Abstract
Background:
Disrupted Ca2+ homeostasis contributes to the development of colonic dysmotility in ulcerative colitis (UC), but the underlying mechanisms are unknown. This study aimed to examine the alteration of colonic smooth muscle (SM) Ca2+ signaling and Ca2+ handling proteins in a rat model of dextran sulfate sodium (DSS)-induced UC.
Methods:
Male Sprague-Dawley rats were randomly divided into control (n = 18) and DSS (n = 17) groups. Acute colitis was induced by 5% DSS in the drinking water for 7 days. Contractility of colonic SM strips (controls, n = 8 and DSS, n = 7) was measured in an organ bath. Cytosolic resting Ca2+ levels (n = 3 in each group) and Ca2+ transients (n = 3 in each group) were measured in single colonic SM cells. Ca2+ handling protein expression was determined by Western blotting (n = 4 in each group). Differences between control and DSS groups were analyzed by a two-sample independent t-test.
Results:
Average tension and amplitude of spontaneous contractions of colonic muscle strips were significantly enhanced in DSS-treated rats compared with controls (1.25 ± 0.08 g vs. 0.96 ± 0.05 g, P = 0.007; and 2.67 ± 0.62 g vs. 0.52 ± 0.10 g, P = 0.013). Average tensions of carbachol-evoked contractions were much weaker in the DSS group (1.08 ± 0.10 g vs. 1.80 ± 0.19 g, P = 0.006). Spontaneous Ca2+ transients were observed in more SM cells from DSS-treated rats (15/30 cells) than from controls (5/36 cells). Peak caffeine-induced intracellular Ca2+ release was lower in SM cells of DSS-treated rats than controls (0.413 ± 0.046 vs. 0.548 ± 0.041, P = 0.033). Finally, several Ca2+ handling proteins in colonic SM were altered by DSS treatment, including sarcoplasmic reticulum calcium-transporting ATPase 2a downregulation and phospholamban and inositol 1,4,5-trisphosphate receptor 1 upregulation.
Conclusions:
Impaired intracellular Ca2+ signaling of colonic SM, caused by alteration of Ca2+ handing proteins, contribute to colonic dysmotility in DSS-induced UC.
Keywords: Calcium; Dextran Sulfate Sodium; Inositol 1; 4,5-trisphosphate Receptor; Large-conductance Calcium-activated Potassium Channels; Phospholamban Protein; Sarcoplasmic Reticulum Calcium-transporting ATPase Calcium ATPase; Ulcerative Colitis
INTRODUCTION
Ulcerative colitis (UC) involves chronic, relapsing inflammation of the gastrointestinal tract. Previous studies showed that, in UC, colon motility was abnormal, with increased propulsive activity and decreased segmenting contractions.[1,2] These motor abnormalities can persist even in patients with quiescent UC.[3] However, the underlying mechanisms of the abnormal motility are unclear. Changes in the enteric nervous system may contribute to the dysmotility.[4,5,6] However, the altered contractility of smooth muscle (SM) also plays a potentially important role.
Increased cytosolic Ca2+ is crucial to SM contraction, which relies on Ca2+ influx from extracellular stores and Ca2+ release from intracellular stores during action potentials. It was reported by some that reduced Ca2+ influx contributed to dysmotility in colitis,[7,8] and by others that disrupted calcium release was the major cause of colonic dysmotility.[9,10,11,12] The alteration of Ca2+ pathways in colonic SM during colitis, therefore, remain unclear.
Inositol 1,4,5-trisphosphate receptor (IP3R) and ryanodine receptor (RyR) are Ca2+ release channels while sarcoplasmic reticulum Ca2+-ATPase (SERCA) is a Ca2+ pump residing on the sarcoplasmic reticulum (SR). Alteration in these channels can impair Ca2+ homeostasis and result in abnormal SM contractility.
The aim of this study was to determine, in a UC model: (1) whether intracellular Ca2+ stores were changed during inflammation, and if so, how this alteration influenced colonic motility; and (2) the underlying molecular mechanisms of impaired intracellular Ca2+ homeostasis.
METHODS
Animals
Male Sprague-Dawley rats (aged 7 weeks and weighing 190–210 g) were purchased from Vital River Laboratories (Beijing, China) and housed in the animal facility of Peking University First Hospital, in accordance with approved ethical guidelines. Animals were exposed to light: dark cycles (12 h: 12 h) with a controlled environmental temperature (22°C) and were acclimatized for 3 days before entering the study.
Animal grouping and induction of colitis
Using a random number table, 35 normal rats were divided into control (n = 18) and dextran sulfate sodium (DSS) (n = 17) groups. Induction of colitis was performed as previously described with minor alteration.[13] Rats of the DSS group were administered 5% DSS (MW 36,000–50,000; MP Biomedicals, LLC, OH, USA) in the drinking water for 7 days. Control, or healthy, rats drank only the same tap water. On day 8, the distal colons were removed from rats anesthetized with 5% chloral hydrate (Sigma-Aldrich, St. Louis, MO, USA). After removing feces, colons were fully cleaned with ice-cold extracellular solution (140 mmol/L NaCl, 2 mmol/L CaCl2, 5.5 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L HEPES, 3 mmol/L glucose, pH 7.4). Colon segments at 5 cm above the anus were harvested and used for further analysis. Using a random number table, 8 control and 7 DSS-treated rats were selected and contractility of the muscle strips was measured. In addition, 3 rats were selected from each group to measure Ca2+ transients and another 3 rats to measure cytosolic Ca2+. The remaining 4 rats in each group were used to harvest samples for Western blotting. The experiment was performed at the National Laboratory of Biomacromolecules, Chinese Academy of Sciences, Beijing, China.
Histological staining
Distal colon segments were fixed in paraformaldehyde (Sigma-Aldrich) for 24 h and were then embedded in paraffin and sectioned (5 µm) onto glass slides. Hematoxylin-eosin staining was performed using standard protocols. Images were acquired on a Leica SCN400 Scanner microscope (Leica, Mannheim, Germany).
Contractile measurement of rat colonic muscle strips in vitro
Contractile measurements were performed as previously described with minor alteration.[14] To measure isometric tension contractions, approximately 0.5 cm freshly prepared muscle strips with mucosa were suspended in the circular direction in a 10-ml organ bath, coupled with a model BL-420F acquisition system (Chengdu TME Technology Co., Ltd., Sichuan, China), and containing oxygenated Krebs solution (119 mmol/L NaCl, 2.5 mmol/L CaCl2, 4.7 mmol/L KCl, 1.17 mmol/L MgSO4, 1.18 mmol/L KH2PO4, 25 mmol/L NaHCO3, 11 mmol/L glucose) at 37°C. The tissues were allowed to equilibrate for 1 h, with the bath fluid changed every 15 min, at a resting tension of 1 g. After equilibration, 1 μmol/L carbachol (Cch) (Sigma-Aldrich) was used to stimulate contraction of the muscle strips. After washing out the Cch, 20 mmol/L KCl was applied to enhance muscle strip contraction. The mean amplitude, frequency and average tension of spontaneous, Cch- and KCl-enhanced contractions were measured from 5 min before until 5 min after application of each treatment.
Isolation of smooth muscle cells
Single SM cells were prepared enzymatically as previously described with minor modifications.[15] The mucosa and serosa of distal colon segments were removed under a microscope. The SM was then minced and incubated in a Ca2+-free enzyme extracellular solution containing 2 mg/ml papain, 1 mg/ml dithiothreitol, and 1 mg/ml bovine serum albumin (BSA) (all from Sigma-Aldrich) for 20 min at 37°C. The solution was then decanted and the cell suspension rinsed three times and placed in a low Ca2+ (0.1 mmol/L) extracellular solution containing 1 mg/ml collagenase H (Roche Diagnostics, Mannheim, Germany), 1 mg/ml dithiothreitol and 1 mg/ml BSA for an additional 15 min at 37°C. Next, collagenase-free low Ca2+ extracellular solution was used to wash the suspended cells to remove all collagenase and single cells were released by gentle agitation. Cells were maintained in the low Ca2+ extracellular solution supplemented with 1 mg/ml BSA at 4°C.
Measurement of Ca2+ fluorescence
Freshly isolated colonic SM cells on glass coverslips were incubated with 5 μmol/L Fluo-4 AM (Molecular Probes, Eugene, OR, USA) for 20 min at 37°C and then perfused for 20 min at room temperature with normal extracellular solution. The 40× oil immersion objective of an inverted microscope (Leica) connected to a software-controlled (Las AF, Leica) cooled charge-coupled camera (Leica SP5 confocal microscope) was used to capture images. X-Y images were obtained every 573 ms for 2 min. To capture spontaneous transients, SM cells were first perfused with normal extracellular solution for about 20 min and then 10 mmol/L caffeine (Sigma-Aldrich) was directly applied to evoke Ca2+ transients and cell contraction.
Custom software written in MATLAB (The Mathworks Inc., Natick, MA, USA) was used to calculate kinetic data, including F0, starting time, rising time, peak F/F0, half-time of decay and final offset using nonlinear least squares fitting routine. Leica Las AF software (Leica, Mannheim, Germany) was used to analyze X-Y images, and fluorescence profiles were constructed and transferred to Microsoft Excel (Microsoft Corp., Redmond, WA, USA). The average fluorescence value of the continuous 20 images without Ca2+ transient activity was calculated as F0. The amplitude and frequency of Ca2+ transients were calculated before and after caffeine application. Microsoft Excel software was used to determine kinetic parameters of the Ca2+ signals.
Cytosolic Ca2+ measurements
Freshly isolated cells were kept in extracellular solution and incubated with 2 μmol/L Fura 2-AM (molecular probes) for 20 min. Then SM cells were placed in chambers mounted on the stage of an inverted microscope (Olympus, Tokyo, Japan). The excitation wavelengths were switched between 340 and 380 nm and fluorescence emission was measured at 510 nm. Cytosolic Ca2+ concentrations were calculated from fluorescence ratios as previously described.[16]
Western blotting analysis
Western blotting analysis was performed as described previously[17] with minor modifications. SM samples were homogenized in RIPA buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) to extract protein. Samples were heated at 90°C for 5 min and centrifuged at 5000 ×g for 5 min. The supernatants were resolved on 10% (for large-conductance calcium-activated potassium channel [BKCa], SERCA2a and nuclear factor-kappa B [NF-κB]) or 8% (for IP3R1) SDS-polyacrylamide gels and 4–12% Bis-Tris Gels (Invitrogen, NY, CA, USA) (for phospholamban [PLB]). The resolved protein bands were transferred to PVDF membranes (Millipore, Bedford, MA, USA) at 300 mA for 1 h (BKCa, NF-κB), 300 mA for 1.5 h (SERCA2a), 400 mA for 1.5 h (IP3R1), or 400 mA for 1 h (PLB). The membranes were blocked with Tris-buffered saline-Tween 20 containing 5% nonfat dry milk (Sigma-Aldrich) at room temperature for 1 h and then incubated at 4°C overnight with anti-SERCA IgG (1:1000, ab2801, Abcam, Cambridge, UK), anti-BKCa IgG (1:1000, ab3587, Abcam), anti-NF-κB IgG (1:2000, 8242, Cell Signaling Technology, Inc.), anti-IP3R1 IgG (1:1000, ab5804, Abcam) or anti-PLB IgG (1:1000, ab2865, Abcam). The membranes were washed and then incubated with the appropriate secondary antibodies at room temperature. After washing, labeled bands were detected using enhanced chemiluminescence solutions 1 and 2 (1:1) (Millipore).
Statistical analyses
Data are expressed as mean ± standard error (SE). Statistical differences between means were determined with SPSS version 16.0 (SPSS, Chicago, IL, USA). Differences between control and DSS groups were analyzed using two-sample independent t-test and comparison within the group before and after infusion of various stimulations were compared by paired t-test. Bonferroni correction was performed in multiple comparisons. N represents the number of cells and n represents the number of rats. P < 0.05 was considered statistically significant.
RESULTS
Morphology and histopathology
Rats drinking DSS solution for 7 days had colitis with diarrhea and bloody stools. The lengths of the large intestines in DSS treated rats (n = 7) were much shorter than in controls (n = 8) (14.4 ± 0.8 cm vs. 18.6 ± 0.3 cm, t = 5.174, P < 0.001) [Figure 1a and 1b]. In contrast, no statistical differences in weight gain were observed between these two groups (28 ± 2% vs. 29 ± 1%, t = 0.432, P = 0.673) [Figure 1c]. Histopathology indicated that several features of colonic inflammation such as superficial focal ulceration, crypt abscess, areas of crypt destruction and infiltration of inflammatory cells in the submucosa were observed in DSS treated but not in control rats [Figure 1d–1g]. NF-κB, a transcription factor regulating expression of various inflammatory cytokines, was upregulated within the SM layer in DSS treated rats (1.56 ± 0.17 vs. 0.81 ± 0.20, t = 2.844, P = 0.029, n = 4) [Supplementary Figure 1 (199.9KB, tif) ].
Figure 1.
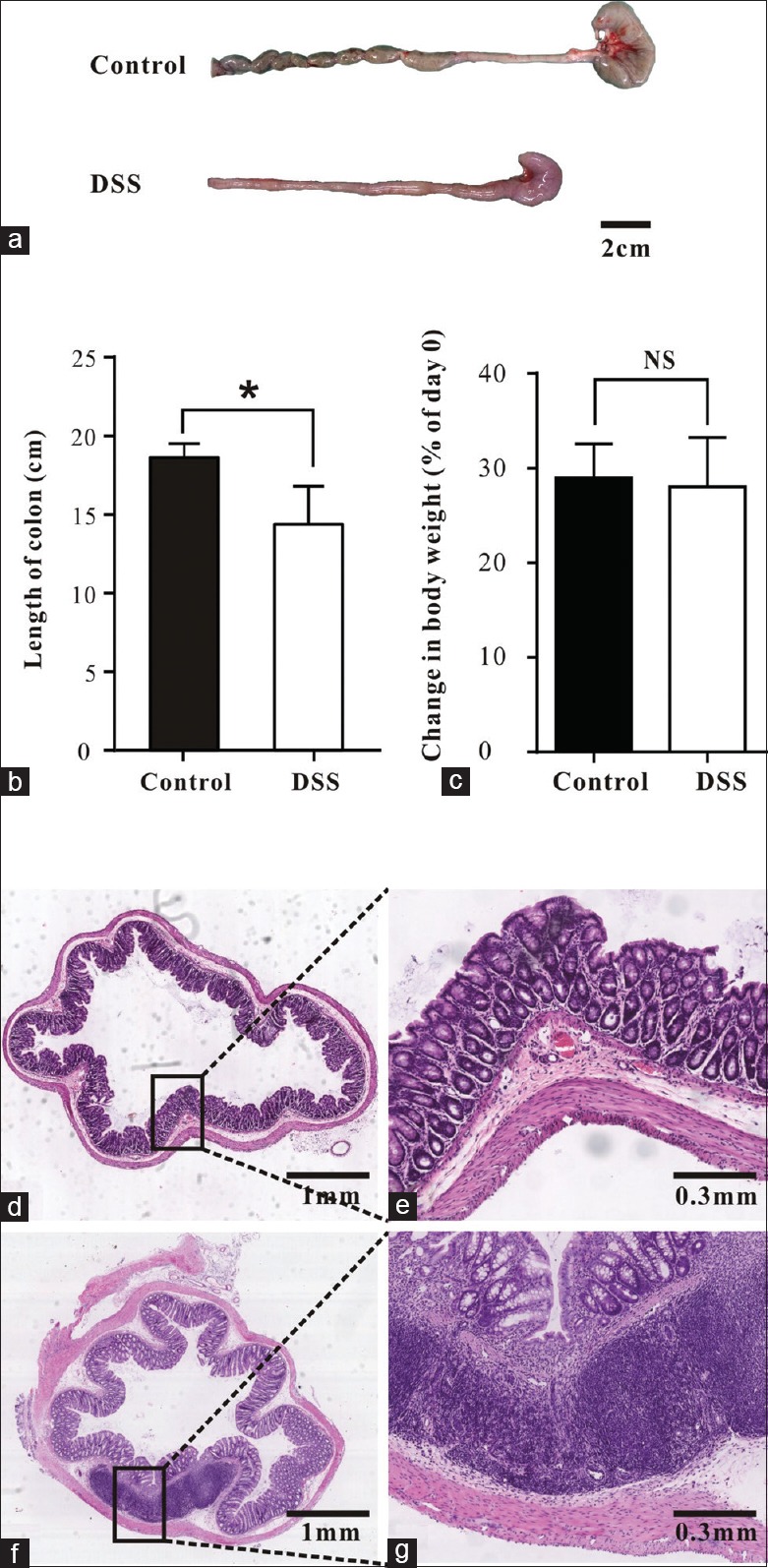
Changes in colonic length, body weight and histology. (a and b) The length of the large intestines from control and DSS treated rats. (c) Changes in body weight shown as percentage of weight on d 0. (d-g) Representative H and E stained sections showing histology of colons from control (d-e) and DSS treated rats (f-g). Data are mean ± SE of n (controls, n = 8 and DSS, n = 7) independent colonic strips. *P < 0.05; NS: Not significantly different. DSS: Dextran sulfate sodium; SE: Standard error.
Upregulated NF-κB expression in colonic SM from DSS treated rats, compared with controls. (a) Representative Western blots for NF-κB. (b) Summary data showing NF-κB expression. Values are mean ± SE of n rats (n = 4 per group). At least 3 separate experiments were performed and showed similar results. *P < 0.05 versus vehicle. SE: Standard error; DSS: Dextran sulfate sodium; SM: Smooth muscle; NF-κB: Nuclear factor-kappa B.
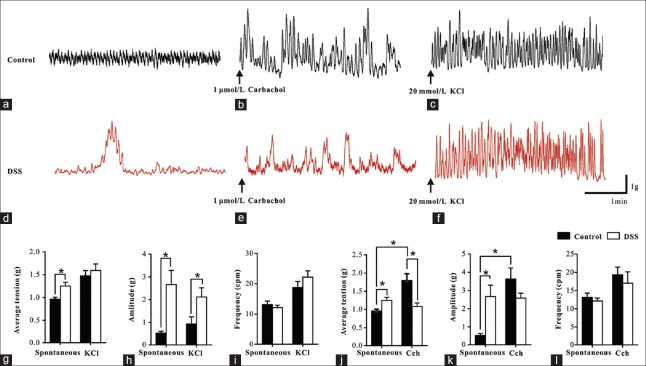
Spontaneous and stimuli-mediated contractions of colonic smooth muscle strips from control and dextran sulfate sodium treated rats
Average tensions and amplitudes of the spontaneous phasic contractions of SM strips from DSS treated rats (n = 7) were significantly increased compared with those from controls (n = 8) (1.25 ± 0.08 g vs. 0.96 ± 0.05 g, t = 3.198, P = 0.007 and 2.67 ± 0.62 g vs. 0.52 ± 0.10 g, t = 3.676, P = 0.013, respectively) [Figure 2a, 2d and 2g–2h]. The frequencies of spontaneous phasic contractions of SM strips from DSS treated rats were similar to controls (12.14 ± 0.82 cycles per minute [cpm] vs. 13.15 ± 1.17 cpm, t = 0.685, P = 0.505) [Figure 2i].
Figure 2.
Alterations of distal colon SM contraction. (a-f) Representative traces of spontaneous, 1 μmol/L Cch induced and 20 mmol/L KCl induced contractions of SM strips. (g-i) Summary data showing the average tension, amplitude, and frequency of spontaneous and KCl enhanced contractions. (j-l) Summary data showing the average tension, amplitude, and frequency of Cch enhanced contractions. Data are mean ± SE of n (controls, n = 8 and DSS, n = 7) independent SM strips. *P < 0.05. SM: Smooth muscle; SE: Standard error; Cch: Carbachol; DSS: Dextran sulfate sodium.
The average tension (1.59 ± 0.14 g vs. 1.48 ± 0.12 g, for DSS treated and control rats, respectively, t = 0.656, P = 0.523) and frequency (22.20 ± 2.07 cpm vs. 18.80 ± 1.96 cpm, for DSS treated and control rats, respectively, t = 1.193, P = 0.254) of KCl (20 mmol/L)-induced contractions of muscle strips between the control and colitis were not markedly different [Figure 2c, 2f, 2g and 2i]. The amplitude of KCl (20 mmol/L)-induced contractions of muscle strips of colotic rats was higher compared to control rats (2.12 ± 0.40 g vs. 0.94 ± 0.31 g, t = 2.359, P = 0.035) [Figure 2h].
In control rats, the Cch (1 μmol/L) induced contractions of SM strips had an average tension that was increased about 2-fold above baseline (from 0.96 ± 0.05 g to 1.80 ± 0.19 g, t = 5.424, P = 0.001) [Figure 2b and 2j] and an amplitude of phasic contraction increased over 7-fold (from 0.52 ± 0.10 g to 3.62 ± 0.60 g, t = 5.005, P = 0.002) [Figure 2k]. However, in SM strips from the DSS group, Cch did not significantly increase either average tension or amplitude of phasic contractions [Figure 2e and 2j–2k]. Thus, compared to control groups, the average tension of Cch-evoked contractions in DSS group were much weaker (1.08 ± 0.10 g vs. 1.80 ± 0.19 g, t = 3.253, P = 0.006). In both DSS and control rats, there was no statistically significant effect of Cch on frequency of SM contractions (17.03 ± 3.11 cpm vs. 19.35 ± 2.10 cpm, t = 0.632, P = 0.538) [Figure 2l].
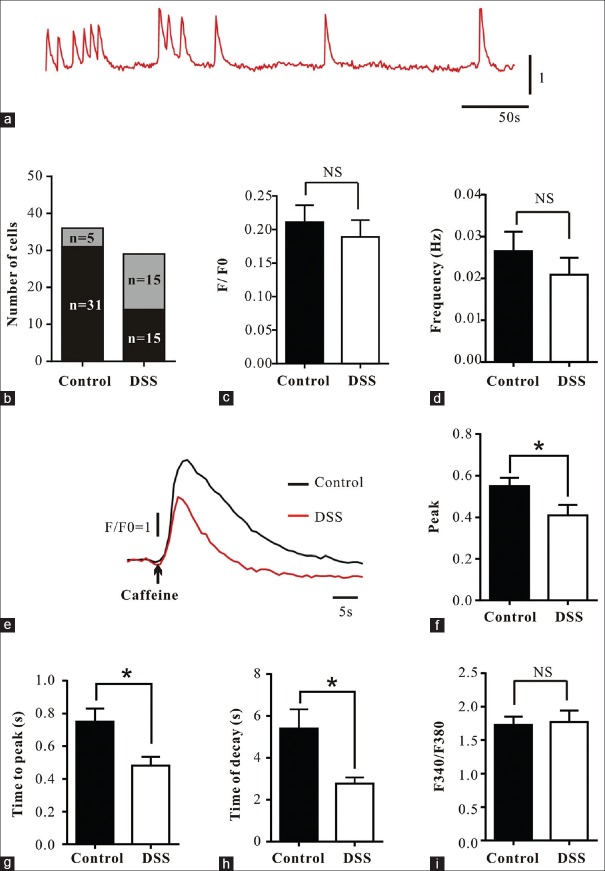
Ca2+ transients were significantly altered in colonic smooth muscle of dextran sulfate sodium treated rats
In Fluo-4 AM-loaded colonic SM cells from both control and DSS treated rats (n = 3) spontaneous Ca2+ transients were observed but these were more likely to occur in cells from the DSS treated rats (50% vs. 14%, N = 30 in DSS group and N = 36 in control group) [Figure 3a and 3b]. The frequency (0.021 ± 0.004 Hz vs. 0.027 ± 0.005 Hz, t = 0.752, P = 0.462) and amplitude (F/F0) (0.189 ± 0.025 vs. 0.211 ± 0.025, t = 0.481, P = 0.636) of Ca2+ transients of SM cells from DSS treated rats were similar to that of control rats [Figure 3c and 3d].
Figure 3.
Impairment of spontaneous and caffeine induced Ca2+ transients. (a-d) Typical fluorescence profiles and summary data showing the number of cells, F/F0 and frequency of spontaneous Ca2+ transients (controls, N = 36; DSS, N = 30). (e-h) Representative fluorescence profiles and summary data showing ΔF/F0, time to peak and time of decay of caffeine-induced Ca2+ transients (controls, N = 34; DSS, N = 24). (i) Summary data showing cytosolic Ca2+ levels in SM cells (controls, N = 50; DSS, N = 58). Data are mean ± SE of N cells isolated from n rats (n = 3). *P < 0.05; NS: Not significantly different. DSS: Dextran sulfate sodium; SE: Standard error.
The amplitudes of caffeine-induced Ca2+ transients from DSS treated rats were significantly lower than controls (0.413 ± 0.046 vs. 0.548 ± 0.041, N = 24 in DSS group and N = 34 in control group, t = 2.184, P = 0.033) [Figure 3e and 3f]. In myocytes from DSS treated rats, time to peak (0.481 ± 0.054 s vs. 0.749 ± 0.083 s, t = 2.459, P = 0.017) and time of decay (2.773 ± 0.294 s vs. 5.399 ± 0.929 s, t = 2.311, P = 0.010) were significantly shorter than those from controls [Figure 3g and 3h].
The cytosolic Ca2+ levels measured in Fura-2 AM-loaded colonic SM cells from DSS treated rats were similar to those in controls (1.768 ± 0.023 vs. 1.727 ± 0.017, N = 58 in DSS group and N = 50 in control group, t = 1.386, P = 0.169) [Figure 3i].
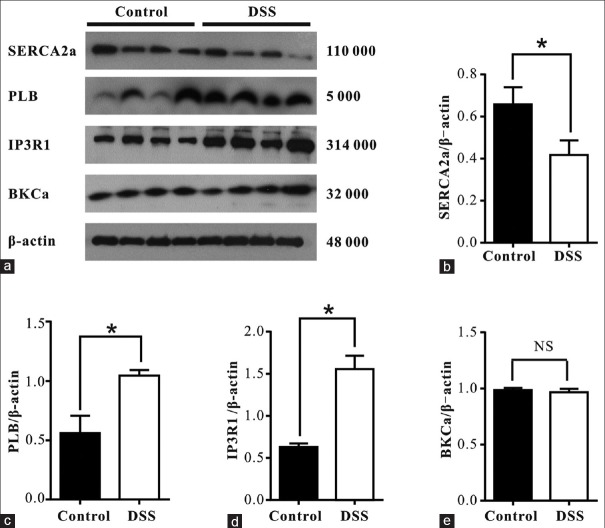
Altered expression of Ca2+ handling proteins
Because SERCA2a, PLB, IP3R1, and BKCa are Ca2+ handling proteins in SM cells, their expression in colonic SM from control and DSS treated rats (n = 4) were analyzed by Western blotting. As shown in Figure 4, SERCA2a expression within the SM layer was significantly lower in DSS treated rats than in controls (0.42 ± 0.05 vs. 0.66 ± 0.06, t = 3.274, P = 0.017) [Figure 4b]. Expression of PLB (1.05 ± 0.05 vs. 0.56 ± 0. 14, t = 3.187, P = 0.019) [Figure 4c] and IP3R1 (1.56 ± 0.16 vs. 0.63 ± 0.04, t = 5.689, P = 0.001) [Figure 4d] was significantly higher in DSS treated rats than in controls. However, BKCa expression was similar in both groups (0.97 ± 0.04 vs. 0.98 ± 0.04, t = 0.310, P = 0.767) [Figure 4e].
Figure 4.
Expression of Ca2+ handling proteins in DSS treated rats was significantly altered. (a) Representative Western blots for SERCA2a, PLB, IP3R1 and BKCa are shown. (b-e) Summary data showing expression of Ca2+ handling proteins. Values are mean ± SE of n rats (n = 4 per group). At least 3 separate experiments were performed and these showed similar results. *P < 0.05; NS: Not significantly different. SE: Standard error; BKCa: Large-conductance calcium-activated potassium channel; PLB: Phospholamban; IP3R1: Inositol 1,4,5-trisphosphate receptor 1; SERCA2a: Sarcoplasmic reticulum calcium-transporting ATPase 2a; DSS: Dextran sulfate sodium.
DISCUSSION
In this study, we used DSS-treated rats to assess the impact of Ca2+ signaling alterations on the disrupted contractility of colonic SM and further investigated the underlying mechanisms of disrupted Ca2+ signaling pathways during UC. DSS-treated rats provide a reliable animal model of the disease, exhibiting some of the clinical and histopathological characteristics of UC.[18,19,20,21] In addition, some therapeutic agents for UC were effective in DSS-induced colitis.[19] All rats treated with DSS in our study had diarrhea and hematochezia. Their colon lengths were shorter than those of control rats and they showed mucosal ulcerations and inflammatory cell infiltrations.
Decreased segmenting colonic contractions and increased propagating contractions in patients with UC[2] were previously demonstrated, but the underlying mechanisms of this process have been controversial. Our results showed that compared with control rats, the amplitude and average tension of spontaneous contractions of the colonic SM from DSS-treated rats were much higher while Cch-evoked contractions were significantly lower. However, contractile responses to KCl were similar in the two groups. Potassium initiates contraction via membrane depolarization and activation of calcium influx through voltage-sensitive channels. Because the response to potassium in the SM strips from DSS-treated rats were similar to that in the control, it is reasonable to speculate that this pathway did not contribute to the alteration of contractility in the inflamed colons. Cch, nevertheless, initiates contraction by the release of calcium from intracellular stores. The decreased Cch-evoked contraction in SM strips from DSS rats demonstrated that altered calcium release from intracellular stores might be responsible for dysmotility in the inflamed colon. Our findings agreed with previous reports suggesting that the handling of intracellular calcium was altered in colitis.[9,10,22,23] However, several studies suggested that defective influx of extracellular Ca2+, caused by reduced L-type Ca2+ channel expression or decreased L-type Ca2+ channel activity, caused the dysregulated contractility of SM during colitis.[7,8,24] The reasons for such contradictions are unknown but might relate to differences in materials, species and the region or severity of inflammation among various studies.
To further test the hypothesis that disrupted Ca2+ utilization and intracellular Ca2+ stores contribute to dysmotility, we examined cytoplasmic Ca2+, monitoring both spontaneous and caffeine-evoked Ca2+ transients in single SM cells isolated from the colons of the experimental animals. Resting cytoplasmic Ca2+ levels in myocytes from DSS rats were identical to those in controls, while the peak of caffeine-induced Ca2+ transients was significantly lower, indicating decreased SR calcium contents in rats with UC. A decreased SR calcium content indicated that Ca2+ release during electrical action potential would be lower, resulting in impaired SM contractility. However, Ca2+ transients, observed in SM from both groups before caffeine application, had a higher incidence in myocytes from inflamed colons (50% vs. 14%). Because Ca2+ transients represent fundamental mechanisms to modulate membrane potential and excitability in myocytes,[25] the finding that SM cells from inflamed colons had a higher incidence of Ca2+ transients supported a speculation that these cells were more excited and more likely to contract. The SM cells from animals with colitis would have much more tendency to contract might explain our observation that colon lengths in DSS-treated rats were significantly shorter than in controls. Furthermore, the decreased intracellular Ca2+ release in colonic SM cells was related to decreased Cch-evoked contractions.
IP3R, RyR, and SERCA are the major Ca2+ channels present on the SR and the function of SERCA is regulated by PLB.[26] To further investigate the underlying mechanisms for disrupted intracellular Ca2+ mobilization in SM cells in colitis, we examined levels of IP3R1, PLB, and SERCA2a, the major isoforms expressed in colonic SM.[14] We found that in SM from DSS compared with control rats, SERCA2a levels were significantly decreased while, in contrast, those of PLB were upregulated. SERCA2a is regulated by PLB, a 5-kD protein. Unphosphorylated PLB inhibits SERCA2a by decreasing its Ca2+ affinity, and this inhibition is relieved by PLB phosphorylation. Our results suggested that both expression and Ca2+ affinity of SERCA2a were decreased, leading to impaired refilling of intracellular Ca2+ stores. Meanwhile, expression levels of IP3R1 were clearly increased in rats with colitis, which might explain the higher incidence, compared with in control animals, of Ca2+ transients in inflamed colonic SM cells and the shortened colon length in DSS-treated rats. These preliminary findings agreed with reports by Al-Jarallah et al.[14] and Qureshi et al.[11]
BKCa can be activated directly by Ca2+ release from the SR in SM cells of the gastrointestinal tract, leading to membrane hyperpolarization and decreased Ca2+ entry through voltage-dependent Ca2+ channels and ultimately resulting in muscle relaxation.[27] In our study, we demonstrated that BKCa expression was similar in DSS and control groups, further eliminating this pathway as a contributor in the decreased SM contractility in UC. That is, decreased extracellular Ca2+ entry is not a likely reason for the dysmotility. However, further investigation will be needed to assess whether BKCa function is altered in UC.
In summary, our study showed that spontaneous contractility increased significantly and Cch-evoked contraction decreased remarkably in DSS-induced UC. Abnormalities in intracellular Ca2+ stores, including increased Ca2+ transients and impaired refilling of intracellular stores, accounted for the observed dysmotility. The underlying mechanism involved increased IP3R1 expression and decreased expression and function of SERCA2a.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by a grant from the National Laboratory of Biomacromolecules (No. 2014KF06).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Pazdrak K, Shi XZ, Sarna SK. TNFalpha suppresses human colonic circular smooth muscle cell contractility by SP1- and NF-kappaB-mediated induction of ICAM-1. Gastroenterology. 2004;127:1096–109. doi: 10.1053/j.gastro.2004.07.008. doi: 10.1053/j.gastro.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Bassotti G, de Roberto G, Chistolini F, Sietchiping-Nzepa F, Morelli O, Morelli A. Twenty-four-hour manometric study of colonic propulsive activity in patients with diarrhea due to inflammatory (ulcerative colitis) and non-inflammatory (irritable bowel syndrome) conditions. Int J Colorectal Dis. 2004;19:493–7. doi: 10.1007/s00384-004-0604-6. doi: 10.1007/s00384-004-0604-6. [DOI] [PubMed] [Google Scholar]
- 3.Bassotti G, Villanacci V, Mazzocchi A, Castellani D, Giuliano V, Corsi S, et al. Colonic propulsive and postprandial motor activity in patients with ulcerative colitis in remission. Eur J Gastroenterol Hepatol. 2006;18:507–10. doi: 10.1097/00042737-200605000-00008. doi: 10.1097/00042737-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Vrees MD, Kirber MT, Fiocchi C, Pricolo VE. Hydrogen peroxide contributes to motor dysfunction in ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G833–43. doi: 10.1152/ajpgi.00414.2003. doi: 10.1152/ajpgi.00414.2003. [DOI] [PubMed] [Google Scholar]
- 5.Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84–90. doi: 10.1136/gut.52.1.84. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanacci V, Bassotti G, Nascimbeni R, Antonelli E, Cadei M, Fisogni S, et al. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol Motil. 2008;20:1009–16. doi: 10.1111/j.1365-2982.2008.01146.x. doi: 10.1111/j1365-2982.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 7.Akbarali HI, Pothoulakis C, Castagliuolo I. Altered ion channel activity in murine colonic smooth muscle myocytes in an experimental colitis model. Biochem Biophys Res Commun. 2000;275:637–42. doi: 10.1006/bbrc.2000.3346. doi: 10.1006/bbrc.2000.3346. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Rusch NJ, Striessnig J, Sarna SK. Down-regulation of L-type calcium channels in inflamed circular smooth muscle cells of the canine colon. Gastroenterology. 2001;120:480–9. doi: 10.1053/gast.2001.21167. doi: 10.1053/gast.2001.21167. [DOI] [PubMed] [Google Scholar]
- 9.Cook TA, Brading AF, Mortensen NJ. Abnormal contractile properties of rectal smooth muscle in chronic ulcerative colitis. Aliment Pharmacol Ther. 2000;14:1287–94. doi: 10.1046/j.1365-2036.2000.00819.x. doi: 10.1046/j.1365-2036.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- 10.Myers BS, Martin JS, Dempsey DT, Parkman HP, Thomas RM, Ryan JP. Acute experimental colitis decreases colonic circular smooth muscle contractility in rats. Am J Physiol. 1997;273(4 Pt 1):G928–36. doi: 10.1152/ajpgi.1997.273.4.G928. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi S, Song J, Lee HT, Koh SD, Hennig GW, Perrino BA. CaM kinase II in colonic smooth muscle contributes to dysmotility in murine DSS-colitis. Neurogastroenterol Motil. 2010;22:186–e64. doi: 10.1111/j.1365-2982.2009.01406.x. doi: 10.1111/j.1365-2982.2009.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrees MD, Pricolo VE, Potenti FM, Cao W. Abnormal motility in patients with ulcerative colitis: The role of inflammatory cytokines. Arch Surg. 2002;137:439–45. doi: 10.1001/archsurg.137.4.439. doi: 10.1001/archsurg.137.4.439. [DOI] [PubMed] [Google Scholar]
- 13.Ye YF, Jin X, Chen SH, Yue M, Li YM. Changes of CD8+ T cells in dextran sulfate sodium-induced colitis mice pretreated with oral immune regulation. Chin Med J. 2012;125:2173–9. doi: 10.3760/cma.j.issn.0366-6999.2012.12.017. [PubMed] [Google Scholar]
- 14.Al-Jarallah A, Oriowo MA, Khan I. Mechanism of reduced colonic contractility in experimental colitis: Role of sarcoplasmic reticulum pump isoform-2. Mol Cell Biochem. 2007;298:169–78. doi: 10.1007/s11010-006-9363-8. doi: 10.1007/s11010-006-9363-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhai K, Chang Y, Wei B, Liu Q, Leblais V, Fischmeister R, et al. Phosphodiesterase types 3 and 4 regulate the phasic contraction of neonatal rat bladder smooth myocytes via distinct mechanisms. Cell Signal. 2014;26:1001–10. doi: 10.1016/j.cellsig.2014.01.020. doi: 10.1016/j.cellsig.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 17.Cao W, Vrees MD, Potenti FM, Harnett KM, Fiocchi C, Pricolo VE. Interleukin 1beta-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J Pharmacol Exp Ther. 2004;311:60–70. doi: 10.1124/jpet.104.068023. doi: 10.1124/jpet.104.068023. [DOI] [PubMed] [Google Scholar]
- 18.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6. doi: 10.1038/nprot.2007.41. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 19.Melgar S, Karlsson L, Rehnström E, Karlsson A, Utkovic H, Jansson L, et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836–44. doi: 10.1016/j.intimp.2008.01.036. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim. 2000;49:9–15. doi: 10.1538/expanim.49.9. doi: 10.1538/expanim.49.9. [DOI] [PubMed] [Google Scholar]
- 21.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 22.Snape WJ., Jr The role of a colonic motility disturbance in ulcerative colitis. Keio J Med. 1991;40:6–8. doi: 10.2302/kjm.406. [PubMed] [Google Scholar]
- 23.Kao HW, Zipser RD. Exaggerated prostaglandin production by colonic smooth muscle in rabbit colitis. Dig Dis Sci. 1988;33:697–704. doi: 10.1007/BF01540433. doi: 10.1007/BF01540433. [DOI] [PubMed] [Google Scholar]
- 24.Shi XZ, Sarna SK. Impairment of Ca(2+) mobilization in circular muscle cells of the inflamed colon. Am J Physiol Gastrointest Liver Physiol. 2000;278:G234–42. doi: 10.1152/ajpgi.2000.278.2.G234. [DOI] [PubMed] [Google Scholar]
- 25.McGeown JG. Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: One store or two? Cell Calcium. 2004;35:613–9. doi: 10.1016/j.ceca.2004.01.016. doi: 10.1016/j.ceca.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–60. doi: 10.1161/CIRCRESAHA.111.259754. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Huang H, Hou D, Liu P, Wei H, Fu X, et al. Mechanosensitivity of STREX-lacking BKCa channels in the colonic smooth muscle of the mouse. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1231–40. doi: 10.1152/ajpgi.00268.2010. doi: 10.1152/ajpgi.00268.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Upregulated NF-κB expression in colonic SM from DSS treated rats, compared with controls. (a) Representative Western blots for NF-κB. (b) Summary data showing NF-κB expression. Values are mean ± SE of n rats (n = 4 per group). At least 3 separate experiments were performed and showed similar results. *P < 0.05 versus vehicle. SE: Standard error; DSS: Dextran sulfate sodium; SM: Smooth muscle; NF-κB: Nuclear factor-kappa B.