Abstract
Lipoprotein (a) [Lp(a)] is independently associated with CVD risk. Evolocumab, a monoclonal antibody (mAb) to proprotein convertase subtilisin/kexin type 9 (PCSK9), decreases Lp(a). The potential mechanisms were assessed. A pooled analysis of Lp(a) and LDL cholesterol (LDL-C) in 3,278 patients from 10 clinical trials (eight phase 2/3; two extensions) was conducted. Within each parent study, biweekly and monthly doses of evolocumab statistically significantly reduced Lp(a) at week 12 versus control (P < 0.001 within each study); pooled median (quartile 1, quartile 3) percent reductions were 24.7% (40.0, 3.6) and 21.7% (39.9, 4.2), respectively. Reductions were maintained through week 52 of the open-label extension, and correlated with LDL-C reductions [with and without correction for Lp(a)-cholesterol] at both time points (P < 0.0001). The effect of LDL and LDL receptor (LDLR) availability on Lp(a) cell-association was measured in HepG2 cells: cell-associated LDL fluorescence was reversed by unlabeled LDL and Lp(a). Lp(a) cell-association was reduced by coincubation with LDL and PCSK9 and reversed by adding PCSK9 mAb. These studies support that reductions in Lp(a) with PCSK9 inhibition are partly due to increased LDLR-mediated uptake. In most situations, Lp(a) appears to compete poorly with LDL for LDLR binding and internalization, but when LDLR expression is increased with evolocumab, particularly in the setting of low circulating LDL, Lp(a) is reduced.
Keywords: lipoprotein (a), low density lipoprotein cholesterol, low density lipoprotein receptor, proprotein convertase subtilisin/kexin type 9
Lipoprotein (a) [Lp(a)] is an LDL-like particle consisting of hepatic synthesized apo(a), a plasminogen-like glycoprotein that is disulfide-linked to the apoB moiety of circulating LDL, most likely at the hepatocellular surface (1). Lp(a) levels are highly variable, primarily genetically determined, and independently associated with CVD. Both epidemiological and genetic studies show that increased levels of Lp(a) are consistently and positively associated with CVD risk (2–4).
Statins, while effective at lowering LDL cholesterol (LDL-C), do not reduce Lp(a) levels and may even increase them slightly (5–13). In addition, the reduction of LDL-C with statins has been shown to markedly decrease or attenuate the CVD relationship with Lp(a) (14). Other lipid-lowering drug therapies that do not primarily lower LDL-C by upregulating LDL receptor (LDLR) activity (e.g., bile acid sequestrants, ezetimibe) have no reported effect on Lp(a), while moderate reductions are seen with drugs that inhibit the production of LDL-C or its precursor [e.g., niacin (13), mipomersen, lomitapide]. Yet Lp(a) is robustly and consistently reduced by treatment with proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies, which reduce LDL-C via upregulation of LDLR activity (15–21). The mechanism(s) by which PCSK9 inhibition reduces Lp(a) has not yet been determined, but potential explanations include decreased apoB synthesis (11), decreased Lp(a) synthesis, reduced availability of apoB-containing lipoproteins (LDL) for linkage to Lp(a), enhanced Lp(a) uptake and clearance by the LDLR (11, 22), or other hepatic receptors in the setting of low LDL-C levels (22).
The Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations (PROFICIO) is a pooled analysis of 3,278 patients on various background lipid-lowering therapies from eight parent phase 2 and phase 3 studies, and two extension studies of evolocumab, a fully human monoclonal antibody (mAb) to PCSK9 (18, 19, 21, 23–28). In this analysis, we (1) evaluated the relationship between posttreatment LDL-C levels with and without correction for Lp(a)-cholesterol [Lp(a)-C] and Lp(a) reductions, and (2) determined whether increased LDLR activity could be responsible for clearance of Lp(a) by examining whether this potential effect could be modulated in vitro.
MATERIALS AND METHODS
Evolocumab clinical trials and pooled analyses
Data from eight randomized placebo-controlled blinded phase 2 and 3 clinical trials [MENDEL-1 (19), MENDEL-2 (23), LAPLACE-TIMI 57 (21), LAPLACE-2 (24), RUTHERFORD-1 (25), RUTHERFORD-2 (26), GAUSS-1 (18), GAUSS-2 (27)] and two open-label extension trials [OSLER-1 (28), OSLER-2] were used in this analysis. Patients who completed one of the phase 2 or 3 clinical trials and agreed to participate in the open-label extension studies were rerandomized to standard of care (SOC) or SOC plus evolocumab (supplementary Table 1, supplementary Fig. 1), and informed consent was obtained from all patients prior to their participation in the studies. All studies were approved by independent ethics committees and institutional review boards, and all were conducted in accordance with applicable country regulations and the Declaration of Helsinki.
Endpoints for this analysis included the percent change from baseline to parent study week 12 and extension study week 52 in Lp(a) and LDL-C and the potential relationship between these changes. Lp(a) was measured by the same standardized isoform-independent method throughout the studies. In the phase 2 studies, LDL-C was determined by both Friedewald formula and ultracentrifugation. In the phase 3 studies LDL-C was calculated using the Friedewald formula, but was measured by ultracentrifugation using the same blood sample if LDL-C by Friedewald was <40 mg/dl or triglycerides were >400 mg/dl; LDL-C was measured by ultracentrifugation using the same blood sample. Patients in the control group included those randomized to receive placebo injections every 2 weeks (Q2W) or monthly (QM), placebo plus ezetimibe, ezetimibe alone, or SOC. Because LDL-C, as measured by ultracentrifugation and calculated by Friedewald formula, includes cholesterol in the form of Lp(a)-C, we corrected for Lp(a)-C by multiplying Lp(a) mass by a factor of 0.3, as approximately 30% of Lp(a) mass is cholesterol (29).
Analyses to compare the efficacy of evolocumab versus control for Lp(a) and LDL-C reductions were performed using the ANCOVA model in each dosing regimen (Q2W or QM). Multiplicity was controlled in the phase 3 studies, but not the phase 2 studies. Correlations between Lp(a) and LDL-C, corrected and uncorrected for Lp(a)-C for all patients, were assessed using Spearman’s correlation coefficient for patients with Lp(a) ≥5 nmol/l (the lower limit of detection) at parent study week 12. All analyses were done with SAS/STAT version 9.2 software (SAS Institute, Cary, NC).
In vivo and in vitro analyses
Anti-PCSK9 and anti-LDLR mAb generation and characterization.
Fully human anti-PCSK9 mAbs (mAb1, mAb2, mAb3, and mAb4) were all of the IgG2 subclass, and were generated as previously described (30, 31). The IgG2 control mAb utilized for in vivo studies was a human anti-keyhole limpet hemocyanin antibody (Amgen Inc., Thousand Oaks, CA). The anti-PCSK9 mAbs differed in binding affinity at neutral and acidic pH, leading to differences in half-life and duration of LDL-C lowering (mAb1 < mAb3 < mAb4 < mAb2) (31). Fully human anti-LDLR antibodies were generated by immunizing XenoMouse with human LDLR extracellular domain and human EGFa:EGFb-Fc and selecting high-affinity antibodies binding at the EGFa:EGFb domain region (data not shown).
Anti-PCSK9 mAb pharmacodynamics in nonhuman primate in vivo studies.
All procedures involving laboratory animals were approved by the institutional animal care and use committee at an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility, and adhered to the National Research Council’s Guide for the Care and Use of Laboratory Animals. Data were generated in three separate single-dose studies in cynomolgus monkeys (Macaca fascicularis) at Valley Biosystems (West Sacramento, CA). Details of these studies are provided in the supplement.
Anti-LDLR antibody specificity.
The specificity of anti-LDLR antibodies, mAbA and mAbB (Amgen Inc.), for the LDLR was determined using ELISAs. Details of this analysis are provided in the supplement. Anti-LDLR antibodies and PCSK9 were also tested for the ability to modulate expression of other cell receptors. Lysates of HepG2 cells grown in a lipoprotein-deficient serum (LPDS) and treated with PCSK9 with or without mAb1, or with mAbB were analyzed using Western blots probed with antibodies to the VLDL receptor (VLDLR) (Thermo Scientific, Rockford, IL), scavenger receptor class B member 1 (SR-B1) (Thermo Scientific), LDLR-related protein 1 (LRP1) (Thermo Scientific), and cluster of differentiation 36 (CD36) (R&D Systems, Minneapolis, MN).
Cell LDL uptake competition assay in vitro
Because PCSK9 inhibition has been shown to increase expression of the LDLR and increase hepatic uptake of LDL (32), studies to ascertain a potential connection between the LDLR and cellular Lp(a) association were performed. The majority of LDL is taken up by the LDLR, although ∼10–20% can associate with cells via other receptors or potential non-receptor-mediated pathways (32); therefore, fluorescently labeled LDL uptake by HepG2 cells was used as a surrogate assay of LDLR expression and activity. Fluorescently labeled LDL (LDL BODIPY; Life Technologies, Carlsbad, CA) was mixed with increasing concentrations of unlabeled LDL (Sigma-Aldrich, St. Louis, MO), Lp(a) (Lee Biosolutions), HDL (Sigma-Aldrich), or PBS (control), and was then incubated with HepG2 cells seeded in 96-well culture plates (Costar; Corning Life Sciences, Tewksbury, MA) for 3 h at 37°C. After five washes with PBS, cell-associated relative fluorescence units were measured using a plate reader (Safire; Tecan Systems, Inc., San Jose, CA).
Lp(a) cell association in vitro
Cell association with Lp(a) was determined by Western blot using anti-human Lp(a) mAb (EPR6474; Abcam PLC, Cambridge, MA) of HepG2 whole cell lysate. Cells were incubated with purified Lp(a) (Lee Biosolutions) for 4 h at 37°C. In the competition studies, 10 μg/ml of Lp(a) was mixed with 1–40 μg/ml of LDL or HDL (Sigma-Aldrich) and incubated with HepG2 cells for 4 h in 6-well plates at 37°C. Cells were washed five times with PBS and lysed. Protein (40 μg/lane) was resolved on 3–8% tris-acetate SDS-PAGE gels (Life Technologies) for Lp(a) and 4–12% bis-tris SDS-PAGE gels (Life Technologies) for LDLR. Proteins were transferred to nitrocellulose membranes and probed with anti-Lp(a) antibody (1:10,000 dilution), anti-LDLR antibody (1:5,000 dilution; Abcam), or β-actin control (anti-actin antibody, 1:2,000 dilution; Cell Signaling Technology, Inc., Danvers, MA).
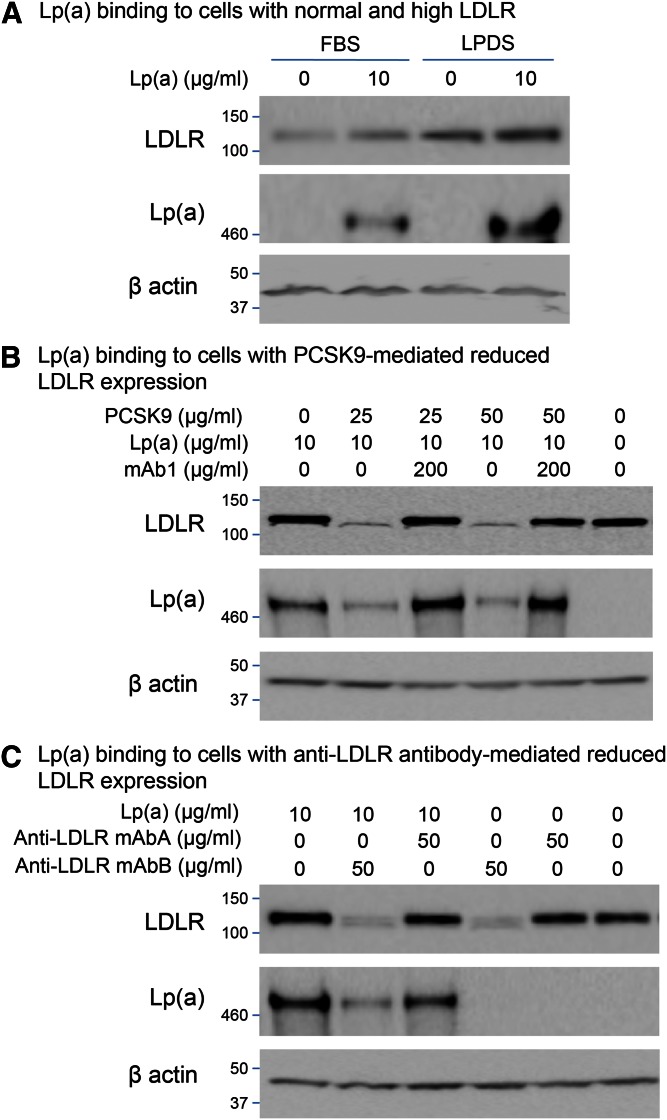
The role of LDLR in Lp(a) cell association was also assessed by manipulating the level of LDLR on HepG2 cells. LDLR expression was increased by culturing HepG2 cells overnight in medium containing 10% LPDS or maintained at normal levels by culturing in medium containing 10% FBS. LDLR levels were reduced by incubating the cells with 25 or 50 μg/ml of recombinant human PCSK9 (Amgen Inc.) in the presence or absence of 200 μg/ml mAb1. Cell LDLR levels were also downregulated using the anti-LDLR antibodies, mAbA and mAbB (Amgen Inc.), at 50 μg/ml. In each experiment, cells were incubated with 10 μg/ml Lp(a) for 4 h at 37°C. Protein lysates were analyzed using Western blots and probed with antibodies to Lp(a), LDLR, and β-actin as described above.
RESULTS
Patients
A total of 3,278 patients received at least one dose of study drug and were included in the analyses (supplementary Table 2). The mean (SD) age was 57.7 (11.2) years; most patients were white (91.6%), half were women (50.1%), and most were treated with statins (69.6%). Baseline median [first and third quartiles (Q1, Q3)] Lp(a) was 37.0 (11.0, 148.0) nmol/l; mean (SD) uncorrected LDL-C was 134.3 (47.9) mg/dl; when corrected for Lp(a)-C, LDL-C was 122.9 (48.9) mg/dl.
Lipid and lipoprotein endpoints
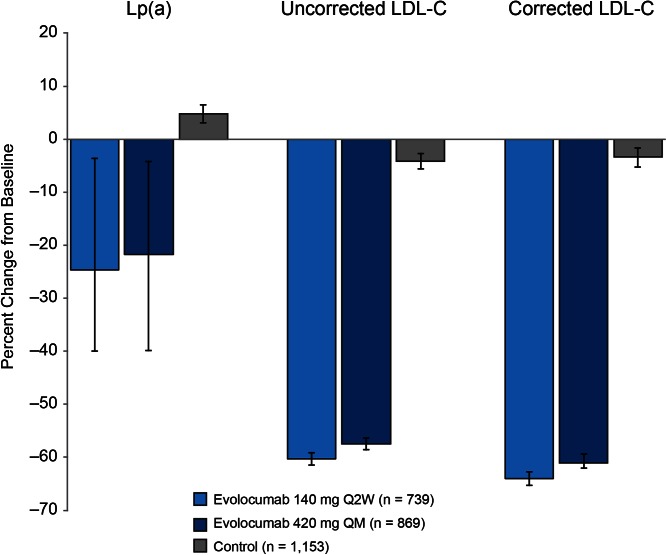
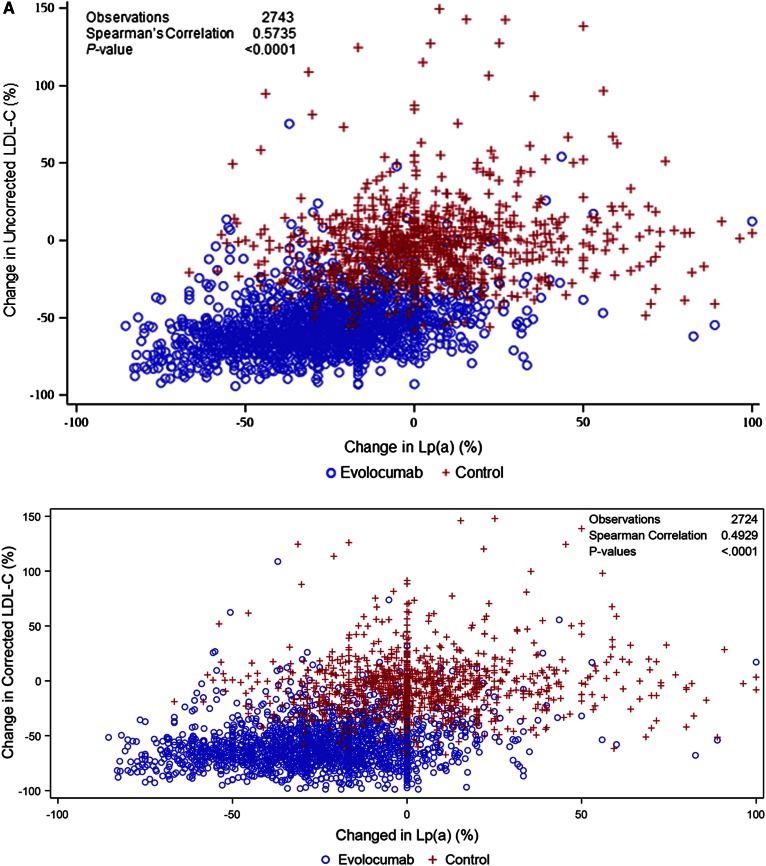
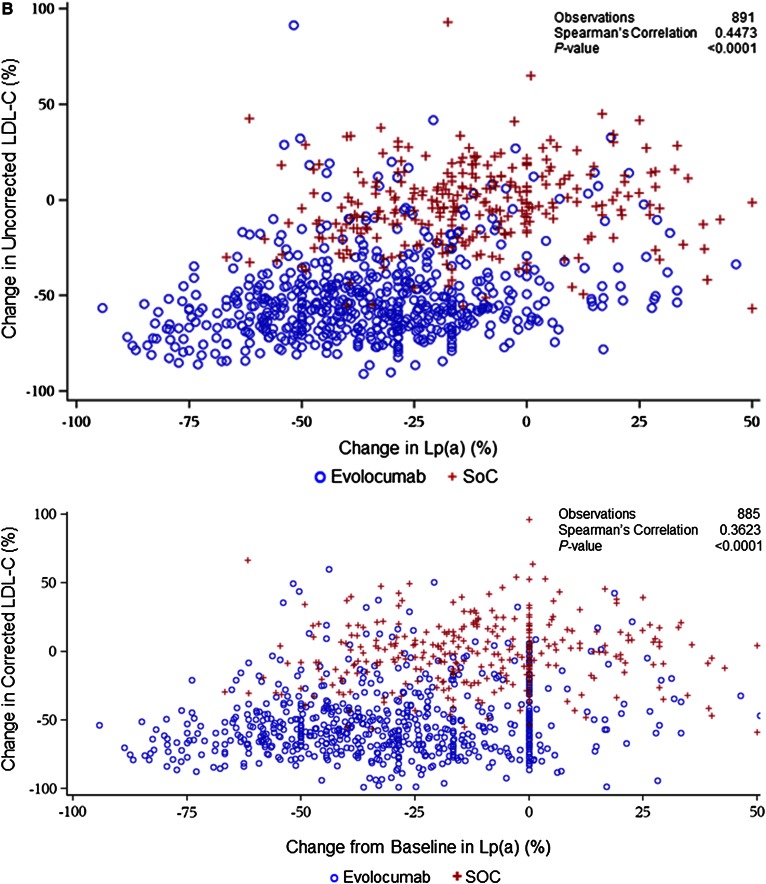
In the pooled dataset, evolocumab (140 mg Q2W and 420 mg QM) resulted in mean (95% confidence interval) percent reductions in uncorrected LDL-C of 60.3% (59.1, 61.5) and 57.4% (56.3, 58.6), respectively. When corrected for Lp(a)-C, reductions in LDL-C were of 64.0% (62.7, 65.3) and 61.0% (59.4, 62.0). Absolute reductions in uncorrected LDL-C were 78.3 mg/dl (75.9, 80.7) and 78.3 mg/dl (75.9, 80.8) at week 12 for the 140 mg Q2W and 420 mg QM doses, respectively, and the Lp(a)-C corrected reductions 77.7 mg/dl (72.8, 75.2) and 77.8 mg/dl (72.9, 75.4) at week 12, respectively. Median (Q1, Q3) percent reductions in Lp(a) for 140 mg Q2W and 420 mg QM dosing were 24.7% (3.6, 40.0) and 21.7% (4.2, 39.9) and absolute reductions were 9.0 nmol/l (1.0, 25.0) and 9.0 nmol/l (1.0, 27.0) at week 12, respectively (Fig. 1). These reductions were maintained for an additional 52 weeks of treatment (supplementary Fig. 2A, B) and were not appreciably affected by background statin use (Table 1). In both the pooled parent and extension studies, reductions in Lp(a) were significantly correlated with reductions in both uncorrected and corrected LDL-C at week 12 (Spearman’s correlation coefficient = 0.5735 and 0.4929, respectively; P < 0.0001 for both; Fig. 2A) and week 52 (Spearman’s correlation coefficient = 0.4473 and 0.3623, respectively, P < 0.0001 for both; Fig. 2B); a similar trend was seen within the evolocumab treatment arms for both the uncorrected and corrected LDL-C at week 12 (P < 0.0001). Analysis of on-treatment LDL-C showed that patients treated with evolocumab with low LDL-C levels (≤40 mg/dl) achieved greater Lp(a) percent reduction compared with patients with LDL-C >70 mg/dl (Table 2). Similar results were seen in patients on statin or no statin background treatment (Table 1).
Fig. 1.
Percent change from baseline in Lp(a) and LDL-C uncorrected and corrected for Lp(a)-C at parent study week 12 (pooled). Data are median (Q1, Q3) values for Lp(a) and mean (95% confidence interval) values for LDL-C (observed data).
TABLE 1.
Percent reductions in Lp(a) at parent study week 12 by baseline Lp(a), statin use, and achieved LDL-C when LDL-C was uncorrected and corrected for Lp(a)-C
| Reduction in Lp(a) at Week 12 (%) | |||
| Statin at Baseline | No Statin at Baseline | ||
| Lp(a) at Baseline | LDL-C ≤40 mg/dl at Week 12 | LDL-C >70 mg/dl at Week 12 | LDL-C >70 mg/dl at Week 12a |
| LDL-C uncorrected for Lp(a)-C | |||
| <75 nmol/l | |||
| N | 393 | 98 | 126 |
| Median (Q1, Q3) | −28.6 (−50.0, 0.0) | −16.7 (−34.6, 0.0) | −22.9 (−38.9, 0.0) |
| 75–125 nmol/l | |||
| N | 27 | 24 | 17 |
| Median (Q1, Q3) | −28.2 (−46.9, −20.9) | −16.6 (−28.4, 0.5) | −26.3 (−33.7, −18.0) |
| >125 nmol/l | |||
| N | 109 | 82 | 47 |
| Median (Q1, Q3) | −25.7 (−34.0, −15.1) | −12.8 (−26.0, −3.3) | −15.5 (−23.7, −3.3) |
| Overall | |||
| N | 1,178 | 432 | |
| Median (Q1, Q3) | −23.3 (−40.0, −5.3) | −21.8 (−39.7, 0.0) | |
| <75 nmol/l | |||
| N | 718 | 309 | |
| Median (Q1, Q3) | −28.1 (−46.7, 0.0) | −23.3 (−44.4, 0.0) | |
| 75–125 nmol/l | |||
| N | 81 | 45 | |
| Median (Q1, Q3) | −26.4 (−40.2, −11.8) | −27.7 (−35.5, −17.5) | |
| >125 nmol/l | |||
| N | 379 | 78 | |
| Median (Q1, Q3) | −18.1 (−29.4, −7.1) | −16.5 (−25.0, −4.6) | |
| LDL-C corrected for Lp(a)-C | |||
| <75 nmol/l | |||
| N | 419 | 91 | 114 |
| Median (Q1, Q3) | −28.6 (−50.0, 0.0) | −16.7 (−36.7, 0.0) | −21.7 (−38.5, 0.0) |
| 75–125 nmol/l | |||
| N | 44 | 19 | 14 |
| Median (Q1, Q3) | −27.1 (−43.6, −12.2) | −12.0 (−20.3, 4.2) | −22.7 (−30.1, −13.0) |
| >125 nmol/l | |||
| N | 274 | 27 | 24 |
| Median (Q1, Q3) | −18.6 (−29.7, −7.0) | −16.8 (−26.4, −7.1) | −17.9 (−26.9, −6.3) |
There were too few patients to analyze in the ≤40 mg/dl group not taking a statin at baseline.
Fig. 2.
Changes in Lp(a) statistically significantly correlated with changes in LDL-C at parent study week 12 (A) and open-label extension study week 52 (B). *Corrected for Lp(a) cholesterol.
TABLE 2.
Percent reductions in Lp(a) at parent study week 12 by baseline Lp(a) and achieved LDL-C when uncorrected and corrected for Lp(a)-C
| Lp(a) at Baseline | Reduction in Lp(a) at Week 12 (%) | |
| LDL-C ≤40 mg/dl at Week 12 | LDL-C >70 mg/dl at Week 12 | |
| LDL-C uncorrected for Lp(a)-C | ||
| <75 nmol/l | ||
| N | 418 | 224 |
| Median (Q1, Q3) | −28.6 (−50.0, 0.0) | −17.8 (−37.5, 0.0) |
| 75–125 nmol/l | ||
| N | 29 | 41 |
| Median (Q1, Q3) | −28.2 (−45.8, −20.9) | −19.4 (−30.9, −12.0) |
| >125 nmol/l | ||
| N | 109 | 129 |
| Median (Q1, Q3) | −25.7 (−34.0, −15.1) | −14.5 (−25.0, −3.3) |
| LDL-C corrected for Lp(a)-C | ||
| <75 nmol/l | ||
| N | 451 | 205 |
| Median (Q1, Q3) | −28.6 (−50.0, 0.0) | −17.2 (−37.5, 0.0) |
| 75–125 nmol/l | ||
| N | 55 | 33 |
| Median (Q1, Q3) | −27.1 (−43.0, −12.7) | −16.9 (−26.0, −3.1) |
| >125 nmol/l | ||
| N | 288 | 51 |
| Median (Q1, Q3) | −18.1 (−29.4, −6.8) | −17.5 (−26.7, −6.5) |
Reductions in Lp(a) and LDL-C with anti-PCSK9 mAbs in vivo
In cynomolgus monkeys, anti-PCSK9 mAb treatment resulted in reductions from baseline in Lp(a), a similar pattern to the changes produced in LDL-C (supplementary Fig. 3A–F). Maximum reduction in Lp(a) was ∼40% from baseline; this was similar across all mAbs. Percent reduction in Lp(a) was approximately one-half of that of LDL-C, although the duration of effect was similar (mAb1 < mAb3 < mAb4 < mAb2). A linear correlation was observed for the percent change in Lp(a) compared with the percent change in LDL-C (Pearson’s correlation coefficient = 0.72; supplementary Fig. 3F).
LDLR competition between LDL and Lp(a) in vitro
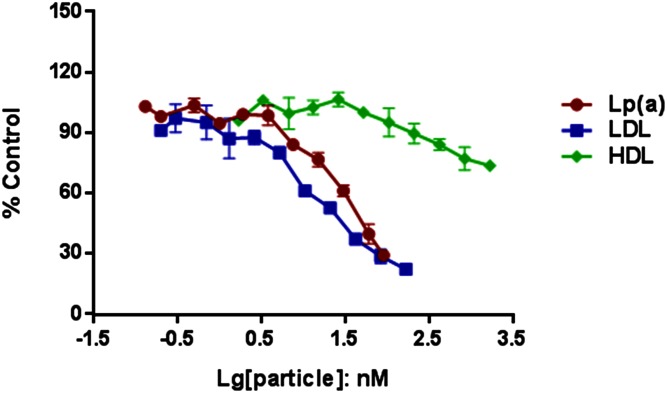
Addition of excess unlabeled LDL resulted in reduction of cell-associated LDL fluorescence to almost background levels, suggesting effective competition for both the LDLR and nonreceptor uptake pathways (Fig. 3A). Increasing concentrations of unlabeled Lp(a) reduced cell-associated LDL fluorescence similar to unlabeled LDL, although effective competition required higher levels of Lp(a) than LDL (Fig. 3B). Unlabeled HDL had a minor effect on reducing fluorescent LDL uptake by HepG2 cells (Fig. 3C).
Fig. 3.
Competition for cell LDL uptake by LDL, Lp(a), and HDL. HepG2 cells were incubated with fluorescently labeled LDL and increasing amounts of unlabeled LDL, Lp(a), and HDL. Data represent values for duplicate wells at each unlabeled lipoprotein concentration as a percentage of control (uptake of fluorescent LDL without unlabeled lipoprotein). Error bars represent SD of the mean. Lg, log.
To better understand the interaction of Lp(a) with HepG2 cells directly, cells were cultured in the presence of purified Lp(a). In the presence of Lp(a), a band at ∼500 kDa could be detected in the HepG2 whole cell lysates consistent with Lp(a) association with the cells; this was effectively eliminated by incubation of the cells with competing and increasing levels of purified LDL (Fig. 4A). This elimination was not seen with HDL (Fig. 4B). LDLR expression was also not altered by the culture conditions.
Fig. 4.
Competition for Lp(a) binding to HepG2 cells incubated with 10 μg/ml Lp(a) (2.5 nM) and increasing levels of LDL (A) or HDL (B). Cell lysates were analyzed by Western blots probed with antibodies to Lp(a), LDLR, and β-actin. Data are representative of several independent experiments.
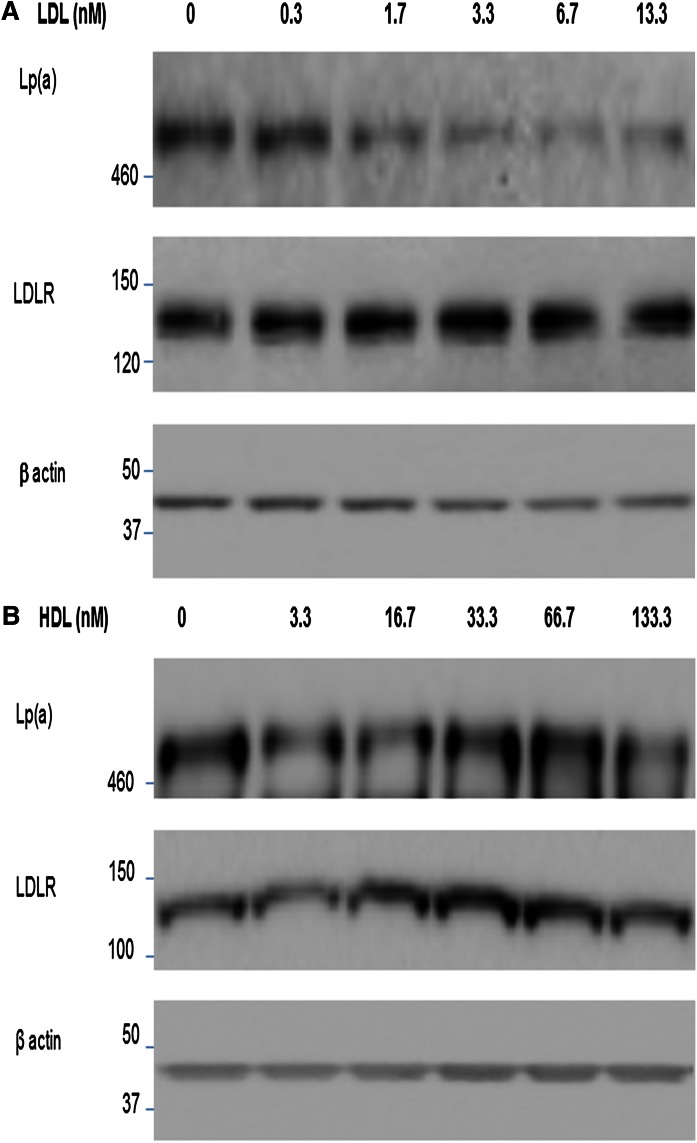
Effects on Lp(a) binding by manipulation of LDLR expression
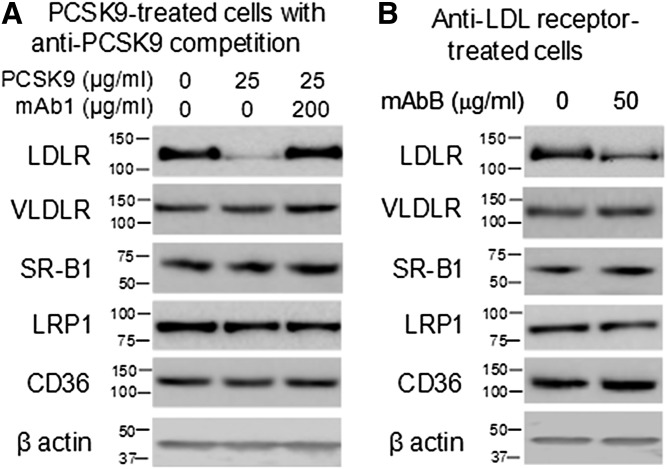
Modulating the level of LDLR activity on HepG2 cells affected the amount of cell-associated Lp(a). Specifically, cell-associated Lp(a) increased when LDLR expression was high (Fig. 5A) and decreased when LDLR expression was low (Fig. 5B, C). The PCSK9-mediated reduction in LDLR expression was abolished by incubating PCSK9 with mAb1, as it effectively blocked the binding of PCSK9 to the LDLR (30). Preventing this PCSK9-mediated reduction in LDLR expression restored the Lp(a) cell association (Fig. 5B). Reduction of LDLR expression by anti-LDLR antibodies also reduced Lp(a) cell association (Fig. 5C). Under the same conditions, neither PCSK9 nor the anti-LDLR antibody, mAbB, affected the expression of the VLDLR, SR-B1, LRP1, or CD36 in HepG2 cells (Fig. 6A, B).
Fig. 5.
Modulation of Lp(a) binding by manipulation of LDLR expression. LDLR expression in HepG2 cells was maintained at normal levels by culturing in 10% FBS, increased by culturing in 10% LPDS (A), or decreased by incubating with PCSK9 (B) or anti-LDLR antibodies (C). Cell lysates were analyzed by Western blots probed with antibodies to Lp(a), LDLR, or β-actin antibody. Data are representative of several independent experiments.
Fig. 6.
Anti-PCSK9 mAb1 and anti-LDLR mAbB do not affect expression of other cell receptors. Protein lysates from HepG2 cells treated with PCSK9 with or without mAb1 (A) or mAbB (B) were separated by SDS-PAGE and analyzed by Western blots probed with antibodies to LDLR, VLDLR, SR-B1, LRP1, CD36, or β-actin.
DISCUSSION
The metabolism of Lp(a) is incompletely understood. Previously, the role for LDLR-mediated clearance of Lp(a) was felt to be minimal or minor (33), a conclusion that was derived from both clinical and biochemical evidence. Statins, a therapy known to upregulate LDLRs, have had little-to-no effect in reducing Lp(a). Further, in vitro studies have shown that the affinity of the remaining LDL component of Lp(a) for LDLRs increased markedly following the cleavage of the disulfide bridge between apo(a) and apoB100 (34).
Our large pooled analyses robustly confirmed the previously reported substantial reductions in Lp(a) and LDL-C in 12 week trials of evolocumab (18, 19, 21, 23–28), again raising the possibility of a role for LDLR clearance of Lp(a). These reductions were maintained up to an additional 52 weeks and significantly correlated with reductions in LDL-C, even when corrected for Lp(a)-C content. However, in the previous analysis, these reductions in LDL-C were not corrected for the cholesterol in Lp(a) (15). In this analysis, we also corrected for Lp(a)-C and found a similar trend of association between reductions in Lp(a) and LDL-C. Yet it should be noted that these corrections are only an approximation. Notably, there was greater Lp(a) percent reduction in patients who achieved lower levels of LDL-C (≤40 mg/dl) during treatment with evolocumab compared with patients with higher levels of LDL-C (>70 mg/dl); this observation is consistent with and supportive of the hypothesis that Lp(a) removal occurs via the LDLR/apoB receptor, particularly when competition for the receptor with LDL-C is reduced. This mechanism of Lp(a) lowering appears to occur during evolocumab treatment regardless of background lipid-lowering therapy. Furthermore, we now provide additional support for the role of the LDLR from in vitro and preclinical studies.
In male cynomolgus monkeys receiving a single subcutaneous injection of anti-PCSK9 mAb (mAb1, mAb2, mAb3, or mAb4), Lp(a) and LDL-C reductions mirrored the changes seen in humans. The varied binding affinity of these mAbs to PCSK9 resulted in differences in the duration of effect of lipid lowering, which was consistent with the characteristics of each mAb. However the pattern of change in LDL-C and Lp(a) suggests that the mechanism(s) involved in LDL-C metabolism affected by PCSK9 inhibition is also relevant to Lp(a) metabolism.
Results from in vitro studies demonstrated that uptake of LDL by HepG2 cells was reduced by both LDL and Lp(a), but much less so by HDL, suggesting that Lp(a) can bind to the LDLR and competes with LDL uptake. Any competition by HDL is somewhat surprising and may relate to the presence of small amounts of other lipoproteins, thus definitive data would require ultrapure lipoprotein preparations. The finding that higher concentrations of Lp(a) were required to compete with LDL confirms that binding of Lp(a) to the LDLR is weaker than that of LDL, and is consistent with enhanced binding and clearance of Lp(a) when competing levels of LDL are reduced, as seen in the clinical trial data (18, 19, 21, 23–28). Western blot analyses also showed that LDL competes with Lp(a) binding to the LDLR and reduces Lp(a) cell association. Competition between LDL and Lp(a) for the LDLR may depend on the apo(a) isoform and its corresponding size and ability to sterically hinder interaction with the ligand binding domains of the receptor. The association of Lp(a) with HepG2 cells was increased when LDLR expression was increased and decreased when LDLR expression was reduced. PCSK9 has been shown to interact with and modulate the expression of other lipoprotein/lipid receptors in addition to LDLR under certain conditions (35–39). Reports have described a role for some of these receptors in cellular uptake of Lp(a), although in our study PCSK9 was not found to modify expression of other lipoprotein receptors, providing further support for this proposed mechanism of Lp(a) clearance being LDLR mediated (40, 41). However, we cannot exclude the possibility that another receptor modulated by PCSK9 that is involved in Lp(a) uptake or cell association might exist.
A possible explanation of previous study findings of little or no change in Lp(a) with statin therapy may be related to the function of PCSK9 itself. Statins upregulate both LDLR expression on hepatocytes and PCSK9 production. Consequently, if PCSK9 binding to the LDLRs specifically reduces the affinity of the large Lp(a) molecule binding to the LDLR or blocks the internalization of the LDLR/Lp(a) complex into the hepatocyte, then statin therapy, despite increasing LDLR, would have less of an effect on Lp(a) than otherwise predicted. Our data support this concept: we found that PCSK9 inhibition resulted in Lp(a) reduction both with or without background statin therapy with percent reductions greater for patients not receiving statin therapy at higher levels of LDL (>70 mg/dl).
Currently, there is limited clinical evidence for the benefit of lowering Lp(a). There is no approved therapy that only lowers Lp(a), although a drug targeted at selective inhibition of apo(a) is in very early development (42); there is epidemiological and genetic evidence suggesting a benefit independent of LDL (2–4). The clinical importance of the effect of anti-PCSK9 therapy on Lp(a) will be better understood following the completion and analysis of ongoing large clinical trials. The 27,500 patient FOURIER trial is studying the effect of PCSK9 inhibition with evolocumab on clinical cardiovascular outcomes (NCT01764633) and is due to report results later in 2016 (43). It will be interesting to see whether the magnitude of the relative risk reduction is proportional to the absolute LDL-C reduction in accordance with the Cholesterol Treatment Trialists’ Collaboration meta-analysis of statins (44). If the observed risk reduction is greater than expected, it may be due to anti-PCSK9’s effect on Lp(a), although it may be difficult to dissect benefits of reductions in Lp(a) from those of reduced LDL-C.
In conclusion, evolocumab treatment reduces both Lp(a) and LDL-C, both uncorrected and corrected for Lp(a)-C, and these reductions are correlated. The greater percent reduction in Lp(a) observed in patients who had lower levels of corrected and uncorrected LDL-C compared with those with higher levels is supportive of the hypothesis that Lp(a) competes with LDL-C for the LDLR/apoB receptor. In vivo and in vitro data in this analysis and others (11) support the hypothesis that the additional upregulation of LDLR activity by PCSK9 mAb also increases the clearance of Lp(a). Thus, LDLR-mediated uptake or cell association of Lp(a), due to a combination of upregulation of LDLR activity along with reduced LDL competition, likely plays a significant role in the reduction of Lp(a) by PCSK9 inhibitors.
Acknowledgments
The authors thank Janice Carlson, PhD (Amgen Inc.) and Julia R. Gage, PhD (on behalf of Amgen Inc.) for assistance with editing the manuscript.
Footnotes
Abbreviations:
- CD36
- cluster of differentiation 36
- LDL-C
- LDL cholesterol
- LDLR
- LDL receptor
- Lp(a)
- lipoprotein (a)
- Lp(a)-C
- lipoprotein (a)-cholesterol
- LPDS
- lipoprotein-deficient serum
- LRP1
- LDL receptor-related protein 1
- mAb
- monoclonal antibody
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- Q1
- quartile 1
- Q2W
- every 2 weeks
- Q3
- quartile 3
- QM
- monthly
- SOC
- standard of care
- SR-B1
- scavenger receptor class B member 1
- VLDLR
- VLDL receptor
This study was funded by Amgen. The authors report the following potential conflicts of interest: F.J.R.: Grants/research support: Amgen, Sanofi; Speakers Bureau: AstraZeneca, Pfizer, Merck, Amgen; Honoraria: AstraZeneca, Pfizer, Merck, Amgen, Sanofi; Consultant/Advisory Board: AstraZeneca, Pfizer, Merck; N.G.S.: Grants: Leducq Foundation grant, Canadian Research Chair. R.P.G.: Grants/research Support: Amgen, Daiichi-Sankyo, Merck; Honoraria: Daiichi-Sankyo, Merck; Consultant/Advisory Board: Amgen, Daiichi-Sankyo, Janssen, Lexicon, Merck, Sanofi, Beckman-Coulter. M.S.S.: Grant/research support: Amgen, AstraZeneca, AstraZeneca/Bristol-Myers Squibb Alliance, Bristol-Myers Squibb/Sanofi-Aventis Joint Venture, Daiichi-Sankyo, Eisai, Genzyme, GlaxoSmithKline, Merck, Sanofi-Aventis, Takeda, Abbott Laboratories, Accumetrics, Critical Diagnostics, Nonsphere, Roche Diagnostics; Consultant/Advisory Board: Aegerion, Amgen, Diasorin, GlaxoSmithKline, Merck, Pfizer, Sanofi-Aventis, AstraZeneca, Vertex. M.J.K.: Grants/research support: Amgen, Regeneron, Sanofi, Roche, Pfizer. D.B.: Honoraria: Amgen, Sanofi-Aventis, MSD, PharmaDynamics, AstraZeneca, Aegerion; Consultant/Advisory Board: Amgen, MSD, Sanofi-Aventis, Aegerion, Gemphire. A.L., N.H., A.X., P.C., S.J., M.D., M.P., R. So., S.M.W., and R. Sc.: Employees of Amgen Inc. E.A.S.: Consultant/Advisory Board: Amgen, Adnexus Therapeutics, Genentech, Regeneron, Sanofi, ISIS, Genzyme.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Utermann G. 1989. The mysteries of lipoprotein(a). Science. 246: 904–910. [DOI] [PubMed] [Google Scholar]
- 2.Erqou S., Kaptoge S., Perry P. L., Di Angelantonio E., Thompson A., White I. R., Marcovina S. M., Collins R., Thompson S. G., and Danesh J.; Emerging Risk Factors Collaboration. 2009. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 302: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordestgaard B. G., Chapman M. J., Ray K., Borén J., Andreotti F., Watts G. F., Ginsberg H., Amarenco P., Catapano A., Descamps O. S., et al. ; European Atherosclerosis Society Consensus Panel. 2010. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur. Heart J. 31: 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke R., Peden J. F., Hopewell J. C., Kyriakou T., Goel A., Heath S. C., Parish S., Barlera S., Franzosi M. G., Rust S., et al. ; PROCARDIS Consortium. 2009. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 5.Hernández C., Francisco G., Ciudin A., Chacón P., Montoro B., Llaverias G., Blanco-Vaca F., and Simó R.. 2011. Effect of atorvastatin on lipoprotein (a) and interleukin-10: a randomized placebo-controlled trial. Diabetes Metab. 37: 124–130. [DOI] [PubMed] [Google Scholar]
- 6.Gonbert S., Malinsky S., Sposito A. C., Laouenan H., Doucet C., Chapman M. J., and Thillet J.. 2002. Atorvastatin lowers lipoprotein(a) but not apolipoprotein(a) fragment levels in hypercholesterolemic subjects at high cardiovascular risk. Atherosclerosis. 164: 305–311. [DOI] [PubMed] [Google Scholar]
- 7.Crouse J. R. 3rd, Frohlich J., Ose L., Mercuri M., and Tobert J. A.. 1999. Effects of high doses of simvastatin and atorvastatin on high-density lipoprotein cholesterol and apolipoprotein A-I. Am. J. Cardiol. 83: 1476–1477, A7. [DOI] [PubMed] [Google Scholar]
- 8.Hunninghake D. B., Stein E. A., and Mellies M. J.. 1993. Effects of one year of treatment with pravastatin, an HMG-CoA reductase inhibitor, on lipoprotein a. J. Clin. Pharmacol. 33: 574–580. [DOI] [PubMed] [Google Scholar]
- 9.Fieseler H. G., Armstrong V. W., Wieland E., Thiery J., Schütz E., Walli A. K., and Seidel D.. 1991. Serum Lp(a) concentrations are unaffected by treatment with the HMG-CoA reductase inhibitor Pravastatin: results of a 2-year investigation. Clin. Chim. Acta. 204: 291–300. [DOI] [PubMed] [Google Scholar]
- 10.Irudayam J. B., Sivaraj R., and Nirmala P.. 2014. Effect of statins on lipoprotein (a) in dyslipidemic patients. Int. J. Basic Clin. Pharmacol. 3: 1024–1029. [Google Scholar]
- 11.Romagnuolo R., Scipione C. A., Boffa M. B., Marcovina S. M., Seidah N. G., and Koschinsky M. L.. 2015. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J. Biol. Chem. 290: 11649–11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostner G. M., Gavish D., Leopold B., Bolzano K., Weintraub M. S., and Breslow J. L.. 1989. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation. 80: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 13.Yeang C., Hung M-Y., Byun Y-S., Clopton P., Yang X., Witztum J. L., and Tsimikas S.. 2016. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J. Clin. Lipidol. In press. [DOI] [PubMed] [Google Scholar]
- 14.Berg K., Dahlén G., Christophersen B., Cook T., Kjekshus J., and Pedersen T.. 1997. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin. Genet. 52: 254–261. [DOI] [PubMed] [Google Scholar]
- 15.Raal F. J., Giugliano R. P., Sabatine M. S., Koren M. J., Langslet G., Bays H., Blom D., Eriksson M., Dent R., Wasserman S. M., et al. 2014. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 63: 1278–1288. [DOI] [PubMed] [Google Scholar]
- 16.Gaudet D., Kereiakes D. J., McKenney J. M., Roth E. M., Hanotin C., Gipe D., Du Y., Ferrand A. C., Ginsberg H. N., and Stein E. A.. 2014. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am. J. Cardiol. 114: 711–715. [DOI] [PubMed] [Google Scholar]
- 17.Dias C. S., Shaywitz A. J., Wasserman S. M., Smith B. P., Gao B., Stolman D. S., Crispino C. P., Smirnakis K. V., Emery M. G., Colbert A., et al. 2012. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J. Am. Coll. Cardiol. 60: 1888–1898. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan D., Olsson A. G., Scott R., Kim J. B., Xue A., Gebski V., Wasserman S. M., and Stein E. A.. 2012. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 308: 2497–2506. [DOI] [PubMed] [Google Scholar]
- 19.Koren M. J., Scott R., Kim J. B., Knusel B., Liu T., Lei L., Bolognese M., and Wasserman S. M.. 2012. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 380: 1995–2006. [DOI] [PubMed] [Google Scholar]
- 20.Stein E. A., Mellis S., Yancopoulos G. D., Stahl N., Logan D., Smith W. B., Lisbon E., Gutierrez M., Webb C., Wu R., et al. 2012. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 366: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano R. P., Desai N. R., Kohli P., Rogers W. J., Somaratne R., Huang F., Liu T., Mohanavelu S., Hoffman E. B., McDonald S. T., et al. ; LAPLACE-TIMI 57 Investigators. 2012. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 380: 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H., Samarghandi A., Zhang N., Yao Z., Xiong M., and Teng B. B.. 2012. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler. Thromb. Vasc. Biol. 32: 1585–1595. [DOI] [PubMed] [Google Scholar]
- 23.Koren M. J., Lundqvist P., Bolognese M., Neutel J. M., Monsalvo M. L., Yang J., Kim J. B., Scott R., Wasserman S. M., and Bays H.; MENDEL-2 Investigators. 2014. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J. Am. Coll. Cardiol. 63: 2531–2540. [DOI] [PubMed] [Google Scholar]
- 24.Robinson J. G., Nedergaard B. S., Rogers W. J., Fialkow J., Neutel J. M., Ramstad D., Somaratne R., Legg J. C., Nelson P., Scott R., et al. ; LAPLACE-2 Investigators. 2014. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 311: 1870–1882. [DOI] [PubMed] [Google Scholar]
- 25.Raal F., Scott R., Somaratne R., Bridges I., Li G., Wasserman S. M., and Stein E. A.. 2012. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 126: 2408–2417. [DOI] [PubMed] [Google Scholar]
- 26.Raal F. J., Stein E. A., Dufour R., Turner T., Civeira F., Burgess L., Langslet G., Scott R., Olsson A. G., Sullivan D., et al. ; RUTHERFORD-2 Investigators. 2015. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 385: 331–340. [DOI] [PubMed] [Google Scholar]
- 27.Stroes E., Colquhoun D., Sullivan D., Civeira F., Rosenson R. S., Watts G. F., Bruckert E., Cho L., Dent R., Knusel B., et al. ; GAUSS-2 Investigators. 2014. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 63: 2541–2548. [DOI] [PubMed] [Google Scholar]
- 28.Koren M. J., Giugliano R. P., Raal F. J., Sullivan D., Bolognese M., Langslet G., Civeira F., Somaratne R., Nelson P., Liu T., et al. ; OSLER Investigators. 2014. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 129: 234–243. [DOI] [PubMed] [Google Scholar]
- 29.Seman L. J., and Breckenridge W. C.. 1986. Isolation and partial characterization of apolipoprotein (a) from human lipoprotein (a). Biochem. Cell Biol. 64: 999–1009. [DOI] [PubMed] [Google Scholar]
- 30.Chan J. C., Piper D. E., Cao Q., Liu D., King C., Wang W., Tang J., Liu Q., Higbee J., Xia Z., et al. 2009. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA. 106: 9820–9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henne K. R., Ason B., Howard M., Wang W., Sun J., Higbee J., Tang J., Matsuda K. C., Xu R., Zhou L., et al. 2015. Anti-PCSK9 antibody pharmacokinetics and low-density lipoprotein-cholesterol pharmacodynamics in nonhuman primates are antigen affinity-dependent and exhibit limited sensitivity to neonatal Fc receptor-binding enhancement. J. Pharmacol. Exp. Ther. 353: 119–131. [DOI] [PubMed] [Google Scholar]
- 32.Dietschy J. M., and Turley S. D.. 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12: 105–112. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong V. W., Harrach B., Robenek H., Helmhold M., Walli A. K., and Seidel D.. 1990. Heterogeneity of human lipoprotein Lp[a]: cytochemical and biochemical studies on the interaction of two Lp[a] species with the LDL receptor. J. Lipid Res. 31: 429–441. [PubMed] [Google Scholar]
- 34.Armstrong V. W., Walli A. K., and Seidel D.. 1985. Isolation, characterization, and uptake in human fibroblasts of an apo(a)-free lipoprotein obtained on reduction of lipoprotein(a). J. Lipid Res. 26: 1314–1323. [PubMed] [Google Scholar]
- 35.Canuel M., Sun X., Asselin M. C., Paramithiotis E., Prat A., and Seidah N. G.. 2013. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS One. 8: e64145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafsen C., Kjolby M., Nyegaard M., Mattheisen M., Lundhede J., Buttenschøn H., Mors O., Bentzon J. F., Madsen P., Nykjaer A., et al. 2014. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 19: 310–318. [DOI] [PubMed] [Google Scholar]
- 37.Liu M., Wu G., Baysarowich J., Kavana M., Addona G. H., Bierilo K. K., Mudgett J. S., Pavlovic G., Sitlani A., Renger J. J., et al. 2010. PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain. J. Lipid Res. 51: 2611–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirier S., Mayer G., Benjannet S., Bergeron E., Marcinkiewicz J., Nassoury N., Mayer H., Nimpf J., Prat A., and Seidah N. G.. 2008. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 283: 2363–2372. [DOI] [PubMed] [Google Scholar]
- 39.Shan L., Pang L., Zhang R., Murgolo N. J., Lan H., and Hedrick J. A.. 2008. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem. Biophys. Res. Commun. 375: 69–73. [DOI] [PubMed] [Google Scholar]
- 40.Argraves K. M., Kozarsky K. F., Fallon J. T., Harpel P. C., and Strickland D. K.. 1997. The atherogenic lipoprotein Lp(a) is internalized and degraded in a process mediated by the VLDL receptor. J. Clin. Invest. 100: 2170–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X. P., Amar M. J., Vaisman B., Bocharov A. V., Vishnyakova T. G., Freeman L. A., Kurlander R. J., Patterson A. P., Becker L. C., and Remaley A. T.. 2013. Scavenger receptor-BI is a receptor for lipoprotein(a). J. Lipid Res. 54: 2450–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsimikas S., Viney N. J., Hughes S. G., Singleton W., Graham M. J., Baker B. F., Burkey J. L., Yang Q., Marcovina S. M., Geary R. S., et al. 2015. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 386: 1472–1483. [DOI] [PubMed] [Google Scholar]
- 43.Sabatine M. S., Giugliano R. P., Keech A., Honarpour N., Wang H., Liu T., Wasserman S. M., Scott R., Sever P. S., and Pedersen T. R.. 2016. Rationale and design of the further cardiovascular outcomes research with PCSK9 Inhibition in subjects with elevated risk trial. Am. Heart J. 173: 94–101. [DOI] [PubMed] [Google Scholar]
- 44.Baigent C., Blackwell L., Emberson J., Holland L. E., Reith C., Bhala N., Peto R., Barnes E. H., Keech A., Simes J., et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration. 2010. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]