroups and between the spondylolysis group and the control group with independent-sample t-test.
Results:
There were no statistically significant differences of all seven sagittal lumbosacral parameters between Subgroup A and Subgroup B. PI, PT, SS, and LL were higher (P < 0.05) in the spondylolysis group than those in the control group, but STA was lower (P < 0.001) in the spondylolysis group.
Conclusions:
Current study results suggest that increased PI and decreased STA may play important roles in the pathology of lumbar spondylolysis in Han Chinese population.
Keywords: Lumbar Spondylolysis, Lumbosacral, Pathogenesis, Sagittal Parameters
INTRODUCTION
Lumbar spondylolysis is a common cause of low back pain in adolescents and young adults.[1] Specific pathogenesis of lumbar spondylolysis is still not entirely clear until now.[2] After years of research, it is widely accepted now that the cause of lumbar spondylolysis is multifactorial, including genetic factors, stress fracture, trauma, changes of sagittal spinopelvic biomechanics, and so on.[1,2,3] Abnormal sagittal spinopelvic parameters could cause continuous low back pain and be central to the development of many spinal disorders, including spondylolysis, spondylolisthesis, and a variety of other spinal pathologies.[4] However, the sagittal spinopelvic parameters of Han Chinese people may be different from those of Westerners. So far, there is still lack of investigation for Han Chinese people concerning the normal range of spinopelvic parameters and relationship between abnormal sagittal parameters and lumbar diseases. In this study, we investigated the difference of the sagittal lumbosacral parameters between patients with symptomatic lumbar spondylolysis and healthy adults in Han Chinese population from China.
METHODS
Subjects
This study was approved by the Ethics Committee of General Hospital of Armed Police Force (Beijing, China). From January 2013 to July 2015, a total of 52 adult patients with symptomatic lumbar spondylolysis (the spondylolysis group) treated in our hospital were identified. All the 52 patients were divided into two subgroups: Subgroup A, 36 patients with simple lumbar spondylolysis; and Subgroup B, 16 patients with lumbar spondylolysis accompanying with mild lumbar spondylolisthesis (slip percentage <30%). Inclusion criteria for the spondylolysis group were: (1) age between 18 and 55 years; (2) lumbar spondylolysis was definitely diagnosed by history taking, physical examination, and lumbar radiographs; (3) slip percentage of lumbar spondylolisthesis under 30%; (4) no history of lumbar infection, lumbar tumor, no history of pelvis or hip joints disease, and no history of previous spine surgery.
Altogether 207 healthy adults, who took regular check-ups in the medical center of our hospital during the same period, were chosen as the control group. Inclusion criteria for the control group were: (1) age between 18 and 55 years; (2) no history of lumbar, pelvis, or hip joints disease; and (3) no abnormal signs in the lumbosacral lateral radiographs.
Lumbosacral parameters
All lumbosacral lateral radiographs of the spondylolysis patients and the control group were taken with the subjects in a Horton et al.[5] position: a standardized erect posture with the knees and hips held in extension, humerus flexed forward, elbows flexed, and hands centered in the fossae, midway between the suprasternal notch and acromion. The radiographs must contain bilateral femoral heads. All radiographic parameters were measured by a single author thrice at different time with Surgimap Spine software (version: 2.2.1, Nemaris, USA). The average of the three measurements would be included in the study.
The following radiographic parameters were measured:[6] (1) pelvic incidence (PI): the angle between the line perpendicular to the superior sacral endplate and the line joining the midpoint of superior sacral endplate and the femoral head's axis, (2) pelvic tilt (PT): the angle between the line connecting the midpoint of the superior sacral endplate to the femoral head's axis and the vertical axis, (3) sacral slope (SS): the angle between the superior sacral endplate and a horizontal line, (4) lumbar lordosis (LL): the angle between the superior sacral endplate and the superior endplate of L1, (5) L5 incidence (L5I): the angle between the line perpendicular to the superior L5 endplate and the line joining the midpoint of superior L5 endplate and the femoral head's axis, (6) L5 slope (L5S): the angle between the superior L5 endplate and a horizontal line, and (7) sacral table angle (STA): the angle between the superior sacral endplate and the trailing edge line of sacrum [Figure 1].
Figure 1.
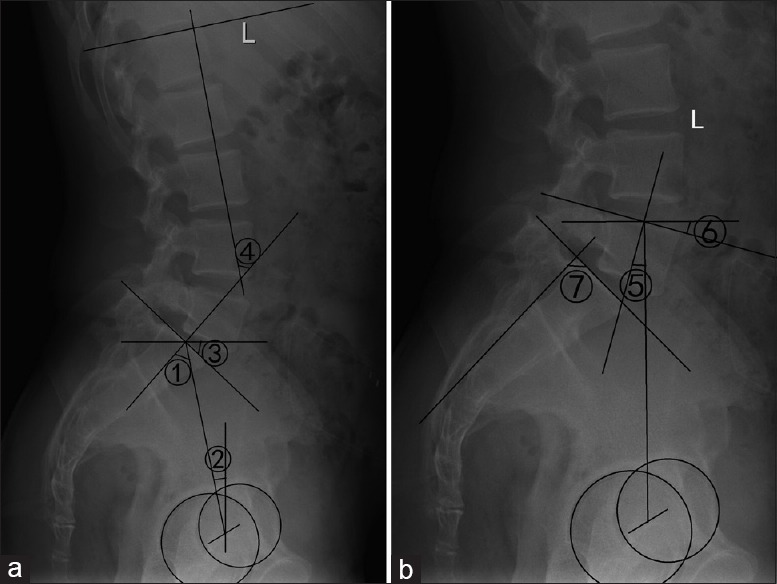
Sagittal lumbosacral parameters. (a) ①: Pelvic incidence, ②: Pelvic tilt, ③: Sacral slope, ④: Lumbar lordosis, (b) ⑤: L5 incidence, ⑥: L5 slope, ⑦: Sacral table angle.
Statistical analysis
All statistical tests were performed using SPSS (IBM Statistics software for Windows, version 19.0, SPSS, Chicago, IL, USA). Continuous variables were described by the formula of mean ± standard deviation (SD). Independent-sample t-test was performed for comparisons between the two subgroups and between the spondylolysis group and the control group. A comparison was considered significant at a value of P < 0.05.
RESULTS
The spondylolysis group consisted of 40 males (76.92%) and 12 females (23.08%) with a mean age of 30.46 years (range: 19–55 years), while the control group consisted of 139 males (67.15%) and 68 females (32.85%) with a mean age of 29.82 years (range: 18–55 years). There was no significant difference of sex ratio between two groups. Subgroup A consisted of 30 males and six females with a mean age of 32.39 years, and Subgroup B consisted of ten males and six females with a mean age of 26.13 years. Distributions of defects in Subgroup A were three bilateral at L4, 32 bilateral at L5, and one bilateral at L4 and L5, and distributions of defects and olisthesis in Subgroup B were 2 bilateral at L4 with L4 olisthesis, 13 bilateral at L5 with L5 olisthesis, and one bilateral at L4 and L5 with L4 and L5 olisthesis.
The range, mean, SD, and standard error of parameters measured of 207 normal adults in the control group are shown in Table 1. Seven sagittal lumbosacral parameters, including PI, PT, SS, LL, L5I, L5S, and STA, were compared between the two Subgroups using independent-sample t- test. No statistically significant difference of all the parameters aforementioned was found between the two subgroups (P > 0.4). When these sagittal lumbosacral parameters were compared between the spondylolysis group and the control group using independent-sample t- test, we found that the PI in the spondylolysis group was strongly higher than that in the control group (P < 0.001), but the STA in the spondylolysis group was strongly lower than that in the control group (P < 0.001). The PT, SS, and LL of the spondylolysis group were also higher (P < 0.05), while the L5I and L5S of the two groups were almost the same, and no statistically significant difference (P > 0.05) [Table 2].
Table 1.
Sagittal lumbosacral parameters of normal adults in the control group (°, n = 207)
| Parameters | Range | Mean ± SD | Standard error |
|---|---|---|---|
| PI | 25 to 68 | 41.84 ± 7.71 | 0.536 |
| PT | −10 to 30 | 10.98 ± 7.47 | 0.519 |
| SS | 9 to 54 | 30.86 ± 7.35 | 0.511 |
| LL | 10 to 69 | 35.86 ± 10.87 | 0.755 |
| L5I | 1 to 38 | 18.57 ± 6.60 | 0.459 |
| L5S | −7 to 33 | 13.97 ± 6.41 | 0.445 |
| STA | 91 to 117 | 102.67 ± 4.80 | 0.334 |
PI: Pelvic incidence; PT: Pelvic tilt; SS: Sacral slope; LL: Lumbar lordosis; L5I: L5 incidence; L5S: L5 slope; STA: Sacral table angle; SD: Standard deviation.
Table 2.
Comparisons of sagittal parameters between the two subgroups and between the spondylolysis group and the control group (°, mean ± SD)
| Parameters | Subgroup A (n = 36) | Subgroup B (n = 16) | Spondylolysis Group (n = 52) | Control group (n = 207) | P1 | P2 |
|---|---|---|---|---|---|---|
| PI | 49.61 ± 10.90 | 48.94 ± 9.49 | 49.40 ± 10.40 | 41.84 ± 7.71 | 0.832 | <0.001 |
| PT | 14.81 ± 6.60 | 13.64 ± 4.23 | 14.45 ± 5.95 | 10.98 ± 7.47 | 0.518 | 0.001 |
| SS | 34.70 ± 9.41 | 34.90 ± 9.60 | 34.76 ± 9.38 | 30.86 ± 7.35 | 0.946 | 0.007 |
| LL | 40.45 ± 13.38 | 40.94 ± 11.84 | 40.60 ± 12.81 | 35.86 ± 10.87 | 0.901 | 0.007 |
| L5I | 20.37 ± 8.76 | 22.08 ± 7.96 | 20.90 ± 8.48 | 18.57 ± 6.60 | 0.507 | 0.069 |
| L5S | 14.63 ± 8.53 | 16.71 ± 7.93 | 15.27 ± 8.33 | 13.97 ± 6.41 | 0.412 | 0.296 |
| STA | 98.12 ± 5.09 | 99.40 ± 5.24 | 98.51 ± 5.12 | 102.67 ± 4.80 | 0.413 | <0.001 |
A value of P < 0.05 was considered significant. P1: comparisons between Subgroup A and Subgroup B, P2: comparisons between the spondylolysis group and the control group. PI: Pelvic incidence; PT: Pelvic tilt; SS: Sacral slope; LL: Lumbar lordosis; L5I: L5 incidence; L5S: L5 slope; STA: Sacral table angle; SD: Standard deviation.
DISCUSSION
With the advancement of studies in recent decades, sagittal lumbosacral parameters have been thought to play important roles in the pathologies of various lumbar spine diseases. PI, first proposed by Duval-Beaupère et al.[7] in 1992, is the most important one of all the sagittal lumbosacral parameters and closely related to the pathogenesis of lumbar spondylolysis, degenerative lumbar spondylolisthesis, lumbar disc herniation, etc.[8] PI is a morphological parameter of the pelvis, has a wide normal range, and does not change with the posture or position of the pelvis.[9] PI of Han Chinese people is not only different from Westerners' but also different from Koreans'.[10,11] As a result, PI is not only related with race but also with gender, region, age, and so on. Normal PI range of Han Chinese people is 40–50°, while Westerners and Koreans have an obviously higher PI [Table 3]. PI is a static morphologic parameter that is consistent throughout a person's lifetime, with an only slight increase during growth. PT is a postural parameter of the pelvis, which reflects the compensatory ability of pelvis in the sagittal plane. PT changes with the posture and position of pelvis. The more the pelvis is retroversion, the greater the PT will be. SS, like PT, is also a postural parameter of pelvis and changes with the posture and position of the pelvis. Geometrically, PI equals the sum of PT and SS. The higher the PI is, the greater the PT or SS, or both will be. Roussouly et al.[11] first brought forth the concept of L5I. They thought that changes of L5I were closely related with isthmic lumbar spondylolisthesis. Especially when a severe isthmic spondylolisthesis occurs, L5I will change dramatically.[11,17] However, in our current study, the L5I did not increase in the Subgroup B patients when compared with that in the Subgroup A patients. Similarly, the L5I was nearly the same in both the spondylolysis group and the control group. Our current study indicates that L5I is a stable sagittal lumbosacral parameter in Han Chinese population, and not related to the pathology of lumbar spondylolysis. STA is a morphological parameter of the sacrum, which remains unchanged after birth.
Table 3.
Sagittal lumbosacral parameters of current and other studies
| Parameters | Current study | Zhu et al.[12] | He et al.[13] | Lee et al.[14] | Lim and Kim[15] | Boulay et al.[16] |
|---|---|---|---|---|---|---|
| Country | China | China | China | Korea | Korea | France |
| n | 207 | 260 | 77 | 86 | 30 | 149 |
| PI (°) | 41.84 ± 7.71 | 44.6 ± 11.2 | 43.6 ± 12.3 | 47.8 ± 9.3 | 49 ± 9 | 53.13 ± 9.04 |
| PT (°) | 10.98 ± 7.47 | 11.2 ± 7.8 | 12.9 ± 7.8 | 11.5 ± 5.3 | 11 ± 6 | 11.96 ± 6.44 |
| SS (°) | 30.86 ± 7.35 | 32.5 ± 6.5 | 29.3 ± 10.9 | 36.3 ± 7.8 | 38 ± 7 | 41.18 ± 6.96 |
| LL (°) | 35.86 ± 10.87 | 48.2 ± 9.6 | 48.2 ± 10.1 | 36.8 ± 7.6 | 48 ± 11 | 66.36 ± 9.47 |
| L5I (°) | 18.57 ± 6.60 | – | 19.2 ± 8.2 | – | – | – |
| L5S (°) | 13.97 ± 6.41 | – | – | – | – | – |
| STA (°) | 102.67 ± 4.80 | – | – | – | – | – |
–: Not enrolled; PI: Pelvic incidence; PT: Pelvic tilt; SS: Sacral slope; LL: Lumbar lordosis; L5I: L5 incidence; L5S: L5 slope; STA: Sacral table angle.
Numerous studies[12,13,14,15,16] have been performed to evaluate sagittal spinopelvic alignment in normal adults and show that those lumbosacral parameters remain relatively stable through adulthood after skeletal maturation. Kim et al.[18] carried out a prospective comparative analysis of sagittal alignment between young and old men without any spinal pathology and found that no significant difference of PI, PT, and SS exist between the normal young and old men. In this study, there was no significant difference of age between the spondylolysis group and the control group.
Recently, some scholars performed a series of studies about the correlation of sagittal lumbosacral parameters between lumbar spondylolysis and normal adults. Roussouly et al.[19] compared 82 lumbar spondylolysis patients with a reference population of 160 normal adult volunteers without symptoms of low back pain or radiographic abnormalities, and noted that PI, PT, SS, and LL were significantly higher in patients with lumbar spondylolysis. In a study from Korean, Oh et al.[20] compared sagittal alignment of a cohort of 198 adults patients with lumbar spondylolysis with 80 age-matched healthy adults without any lumbar diseases, and demonstrated that patients with lumbar spondylolysis showed greater PI and SS but lower STA than healthy adults. These two studies all emphasized the importance of increased PI for lumbar spondylolysis.
In the present study, we observed that PI, PT, SS, and LL were higher in the spondylolysis group than those in the control group, but STA was lower in the spondylolysis group. PI of the spondylolysis group was 49.40° ± 10.40°, while PI of the control group was 41.84° ± 7.71°, and the difference was obviously statistically significant (P < 0.001) [Table 2]. We believe that high PI can be an important factor contributing to the development of lumbar spondylolysis. Because of the geometrical relationship of PI = PT + SS and a narrow normal range of PT (10–15° generally), when PI increases, SS shows a greater increase than PT. When PI increases, LL also increases. The higher the PI is, the more vertical the sacrum will be. Under the circumstances, a higher LL is needed for the spine to maintain an overall balance. On the contrary, the lower the PI is, the more horizontal the sacrum and the lower the LL will be. In our study, the LL of 52 spondylolysis patients was 40.60° ± 12.81°, and the LL of 207 normal adults was 35.86° ± 10.87°. LL of the spondylolysis group was significantly higher than LL of the control group (P = 0.007). Stagnara et al.[21] reported that LL increases linearly with SS. Labelle et al.[22] concluded that among people with high PI, the increase of LL is to maintain the center of gravity and C7 plumb line behind the femoral heads and to sustain a balanced posture. L5 vertebra lies over the oblique endplate of S1. Furthermore, L4 and L5 are not only the junction of lumbar and sacrum anatomically but also a transitive hub of gravity.[23] Gravity loads on the spine are divided into two parts at L5/S1, one being the forward shear force along the superior endplate of S1, and the other being the downward compression load perpendicular to the superior endplate of S1. Moreover, the shear force increases from L1 to L5 gradually, reaching a maximum value on L5.[24] SS and LL are also high in people with high PI. The higher the SS and LL are, the greater the shear force and stress on L5 pars interarticularis will produce. Hence, people with high PI suffer from lumbar spondylolysis or lumbar spondylolisthesis more easily.
In addition, our study found that the STA of spondylolysis group was lower than the normal control group, and the mean STA of the spondylolysis group was 98.51° ± 5.12°, while the mean STA of the control group was 102.67° ± 4.80° (P < 0.001) [Table 2]. Inoue et al.[25] in 2002 reported that STA showed a significant decrease in the patients with spondylolysis and subsequent spondylolisthesis. Whitesides et al.[26] in 2005 concluded that people with lower STA were more likely to develop a pars defect, and even thought that STA was more strongly associated with lumbar spondylolysis than PI. In 2013, after comparing STA of 198 lumbar spondylolysis patients with 80 normal adults, Oh et al.[20] found that STA of the spondylolysis patients was 94.4° ± 5.8° while STA of the normal adults was 100.5° ± 4.7°, and the difference was statistically significant (P < 0.001). All the results of the studies mentioned above are consistent with ours. Low STA leads to the increase of shear force in the lumbar spine, especially in the lower lumbar spine, which produces more stress on pars interarticularis and finally results in spondylolysis.
It is well known that spondylolysis almost all occurs in childhood and adolescence.[27,28] However, all radiographic data collected in the current study derived from an adult population. As such, a question is subsequently brought forth that whether conclusions obtained from adults in our study can be applied to children and adolescents or not? After evaluation of sagittal alignment in a cohort of 341 normal children and adolescents (range: 3–18 years), Mac-Thiong et al.[29] concluded that mean values of PI, PT, SS, LL, and other parameters were slightly smaller than those of normal adults, but the children and adolescents displayed similar sagittal biomechanics and relationships between sagittal parameters when compared with adults. PI was also closely correlated with PT and SS, and high PI or low STA also lead to the increase of lumbosacral shear force and put more stress on pars interarticularis in the children and adolescents. The results from Ghandhari et al.[30] were in accordance with that from Mac-Thiong et al.[29] after their study of 98 children and adolescents (range: 8–19 years) in sagittal parameters. Therefore, like in adults, high PI or low STA make lumbar spondylolysis occur more easily in children and adolescents too.
In conclusion, our current study found that PI, PT, SS, and LL were higher in the spondylolysis group than those in the control group, and L5I and L5S were almost the same in both groups, but STA was lower in the spondylolysis group than that in the control group. The results suggest that higher PI and lower STA may play more important roles in the pathology of lumbar spondylolysis in Han Chinese population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Altaf F, Heran MK, Wilson LF. Back pain in children and adolescents. Bone Joint J. 2014;96-B:717–23. doi: 10.1302/0301-620X.96B6.33075. doi: 10.1302/0301-620X.96B6.33075. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Green DW. Spondylolysis in the adolescent athlete. Curr Opin Pediatr. 2011;23:68–72. doi: 10.1097/MOP.0b013e32834255c2. doi: 10.1097/MOP.0b013e32834255c2. [DOI] [PubMed] [Google Scholar]
- 3.Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: A review. Skeletal Radiol. 2011;40:683–700. doi: 10.1007/s00256-010-0942-0. doi: 10.1007/s00256-010-0942-0. [DOI] [PubMed] [Google Scholar]
- 4.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1459–67. doi: 10.1007/s00586-006-0294-6. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horton WC, Brown CW, Bridwell KH, Glassman SD, Suk SI, Cha CW. Is there an optimal patient stance for obtaining a lateral 36” radiograph? A critical comparison of three techniques. Spine (Phila Pa 1976) 2005;30:427–33. doi: 10.1097/01.brs.0000153698.94091.f8. [DOI] [PubMed] [Google Scholar]
- 6.Klineberg E, Schwab F, Smith JS, Gupta MC, Lafage V, Bess S. Sagittal spinal pelvic alignment. Neurosurg Clin N Am. 2013;24:157–62. doi: 10.1016/j.nec.2012.12.003. doi: 10.1016/j.nec.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Duval-Beaupère G, Schmidt C, Cosson P. A Barycentremetric study of the sagittal shape of spine and pelvis: The conditions required for an economic standing position. Ann Biomed Eng. 1992;20:451–62. doi: 10.1007/BF02368136. [DOI] [PubMed] [Google Scholar]
- 8.Mehta VA, Amin A, Omeis I, Gokaslan ZL, Gottfried ON. Implications of spinopelvic alignment for the spine surgeon. Neurosurgery. 2012;70:707–21. doi: 10.1227/NEU.0b013e31823262ea. doi: 10.1227/01.neu.0000462077.50830.1a. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Hresko MT. Radiographic analysis of spondylolisthesis and sagittal spinopelvic deformity. J Am Acad Orthop Surg. 2012;20:194–205. doi: 10.5435/JAAOS-20-04-194. doi: 10.5435/JAAOS-20-04-194. [DOI] [PubMed] [Google Scholar]
- 10.Li WS, Li G, Chen ZQ, Wood KB. Sagittal plane analysis of the spine and pelvis in adult idiopathic scoliosis. Chin Med J. 2010;123:2978–82. doi: 10.3760/cma.j.issn.0366-6999.2010.21.005. [PubMed] [Google Scholar]
- 11.Roussouly P, Gollogly S, Berthonnaud E, Labelle H, Weidenbaum M. Sagittal alignment of the spine and pelvis in the presence of L5-s1 isthmic lysis and low-grade spondylolisthesis. Spine (Phila Pa 1976) 2006;31:2484–90. doi: 10.1097/01.brs.0000239155.37261.69. doi: 10.1097/01.brs.0000239155.37261.69. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Xu L, Zhu F, Jiang L, Wang Z, Liu Z, et al. Sagittal alignment of spine and pelvis in asymptomatic adults: Norms in Chinese populations. Spine (Phila Pa 1976) 2013;39:E1–6. doi: 10.1097/BRS.0000000000000022. doi: 10.1097/BRS.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 13.He S, Zhu F, Qiu Y, Zhu Z, Bao H, Sun X, et al. The change and clinical significance of sagittal spino-pelvic parameters of adult spondylolisthesis. Chin J Spine Cord. 2014;24:109–15. doi: 10.3969/j.issn.1004-406X.2014.02.03. [Google Scholar]
- 14.Lee CS, Chung SS, Kang KC, Park SJ, Shin SK. Normal patterns of sagittal alignment of the spine in young adults radiological analysis in a Korean population. Spine (Phila Pa 1976) 2011;36:E1648–54. doi: 10.1097/BRS.0b013e318216b0fd. doi: 10.1097/BRS.0b013e318216b0fd. [DOI] [PubMed] [Google Scholar]
- 15.Lim JK, Kim SM. Difference of sagittal spinopelvic alignments between degenerative spondylolisthesis and isthmic spondylolisthesis. J Korean Neurosurg Soc. 2013;53:96–101. doi: 10.3340/jkns.2013.53.2.96. doi: 10.3340/jkns.2013.53.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulay C, Tardieu C, Hecquet J, Benaim C, Mouilleseaux B, Marty C, et al. Sagittal alignment of spine and pelvis regulated by pelvic incidence: Standard values and prediction of lordosis. Eur Spine J. 2006;15:415–22. doi: 10.1007/s00586-005-0984-5. doi: 10.1007/s00586-005-0984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu F, Bao H, Liu Z, Mao S, He S, Zhu Z, et al. Analysis of L5 incidence in normal population use of L5 incidence as a guide in reconstruction of lumbosacral alignment. Spine (Phila Pa 1976) 2014;39:E140–6. doi: 10.1097/BRS.0000000000000069. doi: 10.1097/BRS.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 18.Kim YB, Kim YJ, Ahn YJ, Kang GB, Yang JH, Lim H, et al. A comparative analysis of sagittal spinopelvic alignment between young and old men without localized disc degeneration. Eur Spine J. 2014;23:1400–6. doi: 10.1007/s00586-014-3236-8. doi: 10.1007/s00586-014-3236-8. [DOI] [PubMed] [Google Scholar]
- 19.Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976) 2005;30:346–53. doi: 10.1097/01.brs.0000152379.54463.65. doi: 10.1097/01.brs.0000152379.54463.65. [DOI] [PubMed] [Google Scholar]
- 20.Oh YM, Choi HY, Eun JP. The comparison of sagittal spinopelvic parameters between young adult patients with L5 spondylolysis and age-matched control group. J Korean Neurosurg Soc. 2013;54:207–10. doi: 10.3340/jkns.2013.54.3.207. doi: 10.3340/jkns.2013.54.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagnara P, De Mauroy JC, Dran G, Gonon GP, Costanzo G, Dimnet J, et al. Reciprocal angulation of vertebral bodies in a sagittal plane: Approach to references for the evaluation of kyphosis and lordosis. Spine (Phila Pa 1976) 1982;7:335–42. doi: 10.1097/00007632-198207000-00003. doi: 10.1097/00007632-198207000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Labelle H, Mac-Thiong JM, Roussouly P. Spino-pelvic sagittal balance of spondylolisthesis: A review and classification. Eur Spine J. 2011;20(Suppl 5):641–6. doi: 10.1007/s00586-011-1932-1. doi: 10.1007/s00586-011-1932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vathana P, Prasartritha T. A biomechanic study of the surgical repair technique of pars defect in spondylolysis. J Med Assoc Thai. 1998;81:824–9. [PubMed] [Google Scholar]
- 24.Hajihosseinali M, Arjmand N, Shirazi-Adl A. Effect of body weight on spinal loads in various activities: A personalized biomechanical modeling approach. J Biomech. 2015;48:276–82. doi: 10.1016/j.jbiomech.2014.11.033. doi: 10.1016/j.jbiomech.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Inoue H, Ohmori K, Miyasaka K. Radiographic classification of L5 isthmic spondylolisthesis as adolescent or adult vertebral slip. Spine (Phila Pa 1976) 2002;27:831–8. doi: 10.1097/00007632-200204150-00010. doi: 10.1097/00007632-200204150-00010. [DOI] [PubMed] [Google Scholar]
- 26.Whitesides TE, Jr, Horton WC, Hutton WC, Hodges L. Spondylolytic spondylolisthesis: A study of pelvic and lumbosacral parameters of possible etiologic effect in two genetically and geographically distinct groups with high occurrence. Spine (Phila Pa. 1976;2005;30 6 Suppl:S12–21. doi: 10.1097/01.brs.0000155574.33693.60. doi: 10.1097/01.brs.0000155574.33693.60. [DOI] [PubMed] [Google Scholar]
- 27.Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66:699–707. [PubMed] [Google Scholar]
- 28.Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: Review of the literature. J Orthop Sci. 2010;15:281–8. doi: 10.1007/s00776-010-1454-4. doi: 10.1007/s00776-010-1454-4. [DOI] [PubMed] [Google Scholar]
- 29.Mac-Thiong JM, Labelle H, Berthonnaud E, Betz RR, Roussouly P. Sagittal spinopelvic balance in normal children and adolescents. Eur Spine J. 2007;16:227–34. doi: 10.1007/s00586-005-0013-8. doi: 10.1007/s00586-005-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghandhari H, Hesarikia H, Ameri E, Noori A. Assessment of normal sagittal alignment of the spine and pelvis in children and adolescents. Biomed Res Int 2013. 2013 doi: 10.1155/2013/842624. 842624. doi: 10.1155/2013/842624. [DOI] [PMC free article] [PubMed] [Google Scholar]


