Abstract
Reduced white adipose tissue (WAT) LPL activity delays plasma clearance of TG-rich lipoproteins (TRLs). We reported the secretion of apoC-I, an LPL inhibitor, from WAT ex vivo in women. Therefore we hypothesized that WAT-secreted apoC-I associates with reduced WAT LPL activity and TRL clearance. WAT apoC-I secretion averaged 86.9 ± 31.4 pmol/g/4 h and 74.1 ± 36.6 pmol/g/4 h in 28 women and 11 men with BMI ≥27 kg/m2, respectively, with no sex differences. Following the ingestion of a 13C-triolein-labeled high-fat meal, subjects with high WAT apoC-I secretion (above median) had delayed postprandial plasma clearance of dietary TRLs, assessed from plasma 13C-triolein-labeled TGs and apoB48. They also had reduced hydrolysis and storage of synthetic 3H-triolein-labeled (3H)-TRLs in WAT ex vivo (i.e., in situ LPL activity). Adjusting for WAT in situ LPL activity eliminated group differences in chylomicron clearance; while adjusting for plasma apoC-I, 3H-NEFA uptake by WAT, or body composition did not. apoC-I inhibited in situ LPL activity in adipocytes in both a concentration- and time-dependent manner. There was no change in postprandial WAT apoC-I secretion. WAT apoC-I secretion may inhibit WAT LPL activity and promote delayed chylomicron clearance in overweight and obese subjects. We propose that reducing WAT apoC-I secretion ameliorates postprandial TRL clearance in humans.
Keywords: adipocytes, lipase/lipoprotein, triglycerides, obesity, lipolysis and fatty acid metabolism, fat storage, cardiometabolic risk, white adipose tissue, apolipoprotein C-I, triglyceride-rich lipoprotein
The cardiometabolic risk associated with reduced HDL cholesterol (HDL-C) and/or elevated LDL cholesterol (LDL-C) has been well-established, the cardiometabolic risk associated with elevated TG-rich lipoproteins (TRLs) has not. However, elevated concentrations of TRLs have received much interest lately given their association with obesity, insulin resistance, and type 2 diabetes (1, 2). Two recent meta-analyses identified hypertriglyceridemia as a predictor of cardiovascular disease (3, 4). Although controversy exists regarding the independent contribution of fasting hypertriglyceridemia because of its inverse relationship with HDL-C (3, 5), that of postprandial hypertriglyceridemia remains sturdy even after adjustment for HDL-C (5–7). Accordingly, much research has now focused on factors that regulate postprandial TRL clearance.
Clearance of postprandial TRLs is highly dependent on white adipose tissue (WAT). A fundamental function of WAT in the postprandial state is the hydrolysis of the TG core of TRLs through the activity of endothelial LPL and the uptake and storage of released NEFAs (8–10). Dysfunctional WAT has reduced metabolic flexibility and is unable to switch promptly from fasting (catabolic) to postprandial (anabolic) state. This leads to delayed TRL clearance, impaired remodeling of circulating lipoproteins, and increased TRL flux to peripheral tissues; thereby increasing cardiometabolic risks (11, 12). The underlying mechanisms promoting WAT dysfunction and delayed TRL clearance are not fully understood.
Postprandial clearance of TRLs is dependent on multiple transferable apolipoproteins that activate or inhibit LPL activity and TRL clearance, including apoC-I (13–16). apoC-I is a 6.6 kDa apolipoprotein that is primarily secreted from the liver and, in the fasting state, is mainly carried on HDLs (∼80%) with a minor fraction carried on TRLs (∼7%) (14, 16, 17). ApoC-I enrichment of HDLs is anti-atherogenic, as it promotes increased HDL size and cholesterol content (16–19). On the other hand, apoC-I enrichment of TRLs promotes delayed plasma clearance of TRLs (13, 14, 19). In murine studies, this is reported to be secondary to apoC-I-induced inhibition of LPL activity (20, 21) independent of apoC-III (22) and apoE-dependent clearance of TRLs by VLDL receptor (23), LDL receptor (24), and LDL receptor-related protein (25). The local production of apoC-I from macrophages taken from mice overexpressing human apoC-I has also been reported to bind NEFAs and reduce their esterification (26).
The role of apoC-I in TRL clearance by WAT is, to our knowledge, not examined in humans. We were the first to report the secretion of apoC-I from a human adipocyte model (27) and by human subcutaneous WAT ex vivo (14). Moreover, we demonstrated that WAT secretion of apoC-I, but not of apoC-II, apoC-III, or apoE, was correlated with delayed postprandial plasma clearance of dietary TRL in postmenopausal overweight and obese women (14), while no data existed for men. Given its negative regulation of LPL activity, we hypothesized that WAT apoC-I secretion reduces LPL activity of WAT and, accordingly, TRL clearance by WAT (14). To explore this hypothesis while accounting for sex differences, we examined the association of WAT apoC-I secretion with both TRL clearance ex vivo in WAT, and postprandial plasma clearance of dietary TRLs in vivo in 39 overweight and obese men and postmenopausal women. We further verified the direct effect of purified human apoC-I on the clearance of synthetic TRLs in vitro in adipocytes.
MATERIALS AND METHODS
Study population
Metabolic studies examining TRL clearance in vivo and ex vivo in WAT were conducted between 2010 and 2014 at the Institut de recherches cliniques de Montréal (IRCM). Subjects were recruited by newspaper advertisement and their inclusion and exclusion criteria were previously reported (14, 28–30). In brief, recruited subjects had BMI ≥27 kg/m2, were aged 45–74 years, were sedentary nonsmokers with low-alcohol consumption (≤2 servings per day), and women were postmenopausal. The exclusion criteria were chronic diseases such as diabetes, cardiovascular, hepatic, or renal diseases, cancer within the past 3 years, problems with blood coagulation, concomitant medications affecting metabolism including lipid lowering and hormone replacement therapy (except thyroid hormone at stable dose), and known substance abuse. All participants signed a consent form before initiating the study, which was approved by the ethics board at IRCM.
Forty-five subjects were recruited, of which six subjects were excluded from the analysis: one woman did not complete the postprandial fat clearance test, two women and two men had yields of WAT biopsies that were too small to be used, and one man had a WAT apoC-I secretion level that was ∼4.5-fold above the average rendering him a clear outlier in many correlations. Thus this analysis included 28 women and 11 men.
Anthropometric and metabolic parameters (N = 39)
Subjects underwent a 4 week weight stabilization period (±2 kg) prior to testing to eliminate possible effects of weight fluctuation on the measured metabolic outcomes. Body composition was measured by dual energy X-ray absorptiometry (General Electric Lunar Corp., version 6.10.019), plasma glucose by an automated analyzer (YSI 2300 Stat Plus), serum insulin and C-peptide by radioimmunoassay kits (Millipore Corp.), plasma lipids and apoB by an automated analyzer COBAS Integra 400 (Roche Diagnostics), plasma chylomicron concentration by an ELISA kit against human apoB48 (Biovendor), and plasma apoC-I by an in-house ELISA, as previously reported (14, 31). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to Matthews et al. (32). Lipoprotein cholesterol content and LDL diameter were measured by a polyacrylamide gel electrophoresis system (FDA-approved, Lipoprint system; Quantimetrix) (14, 29).
Dietary intake (N = 27)
Dietary intake was assessed using 3 day dietary recall (two weekdays and one weekend day) on a subpopulation of subjects (16 women, 11 men), as previously reported (33). Nutritional data was analyzed using the Food Processor software with a Canadian database version 10 (Esha Research, Salem, OR).
Postprandial fat metabolism (N = 39)
Postprandial dietary fat metabolism was assessed as previously reported (14, 29). In brief, subjects consumed a high-fat meal labeled with 13C-triolein [glycerol tri(oleate-1-13C), 99 atom% 13C; Sigma-Aldrich Canada] standardized according to body surface area (600 kcal/m2, 0.017 g 13C-triolein per gram fat, 68% fat, 18% carbohydrate). Plasma concentrations of 13C-triolein-labeled (13C-)TG and 13C-NEFA were measured over 0, 1, 2, 4, and 6 h using isotope ratio mass spectrometry (14, 29). The postprandial clearance rates of plasma total TG, 13C-TG, and 13C-NEFA were calculated as the area under the 6 h curve of the incremental increase above baseline levels of plasma lipids (iAUC6hrs). The postprandial clearance rate of chylomicron particles was also calculated as iAUC6hrs of plasma apoB48.
ApoC-I secretion from subjects’ WAT samples ex vivo (N = 39)
Subjects’ fasting WAT samples were collected by needle biopsy from the right hip (gynoid) area under local anesthesia (Xylocaine, 20 mg/ml; AstraZeneca) as previously described (14, 29). WAT samples were cleaned and preincubated in 1 ml HBSS for 30 min to wash out products of cellular damage induced by the biopsy. WAT samples were blotted dry, weighed, and then incubated in DMEM/F12 containing 5% fetal bovine serum. Fasting WAT apoC-I secretion was assessed as the 4 h accumulation of apoC-I in the incubation medium using an in-house ELISA, as previously reported (14, 31). Postprandial WAT samples were also collected from the left hip 4 h after the ingestion of the high-fat meal in a subpopulation of 19 subjects (11 men, 8 women), and postprandial WAT apoC-I secretion was measured. WAT apoC-I secretion in the fasting and postprandial states represents average WAT apoC-I accumulation in three to four wells, with two to four WAT pieces per well, for a total of 5–10 mg WAT per well.
WAT in situ LPL activity and NEFA uptake ex vivo (N = 33)
The hydrolysis of synthetic 3H-triolein-labeled (3H-)TRL and the uptake and incorporation of the released 3H-NEFA is a reflection of in situ LPL activity. It was assessed in subjects’ WAT samples, as previously published (12, 29, 34). Briefly, fasting WAT samples were cleaned, blotted dry, weighed and preincubated in HBSS buffer for ∼30 min, then transferred into wells containing 500 μl 3H-TRL substrate [95% TG, 1.27 mmol/l TG, and 0.54 mmol/l Tris-HCL (pH 7.2) in DMEM/F12, 5.1% BSA, and 7.5% fasting human serum, emulsified by sonication] and incubated for an additional 4 h at 37°C on a shaking plate at 300 rpm. WAT 3H-lipids (representing 3H-TRL hydrolysis and uptake) and medium 3H-NEFA (representing 3H-TRL hydrolysis and release) were extracted, counted, and expressed as 3H-TG substrate hydrolyzed per milligram WAT (12, 28, 29, 34). As a negative control, we measured the background medium 3H-NEFA content after incubation of the 3H-TRL substrate for 4 h in the absence of WAT. Medium 3H-NEFA represented a negligible amount of the total 3H-dose added (∼0.70 ± 0.01%), indicating that medium 3H-NEFA and WAT 3H-lipids were the result of 3H-TRL hydrolysis. Moreover, we previously reported that the addition of the LPL inhibitor, tetrahydrolipstatin, to the 3H-TRL substrate markedly reduced intracellular 3H-lipids in 3T3-L1 adipocytes, indicating that the accumulation of intracellular 3H-lipids from 3H-TRL substrate is dependent on LPL activity (34).
To assess NEFA uptake independently of LPL activity, a second set of WAT samples was cleaned, blotted dry, weighed, and preincubated in HBSS buffer for ∼30 min, then transferred into wells containing 500 μl 3H-oleate-labeled NEFA bound to BSA (1 mmol/l oleate:0.167 mmol/l BSA in DMEM/F12) and incubated for an additional 4 h at 37°C on a shaking plate at 300 rpm (12, 29). WAT 3H-lipids were extracted, counted, and expressed as 3H-NEFA uptake per WAT (12, 29). Due to insufficient WAT yields in all included subjects (N = 39), 33 subjects had data for WAT experiments with 3H-TRL and 28 had data for WAT experiments with 3H-NEFA. WAT experiments with 3H-TRL and 3H-NEFA represent the average of WAT samples in three to six wells, with two to four WAT pieces per well, for a total of 5–10 mg WAT per well.
Adipocyte area (N = 39)
The areas of adipocytes of the fasting hip WAT samples were measured by digital imaging as previously reported (14, 28, 29). The average surface area of 986 ± 399 adipocytes, in six fields of view in three WAT slides, is reported per subject.
Direct effect of apoC-I on in situ LPL activity in 3T3-L1 adipocytes
The in situ LPL activity of 3T3-L1 adipocytes was determined in the presence or absence of human VLDL-extracted apoC-I (Calbiochem, La Jolla, CA), as described above. These experiments model the in vivo interaction of TRL with adipocyte-secreted LPL and apoC-I, as on the luminal surface of endothelial cells. Cells were differentiated for 7 days, as previously described (29, 34), then incubated with the 3H-TRL substrate (0.56 mmol/l TG) in the presence or absence of apoC-I (15 μmol/l) over 5–360 min. To test whether the effect of apoC-I was concentration dependent, adipocytes were incubated with the 3H-TRL substrate in the presence or absence of apoC-I at physiological concentrations (1.5–30 μmol/l) for 4 h. Four hours was selected to simulate the duration of WAT apoC-I secretion. After the 4 h experiment, medium 3H-TG (non-hydrolyzed 3H-TRL substrate), medium 3H-NEFA (hydrolyzed 3H-TRL substrate and released 3H-NEFA), and intracellular 3H-lipids (hydrolyzed 3H-TRL substrate and incorporated 3H-NEFA into adipocyte lipids) were extracted, counted, and expressed per well.
Statistical analysis
Data are presented as mean ± SD. Group differences were assessed by Student’s t-test. Pearson correlations were used to examine the association between the measured outcomes. Slope analysis indicated no significant sex differences in the direction of association of the independent variables to dependent variables as shown in Table 2, Fig. 2, and supplementary Fig. 2; accordingly, data for both sexes were pooled. Data were log-transformed (base 10) and then entered in the analysis when the test for equal variance of residues failed. Univariate ANOVA analysis was used to adjust the differences between the two groups of low versus high WAT apoC-I secretion for the different parameters. Statistical analysis was performed using SPSS V20 (IBM) and curve analysis using Prism V6.0f (GraphPad Software). Significance was set at P < 0.05.
TABLE 2.
Pearson correlation of anthropometric parameters with measured outcomes
| Body Composition | WAT apoC-I Secretion (pmol/g/4 h) | Postprandial Plasma Parameters | WAT in situ LPL Activity (i.e., 3H-TRL Substrate) | WAT 3H-NEFA Uptake (i.e., 3H-NEFA:BSA Substrate) | ||||
| iAUC6hrs TG (mmol/l) | LOG10 iAUC6hrs 13C-TG (μmol/l) | iAUC6hrs 13C-NEFA (μmol/l) | iAUC6hrs apoB48b (mg/l) | WAT 3H-lipidsc (nmol 3H-TG/mg) | LOG10 Medium 3H-NEFAd (nmol 3H-TG/mg) | WAT 3H-lipidse (nmol 3H-NEFA/mg) | ||
| Weight (kg) | −0.085 | −0.203 | 0.094 | −0.239 | 0.160 | 0.016 | 0.213 | −0.197 |
| BMI (kg/m2) | −0.025 | −0.168 | −0.118 | −0.292 | 0.111 | −0.091 | 0.168 | −0.341 |
| Waist (cm) | −0.135 | −0.100 | −0.011 | −0.244 | 0.156 | 0.012 | 0.209 | −0.309 |
| Hip (cm) | −0.028 | −0.187 | −0.198 | −0.219 | 0.055 | −0.126 | −0.033 | −0.344 |
| Waist/hip ratio | −0.150 | 0.043 | 0.177 | −0.136 | 0.193 | 0.136 | 0.303 | −0.136 |
| Total fat (kg) | −0.007 | −0.219 | −0.129 | −0.259 | 0.091 | −0.054 | 0.215 | −0.316 |
| Android fat (kg) | −0.098 | −0.210 | 0.001 | −0.282 | 0.131 | 0.023 | 0.002 | −0.341 |
| Gynoid fat (kg) | 0.069 | −0.152 | −0.362a | −0.204 | −0.064 | 0.005 | 0.130 | -0.419a |
| Android/gynoid fat | −0.127 | −0.110 | 0.373a | −0.210 | 0.333a | 0.057 | 0.213 | −0.043 |
Data in bold represent parameters with significant correlation.
P ≤ 0.05 (two-tailed).
N = 38 for missing data.
N = 33 for missing data.
N = 32 for missing data.
N = 28 for missing data.
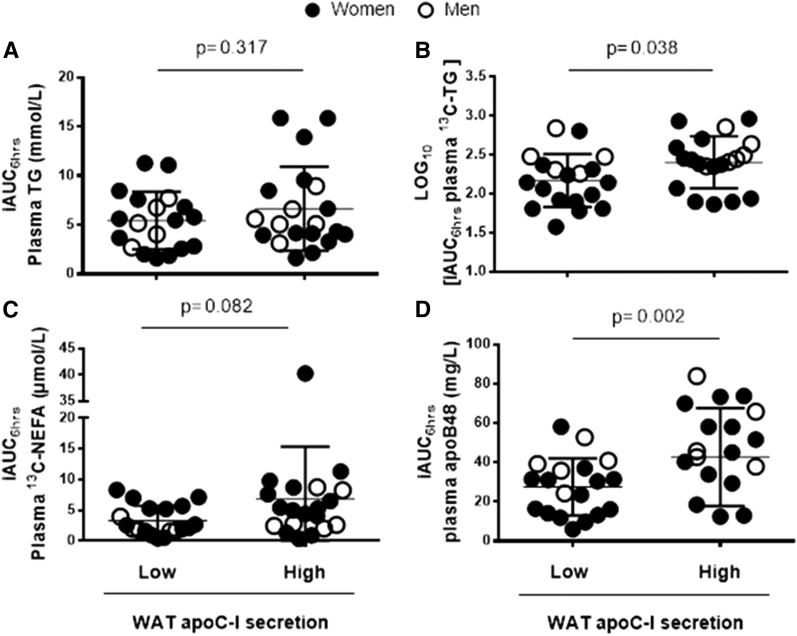
Fig. 2.
Group differences in postprandial plasma clearance of TG (A), 13C-TG (B), 13C-NEFA (C), and chylomicrons (apoB48) (D) in subjects with low (N = 19) versus high (N = 20) WAT apoC-I secretion (except for (D) where N = 5 men in the high group for missing data). Women are represented as solid circles and men as open circles.
RESULTS
Baseline characteristics of the study population are presented in Table 1. Both groups were obese, but men had higher indices of insulin resistance (fasting plasma insulin and HOMA-IR), blood pressure, and adiposity (BMI, weight, and fat mass), particularly central (waist circumference, waist/hip ratio, android fat, and android/gynoid fat ratio). Women had higher total IDL and HDL-C. Men had a higher total daily energy intake; however there were no sex-differences in the percent of energy from carbohydrate, fat, protein, alcohol, or saturated fat, nor in relation to total daily intake of fiber and cholesterol. Notably, although women had higher total plasma apoC-I, there were no sex differences in fasting WAT apoC-I secretion, suggesting that other apoC-I sources, such as the liver, may be different between the two sexes.
TABLE 1.
Fasting baseline characteristics of the study population (N = 39)
| Women (N = 28) | Men (N = 11) | P | |
| Age (years) | 58.5 ± 4.3 | 58.5 ± 6.4 | 0.979 |
| Anthropometric parameters | |||
| Weight (kg) | 77.9 ± 13.4 | 108.8 ± 25.8 | <0.001 |
| BMI (kg/m2) | 31.4 ± 4.4 | 36.1 ± 7.0 | 0.017 |
| Waist circumference (cm) | 102.7 ± 10.7 | 122.3 ± 14.8 | <0.001 |
| Hip circumference (cm) | 111.6 ± 9.0 | 116.3 ± 13.0 | 0.201 |
| Waist/hip ratio | 0.92 ± 0.06 | 1.05 ± 0.08 | <0.001 |
| Total fat (kg) | 35.2 ± 9.4 | 44.2 ± 16.6 | 0.040 |
| Android fat (kg) | 3.36 ± 1.05 | 5.37 ± 2.01 | <0.001 |
| Gynoid fat (kg) | 6.15 ± 1.61 | 5.85 ± 2.09 | 0.631 |
| Android/gynoid fat | 0.55 ± 0.10 | 0.92 ± 0.10 | <0.001 |
| Adipocyte area (μm2) | 3,135 ± 704 | 3,404 ± 982 | 0.345 |
| Metabolic parameters | |||
| Systolic blood pressure (mmHg) | 120.4 ± 17.9 | 136.5 ± 12.2 | 0.011 |
| Diastolic blood pressure (mmHg) | 76.5 ± 8.0 | 84.3 ± 6.8 | 0.009 |
| Plasma glucose (mmol/l) | 5.13 ± 0.47 | 5.15 ± 0.25 | 0.860 |
| Plasma insulin (μU/ml) | 14.2 ± 5.4 | 22.6 ± 17.9 | 0.031 |
| HOMA-IR [(mmol/l) × (mU/l)] | 3.27 ± 1.35 | 5.11 ± 3.96 | 0.035 |
| Plasma cholesterol (mmol/l) | 5.93 ± 1.06 | 4.96 ± 1.20 | 0.015 |
| Plasma VLDL-C (mmol/l) | 1.01 ± 0.32 | 1.05 ± 0.36 | 0.703 |
| Plasma IDL-C (mmol/l) | 1.69 ± 0.41 | 1.34 ± 0.32 | 0.015 |
| Plasma LDL-C (mmol/l) | 1.95 ± 0.56 | 1.64 ± 0.63 | 0.146 |
| Plasma HDL-C (mmol/l) | 1.46 ± 0.35 | 1.07 ± 0.26 | 0.002 |
| Plasma TG (mmol/l) | 1.52 ± 0.84 | 1.76 ± 0.89 | 0.427 |
| Plasma apoB (g/l) | 1.00 ± 0.26 | 1.03 ± 0.27 | 0.829 |
| Plasma apoB48 (mg/l) | 6.62 ± 4.15 | 7.87 ± 4.45 | 0.421 |
| LDL diameter (Å) | 269 ± 6 | 266 ± 7 | 0.157 |
| Plasma apoC-I (μmol/l) | 23.6 ± 5.7 | 17.5 ± 3.9 | 0.004 |
| WAT apoC-I secretion (pmol/g/4 h) | 86.9 ± 31.4 | 74.1 ± 36.6 | 0.279 |
| WAT apoC-I secretion (min–max) | 36.6–155.3 | 23.6–126.9 | — |
| Nutritional parametersa | |||
| Total energy intake (kcal/day) | 1,895 ± 541 | 2,493 ± 528 | 0.009 |
| Carbohydrates (%) | 46.3 ± 5.4 | 47.0 ± 8.8 | 0.792 |
| Fat (%) | 36.1 ± 5.1 | 33.2 ± 6.8 | 0.210 |
| Protein (%) | 16.0 ± 2.1 | 17.0 ± 4.0 | 0.412 |
| Alcohol (%) | 1.64 ± 2.81 | 2.85 ± 3.09 | 0.298 |
| Saturated fat (%) | 11.1 ± 2.6 | 10.1 ± 2.9 | 0.361 |
| Fiber (g/day) | 22.0 ± 7.2 | 25.6 ± 8.8 | 0.260 |
| Cholesterol (mg/day) | 253 ± 117 | 359 ± 169 | 0.065 |
Data are presented as average ± SD. min, minimum; max, maximum. Data in bold represent parameters with significant group differences.
Data from 16 women and 11 men.
Given the phenotype of this population, we examined whether adiposity or fat distribution correlated with the measured outcomes (Table 2, supplementary Fig. 1). There were no associations between several markers of adiposity with total apoC-I, WAT apoC-I, postprandial fat clearance (total TG, dietary 13C-TG, dietary 13C-NEFA, or apoB48), WAT in situ LPL activity (WAT 3H-lipids and medium 3H-NEFA generated from the hydrolysis of 3H-TRL substrate), or 3H-NEFA uptake (from 3H-NEFA:BSA substrate). Gynoid fat mass, which is metabolically protective, was associated with faster postprandial plasma clearance of 13C-TG and lower WAT 3H-NEFA:BSA uptake. On the other hand, higher abdominal relative to gynoid adiposity was associated with delayed postprandial plasma clearance of 13C-TG and apoB48.
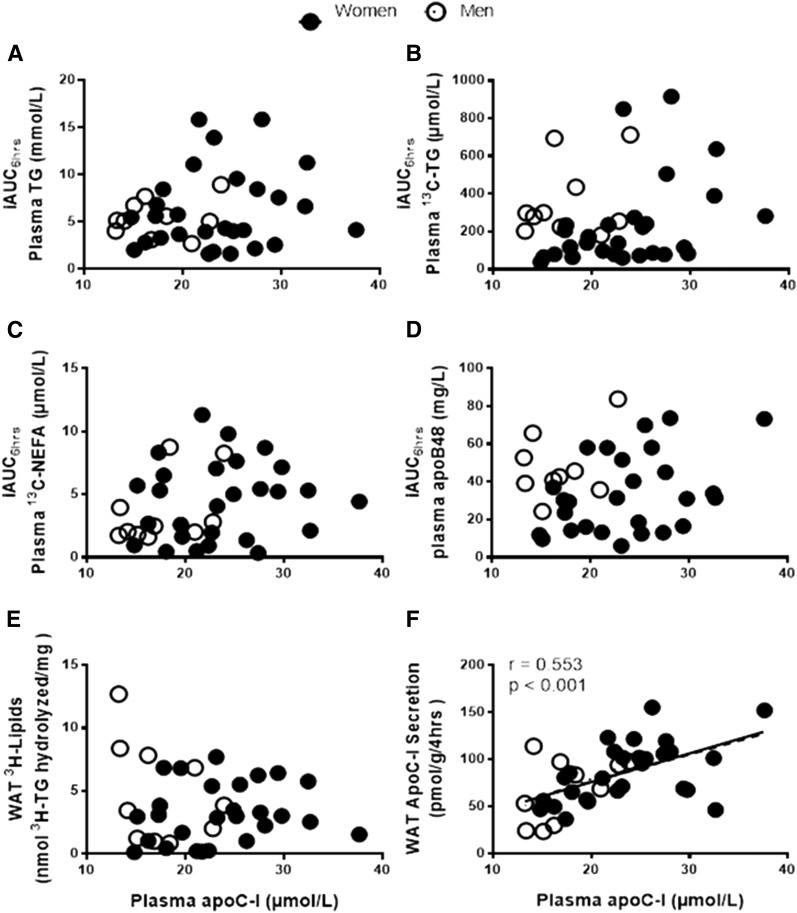
Plasma apoC-I is not correlated with clearance of TRL in vivo or by WAT ex vivo
Given the known role of apoC-I in reducing TRL clearance (when on TRL), we first examined whether fasting plasma apoC-I was correlated with indices of delayed postprandial fat clearance in this population. As presented in Fig. 1, there was no association of plasma apoC-I with postprandial plasma clearance of total TG, dietary 13C-TG, dietary 13C-NEFA, or plasma apoB48 (Fig. 1A–D). Moreover, there was no correlation between plasma apoC-I with in situ LPL activity in WAT ex vivo (Fig. 1E). On the other hand and as previously reported in women, plasma apoC-I correlated with WAT apoC-I secretion and there was no sex-difference in the direction of this association (r = 0.553 P < 0.001; Fig. 1F). Plasma apoC-I correlated with all measures of fasting total plasma cholesterol (supplementary Fig. 2A) and lipoprotein-specific cholesterol (supplementary Fig. 2B–E), but not with plasma TG (supplementary Fig. 2F). It also correlated with fasting concentrations of apoB-lipoproteins, total apoB (supplementary Fig. 2G) and apoB48 (supplementary Fig. 2H), and with fasting plasma NEFA (supplementary Fig. 2I), with no sex differences in the direction of the associations.
Fig. 1.
Association of fasting plasma apoC-I with iAUC6hrs of plasma TG (A), 13C-TG (B), 13C-NEFA (C), and chylomicrons (apoB48) (D), and with WAT in situ LPL activity measured as WAT 3H-lipids (E) and WAT apoC-I secretion (F) in women (N = 28, closed circles with dashed regression line) and men (N = 11, open circles with dotted regression lines). Solid regression line represents pooled data analysis.
Subjects with high WAT apoC-I secretion ex vivo have delayed postprandial plasma clearance of dietary TRL in vivo
We previously reported that postmenopausal obese women with high WAT apoC-I secretion had delayed postprandial plasma clearance of dietary TG when compared with women with low WAT apoC-I secretion (14). We first examined whether the same conclusion could be drawn for both sexes. Subjects were divided into two groups based on median WAT apoC-I secretion per sex (women = 83.2 pmol/g/4 h and men = 83.8 pmol/g/4 h) to assure an equal number of men and women in each group and eliminate possible sex-differences in the measured outcomes. As presented in Table 3, there were no group differences in any of the measured parameters related to adiposity, fat distribution, or adipocyte size. There were no group differences in fasting plasma metabolic parameters, lipids, apoB-lipoprotein particle numbers (total apoB or apoB48), total daily energy intake, and nutritional parameters. Subjects with high WAT apoC-I secretion had higher fasting plasma apoC-I and VLDL cholesterol (VLDL-C). This is in line with the positive correlation of WAT apoC-I with delayed TRL clearance shown previously (14). Thus overall, the two groups had similar anthropometric, metabolic, and nutritional characteristics in the fasting state (Table 3).
TABLE 3.
Fasting baseline characteristics of the two groups with low versus high WAT apoC-I secretion separated based on median WAT apoC-I secretion per sex
| Low WAT apoC-I | High WAT apoC-I | P | |
| Women:men | 14:5 | 14:6 | — |
| WAT apoC-I secretion (pmol/g/4 h) | 55.3 ± 17.3 | 109.8 ± 19.1 | <0.001 |
| WAT apoC-I secretion (min–max) | 23.6–80.72 | 83.8–155.3 | — |
| Age (years) | 56.8 ± 4.9 | 60.0 ± 4.6 | 0.051 |
| Anthropometric parameters | |||
| Weight (kg) | 87.8 ± 25.3 | 85.6 ± 19.7 | 0.759 |
| BMI (kg/m2) | 33.4 ± 6.2 | 32.1 ± 5.1 | 0.482 |
| Waist circumference (cm) | 109.7 ± 15.7 | 106.8 ± 14.1 | 0.551 |
| Hip circumference (cm) | 113.9 ± 9.8 | 112.0 ± 11.1 | 0.574 |
| Waist/hip ratio | 0.96 ± 0.09 | 0.95 ± 0.10 | 0.830 |
| Total fat (kg) | 38.6 ± 12.7 | 37.0 ± 12.3 | 0.674 |
| Android fat (kg) | 4.05 ± 1.87 | 3.81 ± 1.43 | 0.653 |
| Gynoid fat (kg) | 6.22 ± 1.44 | 5.92 ± 2.01 | 0.602 |
| Android/gynoid fat | 0.64 ± 0.20 | 0.66 ± 0.19 | 0.752 |
| Adipocyte area (μm2) | 3,172 ± 876 | 3,254 ± 715 | 0.751 |
| Metabolic parameters | |||
| Systolic blood pressure (mmHg) | 120.5 ± 15.4 | 129.2 ± 19.4 | 0.133 |
| Diastolic blood pressure (mmHg) | 77.1 ± 9.1 | 80.3 ± 7.5 | 0.236 |
| Plasma glucose (mmol/l) | 5.03 ± 0.37 | 5.23 ± 0.45 | 0.144 |
| Plasma insulin (μU/ml) | 19.4 ± 14.7 | 13.9 ± 4.6 | 0.124 |
| HOMA-IR [(mmol/l) × (mU/l)] | 4.33 ± 3.29 | 3.28 ± 1.20 | 0.186 |
| Plasma cholesterol (mmol/l) | 5.32 ± 0.97 | 5.99 ± 1.27 | 0.073 |
| Plasma VLDL-C (mmol/l) | 0.92 ± 0.27 | 1.12 ± 0.35 | 0.048 |
| Plasma IDL-C (mmol/l) | 1.52 ± 0.41 | 1.67 ± 0.42 | 0.287 |
| Plasma LDL-C (mmol/l) | 1.75 ± 0.47 | 1.98 ± 0.68 | 0.216 |
| Plasma HDL-C (mmol/l) | 1.27 ± 0.28 | 1.43 ± 0.43 | 0.186 |
| Plasma TG (mmol/l) | 1.42 ± 0.71 | 1.75 ± 0.95 | 0.229 |
| Plasma apoB (g/l) | 0.93 ± 0.24 | 1.07 ± 0.28 | 0.123 |
| Plasma apoB48a (mg/l) | 6.29 ± 4.10 | 7.61 ± 4.31 | 0.337 |
| LDL diameter (Å) | 269 ± 5 | 267 ± 6 | 0.351 |
| Plasma apoC-I (μmol/l) | 19.8 ± 5.6 | 24.0 ± 5.4 | 0.024 |
| Plasma apoC-I (min–max) | 13.2–32.7 | 14.1–37.6 | — |
| Nutritional parametersb | |||
| Total energy intake (kcal/day) | 2,067 ± 659 | 2,205 ± 565 | 0.563 |
| Carbohydrates (%) | 46.8 ± 6.7 | 46.5 ± 7.7 | 0.917 |
| Fat (%) | 36.2 ± 4.1 | 33.7 ± 7.1 | 0.291 |
| Protein (%) | 15.3 ± 2.4 | 17.4 ± 3.2 | 0.072 |
| Alcohol (%) | 1.79 ± 2.00 | 2.45 ± 3.65 | 0.565 |
| Saturated fat (%) | 11.2 ± 2.7 | 10.2 ± 2.8 | 0.342 |
| Fiber (g/day) | 23.7 ± 9.7 | 23.2 ± 6.1 | 0.878 |
| Cholesterol (mg/day) | 271 ± 123 | 320 ± 168 | 0.403 |
Data in bold represent parameters with significant group differences. min, minimum; max, maximum.
N = 5 men in high WAT apoC-I secretion group.
N = 13 (five men) and N = 14 (six men) in low versus high WAT apoC-I secretion groups, respectively.
Subjects with high WAT secretion had delayed postprandial plasma clearance of dietary TG (Fig. 2B) and apoB48 (Fig. 2D), with no group differences in that of total TG (Fig. 2A) or dietary 13C-NEFA (Fig. 2C) (Note that removing the outlier point in plasma 13C-NEFA in the high secretion group, which is ∼8-fold higher than the average, has no effect on the group differences).
Given that WAT is a source of apoC-I, we examined whether adjusting the group differences (high vs. low WAT apoC-I secretors) for adiposity affected the outcomes. Adjusting for BMI, total fat, android fat, gynoid fat, or android/gynoid ratio retained group differences in plasma 13C-TG and chylomicrons (Fig. 2, P ≤ 0.05 for all). Adjusting for android/gynoid fat ratio rendered group differences in iAUC6hrs of 13C-NEFA significant (P = 0.038, with exclusion of the outlier point in the high group), and further increased the significance of the group differences in iAUC6hrs of apoB48. Adjusting for plasma apoC-I had no effect on the group differences in clearance of apoB48 (P = 0.005), but eliminated group differences in dietary 13C-TG. Thus the association of WAT apoC-I secretion with postprandial plasma clearance of chylomicrons was independent of adiposity, fat distribution, and fasting plasma apoC-I.
Subjects with high WAT apoC-I secretion have reduced WAT in situ LPL activity ex vivo
To test the hypothesis that local WAT apoC-I secretion associates with reduced WAT in situ LPL activity, we measured the hydrolysis of 3H-TRL and uptake and incorporation of LPL-generated 3H-NEFA as WAT 3H-lipids. We also measured the accumulation of 3H-NEFA in the medium over 4 h. As presented in Fig. 3, subjects with high WAT apoC-I had reduced WAT 3H-lipids compared with subjects with low WAT apoC-I (high = 3.15 ± 1.97 nmol vs. low = 5.09 ± 3.21 nmol 3H-TG hydrolyzed per milligram WAT, P = 0.043; Fig. 3A). However, there were no group differences in medium 3H-NEFA (Fig. 3B, equivalent to high = 1.00 ± 0.70 nmol vs. low = 2.19 ± 2.68 nmol 3H-TG hydrolyzed per milligram WAT).
Fig. 3.
Group differences in in situ LPL activity measured as WAT 3H-lipids (A) and as medium 3H-NEFA (B) using 3H-TRL substrate (1.27 mmol/l TG) and 3H-NEFA uptake and incorporation into WAT 3H-lipids using 3H-NEFA:BSA substrate (C) in subjects with low (N = 16) versus high (N = 17) WAT apoC-I secretion for (A, B), and low (N = 13) versus high (N = 15) WAT apoC-I secretion for (C). Women are represented as solid circles and men as open circles.
To verify whether the group difference in WAT 3H-lipids was dependent on LPL activity, we incubated a separate set of WAT samples with 3H-NEFA:BSA for 4 h. In contrast to when 3H-TRL was used, there were no group-differences in WAT 3H-lipids when 3H-NEFA:BSA was used (high = 3.62 ± 2.46 nmol vs. low = 3.71 ± 1.56 nmol 3H-NEFA per milligram WAT, P = 0.911, Fig. 3C). Adjusting for BMI; total, android, or gynoid fat; or android/gynoid ratio did not affect group differences in Fig. 3A–C. This suggests that the effect of WAT apoC-I on TRL clearance ex vivo in WAT is LPL dependent.
To verify whether the group differences in postprandial plasma clearance of dietary TRL were dependent on WAT in situ LPL activity, we adjusted for in situ LPL activity. Adjustment for WAT 3H-lipids (i.e., Fig. 3A) eliminated group differences in iAUC6hrs plasma 13C-TG and apoB48, while adjustment for LPL-released 3H-NEFA in the medium (i.e., Fig. 3B) only eliminated group differences in plasma clearance of 13C-TG, but not apoB48 (P = 0.038). Adjustment for WAT 3H-lipids from 3H-NEFA:BSA sources (i.e., Fig. 3C) also had no effect. Taken together, these data suggest that delayed postprandial plasma clearance of dietary TRL in subjects with high WAT apoC-I secretion may be dependent on reduced WAT LPL activity and not NEFA uptake (whether NEFA was LPL-released or BSA-bound).
WAT apoC-I secretion is not subject to postprandial fluctuation
We examined whether WAT apoC-I secretion was changed postprandially in a subpopulation of 19 subjects. There were no significant postprandial changes in WAT-apoC-I secretion in the entire group (fasting: 86.2 ± 40.5 pmol/g/4 h vs. postprandial: 80.4 ± 60.5 pmol/g/4 h, N = 19) nor per group of low WAT apoC-I secretion (fasting: 46.6 ± 18.2 pmol/g/4 h vs. postprandial: 58.4 ± 44.4 pmol/g/4 h, N = 8) or high WAT apoC-I secretion (fasting: 115.0 ± 23.2 pmol/g/4 h vs. postprandial: 96.4 ± 67.3 pmol/g/4 h, N = 11). Moreover, as in the fasting state, there were no sex-differences in postprandial WAT apoC-I secretion (11 men: 72.4 ± 72.8 pmol/g/4 h vs. 8 women: 91.4 ± 39.8 pmol/g/4 h). There were small associations between postprandial WAT apoC-I secretion with the measured outcomes related to TRL clearance in plasma and in WAT, likely due to the smaller sample size. However, postprandial WAT apoC-I secretion was negatively associated with 3H-TRL hydrolysis by WAT over 4 h (r = −0.832, P < 0.001). The postprandial changes in WAT apoC-I secretion, whether expressed as absolute or percent changes, were not associated with any of the measured outcomes.
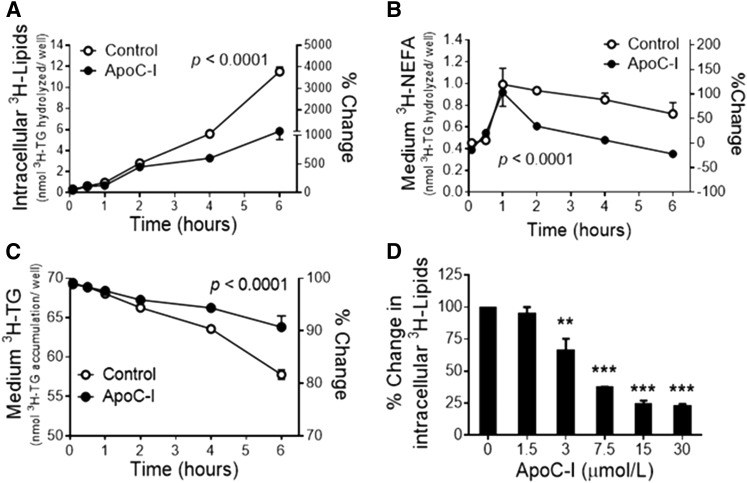
Direct effect of human apoC-I on in situ LPL activity in 3T3-L1 adipocytes
To explore the direct effect of apoC-I on 3H-TRL hydrolysis by adipocytes’ secreted LPL and their storage by adipocytes, we measured in situ LPL activity in 3T3-L1 adipocytes in the presence or absence of human VLDL-extracted apoC-I. Synthetic 3H-TRLs (0.56 mmol/l TG) were incubated with differentiated 3T3-L1 adipocytes with or without physiological concentrations of apoC-I (15 μmol/l) for 6 h. ApoC-I induced a time-dependent reduction in the hydrolysis and incorporation of 3H-TRL substrate as intracellular 3H-lipids by ∼4-fold (Fig. 4A), and reduced the accumulation of LPL-generated 3H-NEFA in the medium by ∼2-fold (Fig. 4B). Subsequently, there was an accumulation of 3H-TRL in the medium of apoC-I-incubated adipocytes (Fig. 4C). The inhibitory effect of apoC-I at 4 h was also concentration dependent, reaching a maximum inhibition of 3H-TRL hydrolysis and incorporation as intracellular 3H-lipids at 15 μM (∼78%, Fig. 4D). Notably, the lack of accumulation of LPL-released 3H-NEFA in the medium of the apoC-I-incubated adipocytes (Fig. 4B) suggests that apoC-I does not hinder the uptake and esterification of NEFA.
Fig. 4.
Time-dependent effects of human VLDL-extracted apoC-I (15 μM) on 3H-TRL hydrolysis and incorporation as intracellular 3H-lipids (A), 3H-TRL hydrolysis and 3H-NEFA release into the medium (B), 3H-TRL accumulation in the medium (C), and concentration-dependent effect of human apoC-I on 3H-TRL hydrolysis and incorporation as intracellular 3H-lipids over 4-h in 3T3-L1 adipocytes (D).
DISCUSSION
In this study of 39 overweight and obese men and postmenopausal women, we present novel data demonstrating that when compared with subjects with low WAT apoC-I, subjects with high WAT apoC-I secretion have: 1) delayed postprandial plasma clearance of dietary, but not total, TG and TRL in vivo; 2) reduced in situ LPL activity ex vivo in WAT; and 3) no differences in NEFA uptake and storage in vivo or ex vivo in WAT. Adjustment for plasma apoC-I, adiposity, fat distribution, or NEFA uptake ex vivo by WAT did not eliminate group differences in apoB48 clearance; however, adjustment for WAT in situ LPL activity did. There were no sex-differences in any measured outcomes related to WAT apoC-I secretion or TRL clearance in vivo or ex vivo in WAT. Finally, human apoC-I directly inhibited in situ LPL activity resulting in the media-accumulation of TRL in both a concentration- and time-dependent manner. Taken together, while the clinical data cannot establish causality, the combination of in vivo, ex vivo, and in vitro studies suggests that local WAT apoC-I secretion inhibits plasma clearance of dietary TRL by reducing LPL activity at the WAT.
Identifying the physiological role of apoC-I in humans has been challenging because, to date, there are no primary dyslipidemias attributed to an apoC-I mutation or polymorphism. In fact, only a single case-report of complete apoC-I deficiency was found in which the patient had combined deficiency of apoC-I and apoC-II and suffered from hypertriglyceridemia, hypercholesterolemia, and type 2 diabetes (35). ApoC-I polymorphisms are equally rare, as only two have been reported: a polymorphism in the promoter region (Hpa1) (36, 37) and an apoC-I structural variant (T45S) (38). However, their effects on lipid metabolism in vivo remain ambiguous and depend on the population examined. For example, reduced plasma apoC-I secondary to the T45S polymorphism was reported to be associated with reduced percent body fat and waist circumference and unchanged BMI, plasma TG, and cholesterol in aboriginal Canadians (39). However, this variant was associated with elevated BMI and diabetes in subjects of American Indian and Mexican ancestry (38). While apoC-I polymorphism was not assessed here, plasma apoC-I was not associated with adiposity or plasma clearance of TRLs (TG or apoB48) in this study. It should be recalled, however, that in humans the major fraction of fasting apoC-I is carried on HDL (∼80%) where it is considered protective and unrelated to TRL clearance (14, 16, 17). Thus, the uneven distribution of apoC-I on plasma lipoproteins may play a major role in the inconsistent relation of plasma apoC-I with lipid metabolism in humans.
Here, we demonstrate for the first time how apoC-I secreted from human WAT impedes in situ LPL activity and TRL clearance in WAT. WAT plays an important role in the clearance of dietary lipids in response to postprandial signals in humans (8, 12, 40). Tracer in vivo human studies reported that the trapping of LPL-generated dietary NEFAs by subcutaneous WAT is almost absent at fasting, increases to ∼100% 1 h after eating, and returns to 10–30% 6 h after eating a meal (10). On the other hand, the role of apoC-I on TRLs has solely been examined in vitro and in murine models. apoC-I was found to inhibit the binding of LPL to lipid emulsions, decreasing TG hydrolysis and rendering unbound-LPL more prone to inactivation (41). ApoE knockout mice overexpressing human apoC-I have reduced uptake of 3H-TG-derived NEFAs from intravenously administered 3H-VLDL-like particles only in WAT (21). Moreover, apoC-I overexpression in wild-type mice was shown to impair the uptake of intravenously injected fatty acid analog by WAT, but not by other tissues (42). Endogenous apoC-I produced locally in vitro from macrophages of mice overexpressing human apoC-I was also reported to bind and reduce NEFA esterification (26). While overexpression of apoC-I was proposed to protect against obesity in mice (26), it consistently led to postprandial hyperlipidemia believed to be secondary to the inhibition of LPL activity, TRL clearance, and NEFA uptake (20, 21, 26, 42, 43), and to the overproduction of hepatic VLDL-TG and apoB without a change of TG absorption (21).
In our hands, WAT apoC-I secretion, but not total plasma apoC-I, was associated with reduced TRL clearance in vivo and ex vivo in human WAT. WAT apoC-I secretion was not, however, associated with NEFA uptake, as measured by the plasma clearance of 13C-NEFAs and the WAT uptake of 3H-NEFAs in subjects with high, compared with low, WAT apoC-I secretion. Moreover, physiological concentrations of human apoC-I did not induce the accumulation of 3H-NEFA in the adipocyte culture medium. Because NEFA accumulation inhibits LPL activity (34, 44–46), the lack of NEFA accumulation with apoC-I addition supports that the inhibition of LPL was due to apoC-I. This also suggests that the principal mechanism by which apoC-I inhibits TRL clearance in WAT is via the inhibition of LPL activity, not NEFA uptake. While this is in line with reduced VLDL-like particle clearance by WAT in mice overexpressing apoC-I (21), it opposes the proposed role of overexpression of apoC-I in hindering NEFA uptake in mice (26, 42).
The in situ experiments with WAT and adipocytes shown here model the in vivo interaction of adipocyte-secreted apoC-I with LPL and chylomicrons. LPL is anchored to the endothelial surface by glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1), where it exerts its lipolytic activity on circulating TRLs (47). The anchoring of LPL on the endothelial surface is vital for its activity because in vivo human studies show that, although plasma LPL mass increases in the postprandial state, it is mostly inactive (>95%) (48). Adipocyte-secreted LPL needs to cross the endothelium and reach the luminal surface in order to exert its lipolytic activity on TRLs (49). Consequently, one would assume that adipocyte-secreted apoC-I also has to cross the endothelium to influence LPL-mediated chylomicron lipolysis. This has yet to be examined.
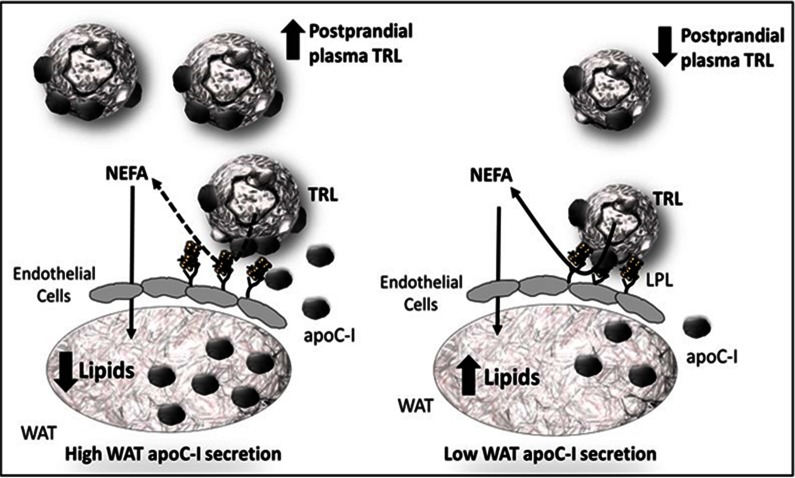
Increased WAT apoC-I secretion was associated with delayed postprandial plasma clearance of dietary TRL particles (13C-TG and apoB48), but not of total plasma TG or apoB. This may be due to the minor contribution of dietary particles to total plasma concentrations (<2%) (29). Alternatively, WAT apoC-I secretion may have a greater effect on chylomicrons than on VLDL clearance because hepatic endoplasmic reticulum-derived VLDL transport vesicles, but not prechylomicrons, were reported to contain apoC-I in rats (50). This suggests that the enrichment of chylomicrons with apoC-I takes place in the plasma. Because the WAT represents a physiological site for the interaction with chylomicrons, it is logical to propose that WAT apoC-I secretion enriches chylomicrons, thereby delaying their clearance by endothelial LPL activity (Fig. 5). This is supported by: 1) our previous findings that WAT apoC-I secretion associates with TRL apoC-I content and that adjustment for TRL apoC-I eliminates the association of WAT apoC-I with delayed 13C-TG clearance in women (14); and 2) by the present study in which adjustment for WAT in situ LPL activity eliminates the association of WAT apoC-I with delayed clearance of dietary TRLs in men and women.
Fig. 5.
Proposed model for the interaction of WAT-secreted apoC-I with TRL and LPL on the endothelial surface of WAT. Increased apoC-I secretion from WAT enriches TRL with apoC-I, which inhibits the activity of LPL on the endothelium of WAT and the hydrolysis of postprandial TRL without affecting the uptake of NEFA into WAT. This results in decreased clearance and incorporation of TRL into WAT lipids and increased postprandial plasma concentrations of TRLs (dashed arrow for inhibited and solid arrow for unaffected process).
Finally, we observed a 7-fold variation in the secretion of apoC-I from WAT among our participants, whereas the plasma apoC-I variation was only 3-fold. Given that WAT apoC-I secretion associates with the fasting and postprandial enrichment of nonHDL lipoproteins with apoC-I (14), increased plasma retention time of TRLs in subjects with high WAT apoC-I secretion may explain higher plasma apoC-I concentrations. Factors affecting the variability of WAT apoC-I secretion, whether genetic or environmental, were not determined in this study. However, none of the examined parameters related to nutritional intake, body composition, and metabolic profile predicted WAT apoC-I secretion. Future studies need to evaluate the regulation of WAT apoC-I secretion by apoC-I polymorphism or variables not examined in this study, such as WAT inflammation, which can modulate both WAT in situ LPL activity and apoC-I secretion.
In conclusion, WAT apoC-I secretion is associated with reduced WAT in situ LPL activity and delayed postprandial plasma clearance of chylomicrons in obese men and postmenopausal women independent of adiposity, body fat distribution, WAT NEFA uptake, or plasma apoC-I. We hypothesize that targeting the reduction in WAT apoC-I secretion ameliorates plasma clearance of TRLs and its associated cardiometabolic risk factors.
Supplementary Material
Acknowledgments
The authors acknowledge the invaluable help of Drs. Remi Rabasa-Lhoret, Alexis Baass, and Robert Dufour in conducting WAT biopsies and subjects’ medical screening and follow-up.
Footnotes
Abbreviations:
- 13C-NEFA
- 13C-triolein-labeled NEFA
- 13C-TG
- 13C-triolein-labeled TG
- HDL-C
- HDL cholesterol
- 3H-NEFA
- 3H-triolein-labeled NEFA
- HOMA-IR
- homeostatic model assessment of insulin resistance
- 3H-TG
- 3H-triolein-labeled TG
- 3H-TRL
- 3H-triolein-labeled TG-rich lipoproteins
- iAUC6hrs
- incremental area under the curve over 6 hours
- IRCM
- Institut de recherches cliniques de Montréal
- LDL-C
- LDL cholesterol
- TRL
- TG-rich lipoprotein
- VLDL-C
- VLDL cholesterol
- WAT
- white adipose tissue
This work was supported by Canadian Institutes of Health Research (CIHR) Grant 93581 to M.F. and a grant from AstraZeneca Canada to J.D. M.F. is the recipient of salary supports from CIHR, Fonds de recherche du Québec - Santé (FRQS), and Leader’s Opportunity Fund from the Canadian Foundation for Innovation (CFI). S.B. and Y.C. are the recipients of Frederick Banting and Charles Best Canada graduate students salary awards from CIHR. V.L. is the recipient of graduate study salary award from FRQS. The authors have no financial conflicts of interest to disclose.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Genest J., McPherson R., Frohlich J., Anderson T., Campbell N., Carpentier A., Couture P., Dufour R., Fodor G., Francis G. A., et al. 2009. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can. J. Cardiol. 25: 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller M., Stone N. J., Ballantyne C., Bittner V., Criqui M. H., Ginsberg H. N., Goldberg A. C., Howard W. J., Jacobson M. S., Kris-Etherton P. M., et al. 2011. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 123: 2292–2333. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S. M., Khaw K. T., and Gudnason V.. 2007. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 115: 450–458. [DOI] [PubMed] [Google Scholar]
- 4.Murad M. H., Hazem A., Coto-Yglesias F., Dzyubak S., Gupta S., Bancos I., Lane M. A., Erwin P. J., Berglund L., Elraiyah T., et al. 2012. The association of hypertriglyceridemia with cardiovascular events and pancreatitis: a systematic review and meta-analysis. BMC Endocr. Disord. 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal S., Buring J. E., Rifai N., Mora S., Sacks F. M., and Ridker P. M.. 2007. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 298: 309–316. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard B. G., Benn M., Schnohr P., and Tybjaerg-Hansen A.. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 298: 299–308. [DOI] [PubMed] [Google Scholar]
- 7.Proctor S. D., and Mamo J. C.. 1998. Retention of fluorescent-labelled chylomicron remnants within the intima of the arterial wall–evidence that plaque cholesterol may be derived from post-prandial lipoproteins. Eur. J. Clin. Invest. 28: 497–503. [DOI] [PubMed] [Google Scholar]
- 8.Faraj M., Lu H. L., and Cianflone K.. 2004. Diabetes, lipids, and adipocyte secretagogues. Biochem. Cell Biol. 82: 170–190. [DOI] [PubMed] [Google Scholar]
- 9.Trujillo M. E., and Scherer P. E.. 2006. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 27: 762–778. [DOI] [PubMed] [Google Scholar]
- 10.Evans K., Burdge G. C., Wootton S. A., Clark M. L., and Frayn K. N.. 2002. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 51: 2684–2690. [DOI] [PubMed] [Google Scholar]
- 11.Guilherme A., Virbasius J. V., Puri V., and Czech M. P.. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraj M., and Cianflone K.. 2004. Differential regulation of fatty acid trapping in mouse adipose tissue and muscle by ASP. Am. J. Physiol. Endocrinol. Metab. 287: E150–E159. [DOI] [PubMed] [Google Scholar]
- 13.Hamsten A., Silveira A., Boquist S., Tang R., Bond M. G., de Faire U., and Bjorkegren J.. 2005. The apolipoprotein CI content of triglyceride-rich lipoproteins independently predicts early atherosclerosis in healthy middle-aged men. J. Am. Coll. Cardiol. 45: 1013–1017. [DOI] [PubMed] [Google Scholar]
- 14.Wassef H., Salem H., Bissonnette S., Baass A., Dufour R., Davignon J., and Faraj M.. 2012. White adipose tissue apolipoprotein C-I secretion in relation to delayed plasma clearance of dietary fat in humans. Arterioscler. Thromb. Vasc. Biol. 32: 2785–2793. [DOI] [PubMed] [Google Scholar]
- 15.Sacks F. M. 2006. The apolipoprotein story. Atheroscler. Suppl. 7: 23–27. [DOI] [PubMed] [Google Scholar]
- 16.Shachter N. S. 2001. Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr. Opin. Lipidol. 12: 297–304. [DOI] [PubMed] [Google Scholar]
- 17.Gautier T., Masson D., de Barros J. P., Athias A., Gambert P., Aunis D., Metz-Boutigue M. H., and Lagrost L.. 2000. Human apolipoprotein C-I accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J. Biol. Chem. 275: 37504–37509. [DOI] [PubMed] [Google Scholar]
- 18.Anuurad E., Yamasaki M., Shachter N., Pearson T. A., and Berglund L.. 2009. ApoE and ApoC-I polymorphisms: association of genotype with cardiovascular disease phenotype in African Americans. J. Lipid Res. 50: 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Björkegren J. 2006. Dual roles of apolipoprotein CI in the formation of atherogenic remnants. Curr. Atheroscler. Rep. 8: 1–2. [DOI] [PubMed] [Google Scholar]
- 20.Berbée J. F., van der Hoogt C. C., Sundararaman D., Havekes L. M., and Rensen P. C.. 2005. Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL. J. Lipid Res. 46: 297–306. [DOI] [PubMed] [Google Scholar]
- 21.Westerterp M., de Haan W., Berbee J. F., Havekes L. M., and Rensen P. C.. 2006. Endogenous apoC-I increases hyperlipidemia in apoE-knockout mice by stimulating VLDL production and inhibiting LPL. J. Lipid Res. 47: 1203–1211. [DOI] [PubMed] [Google Scholar]
- 22.van der Hoogt C. C., Berbee J. F., Espirito Santo S. M., Gerritsen G., Krom Y. D., van der Zee A., Havekes L. M., van Dijk K. W., and Rensen P. C.. 2006. Apolipoprotein CI causes hypertriglyceridemia independent of the very-low-density lipoprotein receptor and apolipoprotein CIII in mice. Biochim. Biophys. Acta. 1761: 213–220. [DOI] [PubMed] [Google Scholar]
- 23.Jong M. C., van Dijk K. W., Dahlmans V. E., Van der Boom H., Kobayashi K., Oka K., Siest G., Chan L., Hofker M. H., and Havekes L. M.. 1999. Reversal of hyperlipidaemia in apolipoprotein C1 transgenic mice by adenovirus-mediated gene delivery of the low-density-lipoprotein receptor, but not by the very-low-density-lipoprotein receptor. Biochem. J. 338: 281–287. [PMC free article] [PubMed] [Google Scholar]
- 24.Sehayek E., and Eisenberg S.. 1991. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 266: 18259–18267. [PubMed] [Google Scholar]
- 25.Weisgraber K. H., Mahley R. W., Kowal R. C., Herz J., Goldstein J. L., and Brown M. S.. 1990. Apolipoprotein C–I modulates the interaction of apolipoprotein E with beta-migrating very low density lipoproteins (beta-VLDL) and inhibits binding of beta-VLDL to low density lipoprotein receptor-related protein. J. Biol. Chem. 265: 22453–22459. [PubMed] [Google Scholar]
- 26.Westerterp M., Berbee J. F., Delsing D. J., Jong M. C., Gijbels M. J., Dahlmans V. E., Offerman E. H., Romijn J. A., Havekes L. M., and Rensen P. C.. 2007. Apolipoprotein C-I binds free fatty acids and reduces their intracellular esterification. J. Lipid Res. 48: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 27.Wassef H., Bernier L., Davignon J., and Cohn J. S.. 2004. Synthesis and secretion of apoC-I and apoE during maturation of human SW872 liposarcoma cells. J. Nutr. 134: 2935–2941. [DOI] [PubMed] [Google Scholar]
- 28.Wassef H., Bissonnette S., Saint-Pierre N., Lamantia V., Cyr Y., Chretien M., and Faraj M.. 2015. The apoB-to-PCSK9 ratio: A new index for metabolic risk in humans. J. Clin. Lipidol. 9: 664–675. [DOI] [PubMed] [Google Scholar]
- 29.Bissonnette S., Salem H., Wassef H., Saint-Pierre N., Tardif A., Baass A., Dufour R., and Faraj M.. 2013. Low density lipoprotein delays clearance of triglyceride-rich lipoprotein by human subcutaneous adipose tissue. J. Lipid Res. 54: 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bissonnette S., Saint-Pierre N., Lamantia V., Cyr Y., Wassef H., and Faraj M.. 2015. Plasma IL-1Ra: linking hyperapoB to risk factors for type 2 diabetes independent of obesity in humans. Nutr. Diabetes. 5: e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassef H., Davignon J., Prud’homme D., Rabasa-Lhoret R., and Faraj M.. 2014. Changes in total and central fat mass after a hypocaloric diet associate with changes of apoC-I in postmenopausal obese women. J. Clin. Lipidol. 8: 510–519. [DOI] [PubMed] [Google Scholar]
- 32.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., and Turner R. C.. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 33.Lavoie M. E., Faraj M., Strychar I., Doucet E., Brochu M., Lavoie J. M., and Rabasa-Lhoret R.. 2013. Synergistic associations of physical activity and diet quality on cardiometabolic risk factors in overweight and obese postmenopausal women. Br. J. Nutr. 109: 605–614. [DOI] [PubMed] [Google Scholar]
- 34.Faraj M., Sniderman A. D., and Cianflone K.. 2004. ASP enhances in situ lipoprotein lipase activity by increasing fatty acid trapping in adipocytes. J. Lipid Res. 45: 657–666. [DOI] [PubMed] [Google Scholar]
- 35.Dumon M. F., and Clerc M.. 1986. Preliminary report on a case of apolipoproteins CI and CII deficiency. Clin. Chim. Acta. 157: 239–248. [DOI] [PubMed] [Google Scholar]
- 36.Smit M., van der Kooij-Meijs E., Woudt L. P., Havekes L. M., and Frants R. R.. 1988. Exact localization of the familial dysbetalipoproteinemia associated HpaI restriction site in the promoter region of the APOC1 gene. Biochem. Biophys. Res. Commun. 152: 1282–1288. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y., Berglund L., Ramakrishnan R., Mayeux R., Ngai C., Holleran S., Tycko B., Leff T., and Shachter N. S.. 1999. A common Hpa I RFLP of apolipoprotein C-I increases gene transcription and exhibits an ethnically distinct pattern of linkage disequilibrium with the alleles of apolipoprotein E. J. Lipid Res. 40: 50–58. [PubMed] [Google Scholar]
- 38.Kasthuri R. S., McMillan K. R., Flood-Urdangarin C., Harvey S. B., Wilson-Grady J. T., and Nelsestuen G. L.. 2007. Correlation of a T45S variant of apolipoprotein C1 with elevated BMI in persons of American Indian and Mexican ancestries. Int. J. Obes. (Lond). 31: 1334–1336. [DOI] [PubMed] [Google Scholar]
- 39.Lahiry P., Cao H., Ban M. R., Pollex R. L., Mamakeesick M., Zinman B., Harris S. B., Hanley A. J., Huff M. W., Connelly P. W., et al. 2010. APOC1 T45S polymorphism is associated with reduced obesity indices and lower plasma concentrations of leptin and apolipoprotein C-I in aboriginal Canadians. J. Lipid Res. 51: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picard F., Naimi N., Richard D., and Deshaies Y.. 1999. Response of adipose tissue lipoprotein lipase to the cephalic phase of insulin secretion. Diabetes. 48: 452–459. [DOI] [PubMed] [Google Scholar]
- 41.Larsson M., Vorrsjo E., Talmud P., Lookene A., and Olivecrona G.. 2013. Apolipoproteins C-I and C–III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J. Biol. Chem. 288: 33997–34008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jong M. C., Voshol P. J., Muurling M., Dahlmans V. E., Romijn J. A., Pijl H., and Havekes L. M.. 2001. Protection from obesity and insulin resistance in mice overexpressing human apolipoprotein C1. Diabetes. 50: 2779–2785. [DOI] [PubMed] [Google Scholar]
- 43.Shachter N. S., Ebara T., Ramakrishnan R., Steiner G., Breslow J. L., Ginsberg H. N., and Smith J. D.. 1996. Combined hyperlipidemia in transgenic mice overexpressing human apolipoprotein CI. J. Clin. Invest. 98: 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena U., Witte L. D., and Goldberg I. J.. 1989. Release of endothelial cell lipoprotein lipase by plasma lipoproteins and free fatty acids. J. Biol. Chem. 264: 4349–4355. [PubMed] [Google Scholar]
- 45.Sasaki A., and Goldberg I. J.. 1992. Lipoprotein lipase release from BFC-1 beta adipocytes. Effects of triglyceride-rich lipoproteins and lipolysis products. J. Biol. Chem. 267: 15198–15204. [PubMed] [Google Scholar]
- 46.Saxena U., and Goldberg I. J.. 1990. Interaction of lipoprotein lipase with glycosaminoglycans and apolipoprotein C-II: effects of free-fatty-acids. Biochim. Biophys. Acta. 1043: 161–168. [DOI] [PubMed] [Google Scholar]
- 47.Adeyo O., Goulbourne C. N., Bensadoun A., Beigneux A. P., Fong L. G., and Young S. G.. 2012. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 and the intravascular processing of triglyceride-rich lipoproteins. J. Intern. Med. 272: 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karpe F., Olivecrona T., Olivecrona G., Samra J. S., Summers L. K., Humphreys S. M., and Frayn K. N.. 1998. Lipoprotein lipase transport in plasma: role of muscle and adipose tissues in regulation of plasma lipoprotein lipase concentrations. J. Lipid Res. 39: 2387–2393. [PubMed] [Google Scholar]
- 49.Kersten S. 2014. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta. 1841: 919–933. [DOI] [PubMed] [Google Scholar]
- 50.Rahim A., Nafi-valencia E., Siddiqi S., Basha R., Runyon C. C., and Siddiqi S. A.. 2012. Proteomic analysis of the very low density lipoprotein (VLDL) transport vesicles. J. Proteomics. 75: 2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.