Abstract
Calcific aortic stenosis (AS) is the most common form of valve disease in the Western world and affects over 2.5 million individuals in North America. Despite the large burden of disease, there are no medical treatments to slow the development of AS, due at least in part to our incomplete understanding of its causes. The Cohorts for Heart and Aging Research in Genetic Epidemiology extra-coronary calcium consortium reported a genome-wide association study demonstrating that genetic variants in LPA are strongly associated with aortic valve (AV) calcium and clinical AS. Using a Mendelian randomization study design, it was demonstrated that the effect of this genetic variant is mediated by plasma lipoprotein (a) [Lp(a)], directly implicating elevations in Lp(a) as a cause of AV calcium and progression to AS. This discovery has sparked intense interest in Lp(a) as a modifiable cause for AV disease. Herein, we will review the mounting epidemiological and genetic findings in support of Lp(a)-mediated valve disease, discuss potential mechanisms underlying this observation, and outline the steps to translate this discovery to a much needed novel preventive and/or therapeutic strategy for AV disease.
Keywords: calcium, drug therapy/hypolipidemic drugs, lipoproteins, oxidized lipids, mendelian randomization
CALCIFIC AORTIC VALVE DISEASE: LARGE BURDEN OF DISEASE WITHOUT KNOWN MEDICAL TREATMENT
Calcific aortic valve disease (CAVD) is currently the third most common cardiovascular disease after ischemic heart disease and hypertension. CAVD encompasses all forms of CAVD, including subclinical forms, such as the presence of aortic valve calcium (AVC), identified by computed tomography (CT) imaging, and aortic sclerosis (i.e., thickening and calcification), identified by echocardiography, as well as the clinical disease of aortic stenosis (AS). In North America, CAVD afflicts over 5 million individuals (1) and is a common cause of death, disability, and health care costs with over 15,000 deaths annually in the US alone (2). AS, which is defined as a progressive narrowing of the aortic valve (AV) (i.e., the valve through which blood is ejected from the left ventricle into the systemic circulation), affects over 2.5 million people (3) and leads to severe impairment in cardiac function consisting of heart failure, angina, and syncope when stenosis becomes severe (see Table 1). Age is the most important risk factor, with 2% of the population over 65 having AS, a figure that rises to 12% [95% confidence interval (CI) 7–18%] after 75 years of age (4). Current treatment consists solely of valve replacement, with nearly 100,000 such procedures performed yearly in the US (5), frequently late in the disease process when patients are generally old and frail, with the attendant high costs and complications associated with intervention at this late stage. Despite the huge burden of disease, there are currently no medical treatments to prevent or retard the progression of this disease and reduce the need for valve replacement.
TABLE 1.
AV disease severity
| Mean Transvalvular Gradient | Peak Aortic Velocity | AV Area | Features | |
| Aortic sclerosis | <20 mm Hg | <2.5 m/s | >2.0 m2 | Echogenic foci of calcification; leaflet thickening |
| AS | ||||
| Mild | <25 mm Hg | 2.6–2.9 m/s | 1.5–2.0 cm2 | Increasing obstruction to flow with progressive calcification and fibrosis |
| Moderate | 25–40 mm Hg | 3.0–4.0 m/s | 1.0–1.5 cm2 | |
| Severe | >40 mm Hg | >4.0 m/s | <1.0 cm2 |
The natural history of CAVD consists of a long clinically silent phase of valve calcification and hardening (“sclerosis”) which lasts generally at least a decade and heralds the clinical disease. AV sclerosis is exceedingly common with a prevalence of 26% after 65 years of age, 40% after 75 years of age, and 75% after 85 years of age (6). AV sclerosis, which was long deemed a benign consequence of ageing, is also now known to confer a 40% increase in the risk of death and a 66% increase in the risk of cardiovascular (CV) death, independent of age and CV risk factors (7). This lesion, which appears to track with vascular disease risk factors, has led to a misconception that aortic sclerosis and vascular atherosclerosis represent the same disease. Although there is significant overlap in the early initiating lesion and certain shared risk factors (8–11), the available evidence indicates major differences in the underlying pathophysiology of these two diseases. First, histopathological evidence demonstrates a more prominent early mineralization phase and a paucity of smooth muscle cells in aortic sclerosis, as compared with vascular atherosclerotic lesions (9). Second, calcification pathways appear to predominate early in valve disease and appear to be independent of the atherosclerotic process, as opposed to vascular disease where calcification develops much later and in parallel with atherosclerosis (12). Third, among individuals undergoing AV replacement for (non-congenital) AS, only 40% have significant coronary artery disease requiring bypass (13), suggesting unique pathological processes. Fourth, lipid lowering agents, which have been remarkably effective for preventing atherosclerosis have not demonstrated any benefit in randomized trials for advanced AV disease in older patient populations, highlighting the major differences in pathophysiology and treatment of these diseases (14–16).
LIPOPROTEIN (a) IS ASSOCIATED WITH CAVD
A major limitation in developing treatments for CAVD stems in large part from our poor understanding of the mediators and causes of this disease. Seminal early pathologic studies performed by Otto et al. (9) clearly demonstrated the importance of lipids in the early initiating lesion of CAVD. They demonstrated that the early sclerotic lesions of AVs were characterized by lipid pools that were absent in adjacent normal valve tissue, implicating lipids in the pathological process (9). In addition, these lipids were likely derived from plasma, as plasma carrier molecules for cholesterol [apoB and apo(a)] were also present in these pools (8). These cholesterol particles colocalized with early foci of calcification in valve tissue providing the first evidence that blood-borne cholesterol, from LDL and/or lipoprotein (a) [Lp(a)], were intimately involved in the early calcification of the AV.
Subsequent epidemiologic studies (summarized in the Table 2) provided further evidence in support of the lipid hypothesis for CAVD. Stewart et al. (10), using cross-sectional baseline data from the 5,201 participants who underwent echocardiography as part of the Cardiovascular Health Study, demonstrated that LDL cholesterol and Lp(a) were associated with a 12% (95% CI 3–23%) and 23% increase (95% CI 14–32%), respectively, in the presence of AS. Gotoh et al. (18) also found that Lp(a) was associated with CAVD in a small cross-sectional case-control study. Among individuals with Lp(a) >30 mg/dl, 36% had aortic sclerosis, as compared with only 13% with Lp(a) <30 mg/dl (P < 0.001). More recently, using a case-control design, Bozbas et al. (19) demonstrated a slightly higher Lp(a) level (27.4 mg/dl) in 112 cases with aortic sclerosis, as compared with 19.9 mg/dl among controls. Finally, Glader et al. (20) also found significantly higher Lp(a) among AS cases than controls.
TABLE 2.
Epidemiologic and genetic associations implicating Lp(a) and LPA variants with CAVD
| Author | Year | Study Design | N | Results |
| Epidemiologic associations | ||||
| Gotoh | 1995 | Cross-sectional | 784 (n = 160 with aortic sclerosis) | 36.1% aortic sclerosis in Lp(a) ≥30 mg/dl versus 12.7% in Lp(a) <30 mg/dl |
| Stewart | 1997 | Cross-sectional | 5,201 (n = 1,405 with sclerosis/stenosis) | OR 1.23 (95% CI 1.14–1.32) for top Lp(a) quartile versus lowest |
| Glader | 2003 | Case-control | 202 (n = 101 with AS) | OR 1.7 (95% CI 0.8–2.9) for Lp(a) >30 mg/dl and 3.4 (95% CI 1.1–11.2) for Lp(a) >48 mg/dl |
| Bozbas | 2007 | Case-control | 285 (n = 112 with AVC) | Lp(a) 27.4 mg/dl in cases versus 19.9 mg/dl in controls |
| Capoulade | 2015 | Cohort | 220 (with mild to moderate AS) followed for 3.5 ± 1.2 years | Lp(a) >58.5 mg/dl was associated with 2.6-fold (95% CI 1.4–5.0; P = 0.003) increase in odds of rapid AS progression |
| Genetic associations | ||||
| Thanassoulis | 2013 | GWAS (AVC) and prospective cohort (AS) | CHARGE: 6,942 (n = 2,245 with AVC) | For AVC, OR per G allele = 2.05 (95% CI 1.66–2.53) for rs10455872 in LPA gene |
| MDCS: 28,193 (n = 308 with AS) | For AS, HR per allele in MDCS, 1.68 (95% CI 1.32–2.15) and HR per allele 1.54 (95% CI 1.05-2.27) in CCHS | |||
| CCHS: 10,400 (n = 192 with AS) | ||||
| Kamstrup | 2014 | Prospective cohort (AS) | 77,680 (n = 454 with AS) combined CCHS and CGHS | HR 1.6 (95% CI 1.2–2.1) for a 10-fold genetic Lp(a) increase |
| Arsenault | 2014 | Prospective cohort and case-control replication (AS) | 17,553 (n = 118 with AS) in EPIC-Norfolk | In incident analysis, HR = 1.78 [1.11–2.87] and HR = 4.83 [1.77–13.20], respectively, for one or two copies of the rs10455872 G allele; in case-control, OR 1.57 (95% CI 1.10–2.26) |
GWAS, genome-wide association study.
Although the above studies support an association between plasma Lp(a) and CAVD, the observational nature of these studies could not establish whether Lp(a) was, in fact, causal for CAVD or simply a bystander in the calcifying process, a critical consideration in determining whether Lp(a) represents a therapeutic target. However, recent genetic studies have provided important insights into the causal nature of these associations.
GENETIC ASSOCIATIONS BETWEEN LPA VARIANTS, Lp(a), AND CAVD
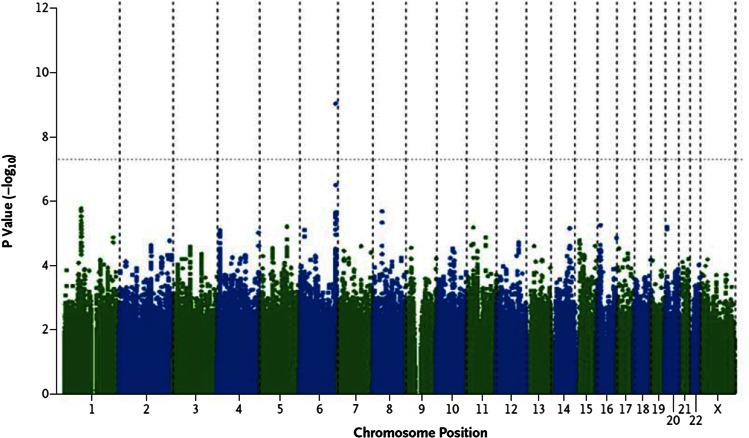
The Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) extra-coronary calcium working group recently reported the first genome-wide association study of valve calcium as detected by CT (21) (Fig. 1). Valve calcium represents a “deep phenotype” that is more proximal to the biological process (i.e., calcification) and reduces phenotypic heterogeneity, as compared with a clinical phenotype such as AS, allowing for improved resolution of genetic signals (22). In the discovery stage, 6,942 participants were included from the Framingham Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Age Gene-Environment Susceptibility-Reykjavik Study, with data on the presence or absence of AVC. A SNP (rs10455872) was identified in the LPA gene that conferred a 2-fold increase in the odds of AV calcification [odds ratio (OR), 2.05; P = 9.0 × 10−10]. Subsequent independent replication in the Heinz-Nixdorf Study confirmed this 2-fold increase in the odds of valve calcium (OR, 2.04; P = 0.018). In a joint meta-analysis combining the discovery and replication cohorts, the rs10455872 SNP in LPA was associated with a doubling in the odds of AVC (P = 2.8 × 10−11), strongly supporting an important association with AVC. Other LPA SNPs, including rs3798220, were also associated with AVC, but did not achieve genome-wide significance due to lower minor allele frequency or smaller effect sizes on Lp(a) levels. To demonstrate relevance with clinical AS, validation in the 28,193 participants from the Malmo Diet Cancer Study (MDCS) was performed. In the MDCS, the rs10455872 was clearly associated with future incident AS [hazard ratio (HR) 1.68, 95% CI 1.32–2.15] and AV replacement for AS (HR 1.54, 95% CI 1.05–2.27). These results were also independently confirmed in the Copenhagen City Heart Study in which the rs10455872 was associated with both incident AS and AV replacement (21). In both cohorts, the association persisted after adjustment for several risk factors and after exclusion of myocardial infarction. To further support the specificity of this association with valve disease rather than vascular atherosclerosis, additional analyses were performed in CHARGE participants discordant for AVC and coronary artery calcium. The rs10455872 tracked consistently with AVC, rather than coronary calcium, demonstrating that the association was not an epiphenomenon of the known Lp(a) association with coronary artery disease.
Fig. 1.
Genome-wide associations with AVC. Reproduced from (21).
The rs10455872 has previously been strongly associated with Lp(a) levels and with myocardial infarction in several studies, which provided the impetus to evaluate the potential role of circulating Lp(a) in valve calcification and CAVD. Given that this SNP correlates with fewer kringle type-IV repeats, which are believed to regulate Lp(a) production and increase Lp(a) levels (23), this SNP provides an excellent opportunity to evaluate the causal nature of life-long genetic elevations in Lp(a) using Mendelian randomization. Mendelian randomization uses the randomization of genetic material at conception to provide largely unconfounded and unbiased inferences for the role of a circulating biomarker on disease outcomes (24, 25). Because genetic associations cannot be confounded by other factors (i.e., lifestyle, diet, etc.) and necessarily precede the development of the outcome, Mendelian randomization provides a more robust measure of the causal association between a genetically elevated biomarker, in this case Lp(a), and the disease of interest, CAVD, and can provide strong supportive evidence that a biomarker may be relevant as a therapeutic target. Based on the known associations between rs10455872 and Lp(a), this SNP was used as a proxy to estimate the association with AVC based on the observed difference in Lp(a) levels among rs10455972 carriers, as compared with noncarriers, using an instrumental variable analysis. Based on this analysis, it was found that for each unit increment in log Lp(a), there was an ∼60% increase in AV calcium, strongly supporting a causal role for Lp(a) and suggesting that lowering of Lp(a) levels may represent a strategy to prevent valve calcification (21).
Following the publication of these findings, several additional Mendelian randomization studies have been reported that are largely in agreement with the findings of the CHARGE consortium and have extended these analyses to clinical AS. In combined analyses of the Copenhagen City Heart Study and the Copenhagen General Population studies, comprising 77,680 individuals with 454 occurrences of AS, Kamstrup, Tybjaerg-Hansen, and Nordestgaard (26) demonstrated a strong graded relationship between Lp(a) levels and AS corresponding to a 60% increase in the risk of AS among individuals with Lp(a) between 20 and 64 mg/dl, a 100% increase when Lp(a) was 65–90 mg/dl, and a 200% increase when Lp(a) was over 90 mg/dl. Using Mendelian randomization by comparing carriers of any genotype that raises Lp(a) levels (including rs10455872, rs3798220, and low number of kringle-4 type 2 repeats) to those without these genotypes, they estimated a 60% increase for each 10-fold increment in Lp(a). Similarly, Arsenault et al. (27) provided further independent replication in the 17,553 participants of the prospective EPIC-Norfolk study. Compared with individuals with no copies of the G risk allele [i.e., Lp(a) increasing], individuals heterozygous and homozygous for the G allele had a HR of 1.78 (95% CI 1.11–2.87) and 4.83 (95% CI 1.77–13.20), respectively, for incident AS. They also provided replication in a case-control study demonstrating that carriers of the G allele had a 57% increase in the odds of AS, as compared with those without the G allele.
Recently, Capoulade et al. (28) have shown that in 220 participants of the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) trial that participants in the top Lp(a) tertile [Lp(a) >58.5 mg/dl] had a faster rate of progression than individuals in the lower tertiles. Interestingly, this effect was more pronounced in younger (≤57 years of age) than in older participants, indicating an important age × Lp(a) interaction (P = 0.04). In multivariable models, Lp(a) >58.5 mg/dl was associated with a 2.6-fold increase in the odds of being a rapid progressor, as defined by an annualized change in maximum aortic velocity >0.2/ms/year. Importantly, in those ≤57 years, the OR for rapid progression was 4.9 (95% CI 1.8–3.7). These results demonstrate robust evidence in support of elevated Lp(a) as a marker of rapid progression of AS, especially in younger individuals, and provide strong evidence to target Lp(a) lowering to this group of patients.
POTENTIAL MECHANISMS OF Lp(a)-MEDIATED CAVD
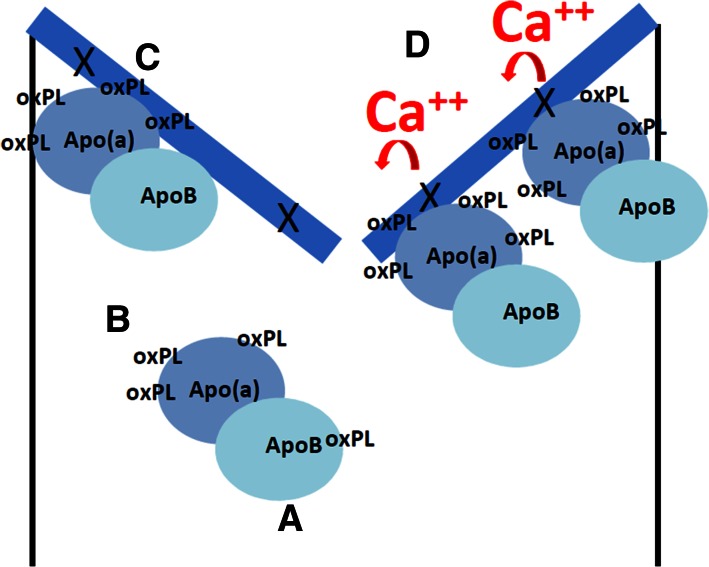
Due to the recent discoveries linking LPA variants with AV disease, there are currently limited molecular and animal studies exploring the mechanisms underlying this association, which represents an important knowledge gap that will need to be addressed in the near future. Nonetheless, Lp(a) has several important properties that could promote AV disease (Fig. 2).
Fig. 2.
Proposed model of Lp(a)-mediated CAVD. A: Lp(a) is a cholesterol-rich lipoprotein in which apoB is covalently linked to apo(a). B: Lp(a) carries OxPLs in plasma (35). C: Lp(a) binds avidly to areas of endothelial injury (29). The AVs (in blue) are particularly susceptible to denudation (denoted by X in the figure) due to hemodynamic stress. D: Deposition of Lp(a) leads to delivery of oxPLs, a known promoter of osteoblastic differentiation (and calcification) (48).
First, Lp(a) is involved in tissue repair. Lp(a), via its apo(a) protein moiety, is comprised of lysine binding sites that can bind to areas of damaged and denuded endothelium (29). Although this may have an important role for maintenance of vascular integrity and possibly for delivery of cholesterol to sites of injury, the AV leaflets may be particularly prone to cellular damage due to the repetitive mechanical stress imposed during the cardiac cycle, in concert with high pressures and shear stress, leading to excessive Lp(a) binding, especially under conditions of Lp(a) excess.
Second, Lp(a) particles can promote foam cell formation and inflammation in early valve lesions. Although pathophysiologically distinct in their mature forms, AV sclerosis and vascular atherosclerosis share a similar initiating phase consisting of subintimal deposition of cholesterol-laden lipoproteins. Native unmodified lipoproteins are poorly internalized by tissue macrophages. However, their oxidation results in unregulated lipoprotein uptake into macrophages, via scavenger receptors (i.e., CD36 and SR-A) (30), leading to foam cell formation, fatty streaks, and atheroma. In appropriate settings, Lp(a), and its associated phosphocholine (PC)-containing oxidized phospholipids (OxPLs), also triggers apoptosis in macrophages, a key process that likely contributes to early valvular lesion progression (31). Furthermore, it was recently shown that Lp(a) binds monocyte chemoattractant protein-1 (MCP-1) in vitro and in vivo (32). The association of MCP-1 may play a role in modulating monocyte trafficking during early valve sclerosis. Lp(a) may also promote inflammation via its apoB component. Recent work has demonstrated that apoB-derived peptides (i.e., apoBDS-1) are danger-associated peptides that are potent promoters of pro-inflammatory cytokines (such as IL-8, IL-6, and CCL2) and other pro-inflammatory mediators (e.g., PGE2) (33). Whether such properties contribute to Lp(a)-mediated valve disease, via the apoB component of Lp(a), remains to be seen.
Third, Lp(a) is the primary carrier of OxPLs in plasma. This property has emerged as a key mediator of Lp(a) pathogenicity and likely plays a major role in CAVD. It has been hypothesized that a physiologic role for Lp(a) may be to bind and transport pro-atherogenic OxPLs in plasma, targeting them for removal from the circulation (34). Although OxPLs have been found on several plasma proteins, including LDL and plasminogen, Lp(a) carries the largest proportion (∼85%) of circulating OxPLs on plasma lipoproteins (35). Lp(a) levels are highly correlated with OxPL/apoB levels (r = 0.85), but this correlation is not consistent for all Lp(a) particles, with the highest correlations observed among the smallest, most atherogenic Lp(a) particles (36, 37). OxPLs appear to be “danger-associated molecular patterns,” which are potent stimulators of innate immunity by interacting with various pattern recognition receptors (12, 30, 39). In addition, bacterial PC (not as a phospholipid) has molecular identity with PC-containing OxPLs (30). OxPL-stimulated endothelial cells have been shown to: 1) upregulate expression of chemoattractants and cell adhesion molecules for inflammatory cell recruitment (41, 42); 2) increase production of reactive oxygen species (43); and 3) increase pro-inflammatory gene expression (IL-1, IL-6, IL-8) (40). This pro-inflammatory phenotype is likely important in early phases of valve sclerosis and likely contributes to the stiffening of the leaflets via recruitment of immune cells and deposition of extracellular matrix proteins.
Finally, Lp(a) is likely capable of inducing valvular calcification, which is the predominant biological feature of AV disease and is also likely mediated, in large part, by OxPLs, but also by native cholesterol. Increased cholesterol delivery, via Lp(a), to the valve leaflets can lead to cholesterol microcrystals which act as nidi for calcification (44). Lp(a), via OxPLs, is also a source of oxidative stress, which is a known promoter of CV calcification (45). Oxidant levels have been found to be elevated around calcified deposits in human valves and also to colocalize with signals of active calcium remodeling (46). Furthermore, oxidative stress induces osteogenic differentiation of endothelial cells by upregulation of runt-related transcription factor-2 (runx-2), a master regulatory transcription factor involved in bone formation (47). OxPLs have also been shown to induce, in a dose-dependent manner, osteoblastic differentiation and production of mineralized matrix (i.e., hydroxyapatite) in vascular cells (48) in vitro. This property may be mediated by lysophophosphatidylcholine, derived from OxPLs, which is released by phospholipases with preferences for short-chain oxidized sn2 fatty acids (e.g., sPLA2 and Lp-PLA2), which are found in high levels in explanted AVs (49). However, the role of these phospholipases is controversial, as several pathological mechanisms of Lp(a) appear to be mediated by an intact OxPL component (e.g., inflammatory gene expression, chemokine expression, and apoptosis), and recent randomized trials in coronary disease with phospholipase inhibitors failed to demonstrate benefit (and actually showed evidence of harm). Furthermore, OxPLs are also ligands for toll-like receptors (TLRs), including TLR-2 and TLR-4 (40, 50), which are major promoters of osteogenic differentiation via bone-morphogenetic protein (BMP)-2, a critical activator of bone and cartilage formation (51). Importantly, TLR-4 is expressed at much higher levels on left-sided valves and may explain, in addition to the higher hemodynamic stress, the increased predilection for OxPLs to induce AV calcification (51). Recently, Bouchareb et al. (52) have also implicated autotaxin (ATX) in Lp(a)-mediated calcification. ATX activity was 60% higher in calcified AVs than controls and colocalized with OxPLs and apo(a). Interestingly, ATX appeared to be transported by Lp(a) into AV tissue, but was also locally secreted by valve interstitial cells. ATX was shown to generate lysophosphatidic acid from available lysophophosphatidylcholine, which promoted valve inflammation and calcification via an NF-κB/IL-6/BMP-dependent pathway.
Based on these properties, Lp(a) may mediate AV disease by binding to areas of denuded valvular tissue, leading to excessive deposition of Lp(a), thus delivering apoB, cholesterol, and highly atherogenic pro-calcifying OxPLs to the leaflet surface, culminating in a pro-inflammatory and pro-calcifying cascade at the valve leaflets (see Fig. 2). This process is likely magnified several fold at the AV and at other areas of high hemodynamic stress (i.e., vascular branch points), which may also explain the higher predilection of calcification at these sites as compared with the remainder of the cardiovascular system.
FUTURE DIRECTIONS: CAN AS BE PREVENTED BY Lp(a) TARGETED THERAPY?
A preventative treatment for CAVD could markedly improve quality of life for older patients, limit the number of valve replacements with a significant cost savings for our health care system, and reduce death and disability in this vulnerable age group. Although the failure of previous randomized trials to prevent AS using lipid-lowering therapies (14–16) has dampened enthusiasm for prevention, CAVD has attractive characteristics for this approach: an easily identified sub-clinical phase that characterizes at-risk individuals; a slow regulated process that begins many years prior to clinical manifestations and provides a time window for intervention; and, at the very least, a partially shared pathophysiology with atherosclerosis, a highly preventable disease, that can be successfully managed with pharmacotherapy.
To date, the only major strategy for the prevention of CAVD has been the lowering of LDL using statins (and ezetimibe in one trial). Four randomized trials of LDL lowering have been completed and the results have been overwhelmingly negative; statins, by lowering LDL, do not appreciably reduce the progression of AV disease (14–16). These results were confirmed by a recent meta-analysis (53), which reported that despite marked lowering of LDL (35–55%) for more than 2 years (2.5–4 years in the different trials) in these trials, there was no evidence for a reduction in echocardiographic parameters in AS progression or in clinical outcomes (i.e., death, AV replacement, hospitalization for AS, etc.). Nonetheless, these trials had major limitations. They were, in large part, performed late in the disease process, could not be performed in individuals with an indication for statin therapy (i.e., many individuals with high cholesterol could not be ethically randomized to placebo), and used statin therapy as the preferred treatment approach, despite some evidence that statins in advanced vascular disease could promote calcification (which may stabilize vascular lesions but worsen AS) (54). Although the relevance of Lp(a) was not known at the time of these trials, statins also do not have any appreciable lowering effect on Lp(a) levels, and may even increase Lp(a), which may have also contributed to this failure. New evidence from Mendelian randomization has shown that LDL is likely causal in valve disease, despite the failure of statins in these randomized controlled trials, and has highlighted the potential role of earlier intervention prior to the development of advanced valve disease (55).
Although the evidence in support of Lp(a)-mediated AV calcification and stenosis are compelling, with genetic data strongly supporting a causal association, the true test of Lp(a) as a causal factor for AS will require a randomized trial targeting Lp(a). Although limited therapeutic options are currently available to lower Lp(a) clinically (e.g., niacin), several new agents are in development. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, new generation cholesteryl ester transfer protein inhibitors, and specific apo(a) antisense molecules are all currently in various stages of development and have been shown in early clinical studies to have Lp(a)-lowering properties (56, 57). Of particular interest, the apo(a) antisense molecules, by virtue of their specificity for Lp(a) lowering, will eventually allow for a clear test of the role of Lp(a) lowering in AV disease and other CV diseases (58). Currently, niacin, in its extended-release formulation, remains one of the few agents that is currently available that lowers Lp(a) in a dose-dependent fashion. Using extended-release niacin in individuals with early valve disease and high Lp(a), the Early Aortic Valve Lipoprotein (a) Lowering (EAVaLL) pilot randomized trial will provide a preliminary test of the Lp(a)-lowering hypothesis in AV disease. If successful, this trial will pave the way for future trials using newer and more targeted Lp(a)-lowering agents (59). In EAVaLL, 238 participants with Lp(a) greater than 50 mg/dl and aortic sclerosis or mild AS will be randomized to extended-release niacin or placebo for 2 years. The primary outcome will be reduction in AVC by CT. Secondary outcomes will include echocardiographic parameters of progression, as well as tolerability and safety.
Recently, an apo(a) antisense developed by ISIS Pharmaceuticals has been shown to lead to a marked dose-dependent decrease in circulating Lp(a), up to 90%, without affecting other lipid markers (60). Such profound and specific Lp(a)-lowering activity has not been previously observed with other agents and represents a unique opportunity to test the Lp(a) hypothesis in CV disease. Such an agent will provide the much needed “magic bullet” to confirm the hypothesis that Lp(a) is causally mediating AV disease, as well as demonstrating that specific Lp(a) lowering could slow disease progression and prevent AS. Due to the profound Lp(a) lowering achievable, such a trial should focus on young patients with marked elevations of Lp(a) with early valve disease, where disease progression is expected to be highest and most closely related to ongoing Lp(a) deposition (rather than other factors, e.g., hemodynamic stress may predominate at later stages) and therefore, where greatest benefit could be demonstrated. Given the profound Lp(a) lowering observed with the apo(a) antisense, and the post hoc ASTRONOMER analysis demonstrating that Lp(a) predicted both progression and clinical outcomes, and that this was most pronounced in the younger population, one might envision a trial targeting younger individuals (i.e., <70 years of age) with moderately advanced disease (i.e., mild and/or moderate AS) with high Lp(a), e.g., >50 mg/dl. Given the expected rates of progression in this group of patients, echocardiographic parameters such as valve area (as well as maximum aortic velocity) would be appropriate end-points with change in valve calcium score as potentially useful secondary outcomes. It would be particularly interesting to also consider state-of-the-art molecular imaging techniques using novel positron emission tomography with sodium fluoride-18, as pioneered by Dweck et al. (61, 62), which measures active osteoblast activity and ongoing calcium deposition at the AV. Validating such an endpoint would also provide new effective approaches to accelerate the testing of potentially novel therapies for AV disease.
In summary, the recent identification of genetic variants in LPA as being strongly associated with CAVD, and the Mendelian randomization studies demonstrating that this genetic association is mediated by circulating Lp(a), as well as our increased understanding of the mechanisms of Lp(a) and their potential role in atherogenesis and cardiovascular calcification, has reignited intense interest in Lp(a) in general, but also as a specific therapeutic target for CAVD. It is hoped that lowering of Lp(a) will demonstrate reduced progression of AV disease and that targeted therapies directed at Lp(a) will become the preferred treatment for AV disease in at-risk individuals with high Lp(a) and, ultimately, will reduce the need for valve replacement and the costs and complications of CAVD.
Footnotes
Abbreviations:
- AS
- aortic stenosis
- ATX
- autotaxin
- AV
- aortic valve
- AVC
- aortic valve calcium
- CAVD
- calcific aortic valve disease
- CHARGE
- Cohorts for Heart and Aging Research in Genetic Epidemiology
- CI
- confidence interval
- CT
- computed tomography
- CV
- cardiovascular
- HR
- hazard ratio
- Lp(a)
- lipoprotein (a)
- MDCS
- Malmo Diet Cancer Study
- OR
- odds ratio
- OxPL
- oxidized phospholipid
- PC
- phosphocholine
- TLR
- toll-like receptor
REFERENCES
- 1.Bach D. S., Radeva J. I., Birnbaum H. G., Fournier A. A., and Tuttle E. G.. 2007. Prevalence, referral patterns, testing, and surgery in aortic valve disease: leaving women and elderly patients behind? J. Heart Valve Dis. 16: 362–369. [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., de Ferranti S., Despres J. P., Fullerton H. J., Howard V. J., et al. 2015. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 131: 434–441. [DOI] [PubMed] [Google Scholar]
- 3.Yutzey K. E., Demer L. L., Body S. C., Huggins G. S., Towler D. A., Giachelli C. M., Hofmann-Bowman M. A., Mortlock D. P., Rogers M. B., Sadeghi M. M., et al. 2014. Calcific aortic valve disease: a consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 34: 2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osnabrugge R. L., Mylotte D., Head S. J., Van Mieghem N. M., Nkomo V. T., LeReun C. M., Bogers A. J., Piazza N., and Kappetein A. P.. 2013. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J. Am. Coll. Cardiol. 62: 1002–1012. [DOI] [PubMed] [Google Scholar]
- 5.Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Blaha M. J., Dai S., Ford E. S., Fox C. S., Franco S., et al. 2014. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindroos M., Kupari M., Heikkila J., and Tilvis R.. 1993. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J. Am. Coll. Cardiol. 21: 1220–1225. [DOI] [PubMed] [Google Scholar]
- 7.Otto C. M., Lind B. K., Kitzman D. W., Gersh B. J., and Siscovick D. S.. 1999. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N. Engl. J. Med. 341: 142–147. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien K. D., Reichenbach D. D., Marcovina S. M., Kuusisto J., Alpers C. E., and Otto C. M.. 1996. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 16: 523–532. [DOI] [PubMed] [Google Scholar]
- 9.Otto C. M., Kuusisto J., Reichenbach D. D., Gown A. M., and O’Brien K. D.. 1994. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 90: 844–853. [DOI] [PubMed] [Google Scholar]
- 10.Stewart B. F., Siscovick D., Lind B. K., Gardin J. M., Gottdiener J. S., Smith V. E., Kitzman D. W., and Otto C. M.. 1997. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 29: 630–634. [DOI] [PubMed] [Google Scholar]
- 11.Thanassoulis G., Massaro J. M., Cury R., Manders E., Benjamin E. J., Vasan R. S., Cupple L. A., Hoffmann U., O’Donnell C. J., and Kathiresan S.. 2010. Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J. Am. Coll. Cardiol. 55: 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demer L., and Tintut Y.. 2011. The roles of lipid oxidation products and receptor activator of nuclear factor-kappaB signaling in atherosclerotic calcification. Circ. Res. 108: 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvidal P., Bergstrom R., Horte L-G., and Stahle E.. 2000. Observed and relative survival after aortic valve replacement. J. Am. Coll. Cardiol. 35: 747–756. [DOI] [PubMed] [Google Scholar]
- 14.Chan K. L., Teo K., Dumesnil J. G., Ni A., and Tam J.; ASTRONOMER Investigators. 2010. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 121: 306–314. [DOI] [PubMed] [Google Scholar]
- 15.Cowell S. J., Newby D. E., Prescott R. J., Bloomfield P., Reid J., Northridge D. B., and Boon N. A.; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. 2005. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 352: 2389–2397. [DOI] [PubMed] [Google Scholar]
- 16.Rossebø A. B., Pedersen T. R., Boman K., Brudi P., Chambers J. B., Egstrup K., Gerdts E., Gohlke-Bärwolf C., Holme I., Kesaniemi Y. A., et al. 2008. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 359: 1343–1356. [DOI] [PubMed] [Google Scholar]
- 17.Deleted in proof. [Google Scholar]
- 18.Gotoh T., Kuroda T., Yamasawa M., Nishinaga M., Mitsuhashi T., Seino Y., Nagoh N., Kayaba K., Yamada S., Matsuo H., et al. 1995. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study). Am. J. Cardiol. 76: 928–932. [DOI] [PubMed] [Google Scholar]
- 19.Bozbas H., Yildirir A., Atar I., Pirat B., Eroglu S., Aydinalp A., Ozin B., and Muderrisoglu H.. 2007. Effects of serum levels of novel atherosclerotic risk factors on aortic valve calcification. J. Heart Valve Dis. 16: 387–393. [PubMed] [Google Scholar]
- 20.Glader C. A., Birgander L. S., Soderberg S., Ildgruben H. P., Saikku P., Waldenstrom A., and Dahlen G. H.. 2003. Lipoprotein(a), Chlamydia pneumoniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur. Heart J. 24: 198–208. [DOI] [PubMed] [Google Scholar]
- 21.Thanassoulis G., Campbell C. Y., Owens D. S., Smith J. G., Smith A. V., Peloso G. M., Kerr K. F., Pechlivanis S., Budoff M. J., Harris T. B., et al. 2013. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 368: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tracy R. P. 2008. ‘Deep phenotyping’: characterizing populations in the era of genomics and systems biology. Curr. Opin. Lipidol. 19: 151–157. [DOI] [PubMed] [Google Scholar]
- 23.Clarke R., Peden J. F., Hopewell J. C., Kyriakou T., Goel A., Heath S. C., Parish S., Barlera S., Franzosi M. G., Rust S., et al. 2009. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 24.Thanassoulis G. 2013. Mendelian randomization: how genetics is pushing the boundaries of epidemiology to identify new causes of heart disease. Can. J. Cardiol. 29: 30–36. [DOI] [PubMed] [Google Scholar]
- 25.Thanassoulis G., and O’Donnell C. J.. 2009. Mendelian randomization: nature’s randomized trial in the post-genome era. JAMA. 301: 2386–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamstrup P. R., Tybjaerg-Hansen A., and Nordestgaard B. G.. 2014. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 63: 470–477. [DOI] [PubMed] [Google Scholar]
- 27.Arsenault B. J., Boekholdt S. M., Dube M. P., Rheaume E., Wareham N. J., Khaw K. T., Sandhu M. S., and Tardif J. C.. 2014. Lipoprotein(a) levels, genotype and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 7: 304–310. [DOI] [PubMed] [Google Scholar]
- 28.Capoulade R., Chan K. L., Yeang C., Mathieu P., Bosse Y., Dumesnil J. G., Tam J. W., Teo K. K., Mahmut A., Yang X., et al. 2015. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 66: 1236–1246. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen L. B., Stender S., Kjeldsen K., and Nordestgaard B. G.. 1996. Specific accumulation of lipoprotein(a) in balloon-injured rabbit aorta in vivo. Circ. Res. 78: 615–626. [DOI] [PubMed] [Google Scholar]
- 30.Miller Y. I., Choi S-H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C. J., Que X., et al. 2011. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108: 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seimon T. A., Nadolski M. J., Liao X., Magallon J., Nguyen M., Feric N. T., Koschinsky M. L., Harkewicz R., Witztum J. L., Tsimikas S., et al. 2010. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12: 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiesner P., Tafelmeier M., Chittka D., Choi S. H., Zhang L., Byun Y. S., Almazan F., Yang X., Iqbal N., Chowdhury P., et al. 2013. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J. Lipid Res. 54: 1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ketelhuth D. F., Rios F. J., Wang Y., Liu H., Johansson M. E., Fredrikson G. N., Hedin U., Gidlund M., Nilsson J., Hansson G. K., et al. 2011. Identification of a danger-associated peptide from apolipoprotein B100 (ApoBDS-1) that triggers innate proatherogenic responses. Circulation. 124: 2433–2443. [DOI] [PubMed] [Google Scholar]
- 34.Tsimikas S., Brilakis E. S., Miller E. R., McConnell J. P., Lennon R. J., Kornman K. S., Witztum J. L., and Berger P. B.. 2005. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353: 46–57. [DOI] [PubMed] [Google Scholar]
- 35.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E. R., Shin M. J., Binder C. J., Horkko S., Krauss R. M., Chapman M. J., et al. 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49: 2230–2239. [DOI] [PubMed] [Google Scholar]
- 36.Tsimikas S., Clopton P., Brilakis E. S., Marcovina S. M., Khera A., Miller E. R., de Lemos J. A., and Witztum J. L.. 2009. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 119: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsimikas S., Kiechl S., Willeit J., Mayr M., Miller E. R., Kronenberg F., Xu Q., Bergmark C., Weger S., Oberhollenzer F., et al. 2006. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J. Am. Coll. Cardiol. 47: 2219–2228. [DOI] [PubMed] [Google Scholar]
- 38.Deleted in proof. [Google Scholar]
- 39.Matzinger P. 2002. The danger model: a renewed sense of self. Science. 296: 301–305. [DOI] [PubMed] [Google Scholar]
- 40.Deleted in proof. [Google Scholar]
- 41.Leitinger N., Tyner T. R., Oslund L., Rizza C., Subbanagounder G., Lee H., Shih P. T., Mackman N., Tigyi G., Territo M. C., et al. 1999. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc. Natl. Acad. Sci. USA. 96: 12010–12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subbanagounder G., Leitinger N., Schwenke D. C., Wong J. W., Lee H., Rizza C., Watson A. D., Faull K. F., Fogelman A. M., and Berliner J. A.. 2000. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler. Thromb. Vasc. Biol. 20: 2248–2254. [DOI] [PubMed] [Google Scholar]
- 43.Bae Y. S., Lee J. H., Choi S. H., Kim S., Almazan F., Witztum J. L., and Miller Y. I.. 2009. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 104: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirsch D., Azoury R., Sarig S., and Kruth H. S.. 1993. Colocalization of cholesterol and hydroxyapatite in human atherosclerotic lesions. Calcif. Tissue Int. 52: 94–98. [DOI] [PubMed] [Google Scholar]
- 45.Mody N., Parhami F., Sarafian T. A., and Demer L. L.. 2001. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 31: 509–519. [DOI] [PubMed] [Google Scholar]
- 46.Liberman M., Bassi E., Martinatti M. K., Lario F. C., Wosniak J. Jr., Pomerantzeff P. M., and Laurindo F. R.. 2008. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 28: 463–470. [DOI] [PubMed] [Google Scholar]
- 47.Ducy P., Zhang R., Geoffroy V., Ridall A. L., and Karsenty G.. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 89: 747–754. [DOI] [PubMed] [Google Scholar]
- 48.Parhami F., Morrow A. D., Balucan J., Leitinger N., Watson A. D., Tintut Y., Berliner J. A., and Demer L. L.. 1997. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation: a possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Thromb. Vasc. Biol. 17: 680–687. [DOI] [PubMed] [Google Scholar]
- 49.Mahmut A., Boulanger M. C., El Husseini D., Fournier D., Bouchareb R., Despres J. P., Pibarot P., Bosse Y., and Mathieu P.. 2014. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: implications for valve mineralization. J. Am. Coll. Cardiol. 63: 460–469. [DOI] [PubMed] [Google Scholar]
- 50.Kadl A., Sharma P. R., Chen W., Agrawal R., Meher A. K., Rudraiah S., Grubbs N., Sharma R., and Leitinger N.. 2011. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic. Biol. Med. 51: 1903–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X., Fullerton D. A., Su X., Ao L., Cleveland J. C. Jr., and Meng X.. 2009. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J. Am. Coll. Cardiol. 53: 491–500. [DOI] [PubMed] [Google Scholar]
- 52.Bouchareb R., Mahmut A., Nsaibia M. J., Boulanger M. C., Dahou A., Lepine J. L., Laflamme M. H., Hadji F., Couture C., Trahan S., et al. 2015. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 132: 677–690. [DOI] [PubMed] [Google Scholar]
- 53.Teo K. K., Corsi D. J., Tam J. W., Dumesnil J. G., and Chan K. L.. 2011. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can. J. Cardiol. 27: 800–808. [DOI] [PubMed] [Google Scholar]
- 54.Mundy G., Garrett R., Harris S., Chan J., Chen D., Rossini G., Boyce B., Zhao M., and Gutierrez G.. 1999. Stimulation of bone formation in vitro and in rodents by statins. Science 286: 1946–1949. [DOI] [PubMed] [Google Scholar]
- 55.Smith J. G., Luk K., Schulz C. A., Engert J. C., Do R., Hindy G., Rukh G., Dufresne L., Almgren P., Owens D. S., et al. 2014. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 312: 1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merki E., Graham M., Taleb A., Leibundgut G., Yang X., Miller E. R., Fu W., Mullick A. E., Lee R., Willeit P., et al. 2011. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipoprotein (a) in transgenic mice. J. Am. Coll. Cardiol. 57: 1611–1621. [DOI] [PubMed] [Google Scholar]
- 57.Raal F. J., Giugliano R. P., Sabatine M. S., Koren M. J., Langslet G., Bays H., Blom D., Eriksson M., Dent R., Wasserman S. M., et al. 2014. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 63: 1278–1288. [DOI] [PubMed] [Google Scholar]
- 58.Graham M. J., Viney N., Crooke R., and Tsimikas S.. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J. Lipid Res. Epub ahead of print. November 4, 2015; doi:10.1194/jlr.R052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thanassoulis G. 2015. Early Aortic Valve Lipoprotein(a) Lowering Trial (EAVaLL). Accessed December 11, 2015, at https://clinicaltrials.gov/ct2/show/NCT02109614.
- 60.Tsimikas S., Viney N. J., Hughes S. G., Singleton W., Graham M. J., Baker B. F., Burkey J. L., Yang Q., Marcovina S. M., Geary R. S., et al. 2015. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 386: 1472–1483. [DOI] [PubMed] [Google Scholar]
- 61.Dweck M. R., Jenkins W. S., Vesey A. T., Pringle M. A., Chin C. W., Malley T. S., Cowie W. J., Tsampasian V., Richardson H., Fletcher A., et al. 2014. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 7: 371–378. [DOI] [PubMed] [Google Scholar]
- 62.Dweck M. R., Jones C., Joshi N. V., Fletcher A. M., Richardson H., White A., Marsden M., Pessotto R., Clark J. C., Wallace W. A., et al. 2012. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 125: 76–86. [DOI] [PubMed] [Google Scholar]