Abstract
Short- and medium-chain fatty acids (SCFAs and MCFAs), independently of their cellular signaling functions, are important substrates of the energy metabolism and anabolic processes in mammals. SCFAs are mostly generated by colonic bacteria and are predominantly metabolized by enterocytes and liver, whereas MCFAs arise mostly from dietary triglycerides, among them milk and dairy products. A common feature of SCFAs and MCFAs is their carnitine-independent uptake and intramitochondrial activation to acyl-CoA thioesters. Contrary to long-chain fatty acids, the cellular metabolism of SCFAs and MCFAs depends to a lesser extent on fatty acid-binding proteins. SCFAs and MCFAs modulate tissue metabolism of carbohydrates and lipids, as manifested by a mostly inhibitory effect on glycolysis and stimulation of lipogenesis or gluconeogenesis. SCFAs and MCFAs exert no or only weak protonophoric and lytic activities in mitochondria and do not significantly impair the electron transport in the respiratory chain. SCFAs and MCFAs modulate mitochondrial energy production by two mechanisms: they provide reducing equivalents to the respiratory chain and partly decrease efficacy of oxidative ATP synthesis.
Keywords: short-chain fatty acids, mitochondria, long-chain fatty acids
Short- and medium-chain fatty acids (SCFAs and MCFAs), along with more abundant long-chain fatty acids (LCFAs), are natural compounds present in both animal and plant tissues that participate in cell metabolism. SCFAs and MCFAs are also important food constituents, where they are mostly in the form of triglycerides in some plant oils and milk (1). Nevertheless, bacterial fermentation of amylase-resistant starch and nonstarch polysaccharides in the gut is probably the most important source of SCFAs in humans and most mammalian species (2–4).
Along with their role as energy-supplying fuel, SCFAs and MCFAs exhibit various regulatory and signaling functions. Butyrate and other SCFAs are known to induce apoptosis under specific conditions and thus to control cell proliferation (5, 6). Currently, increasing attention is given to SCFAs with respect to their putative role in the pathogenesis of allergies, as well as autoimmune, metabolic, and neurological diseases [reviewed in (7)]. In the last two decades, the role of MCFAs as agonists of peroxisome proliferator-activated receptors has also been characterized (8). Moreover, accumulating evidence indicates that SCFAs generated by the gut microbiota exert influence on food intake, thereby regulating energy homeostasis and body weight [reviewed in (9–11)]. SCFAs and MCFAs also play an important role in intracellular signaling and contribute to the regulation of cell metabolism [reviewed in (12–16)]. Finally, MCFAs and SCFAs can control cell death and survival (17–20). These important regulatory functions of MCFAs and SCFAs and their implications to human health and pathologies are the subject of a number of excellent comprehensive reviews (1, 7, 21, 22). Here, we want to concentrate on some peculiarities of the metabolic features of SCFAs and MCFAs that differ from those of LCFAs and to sum up the current understanding of their role in cellular energy metabolism. Some aspects of SCFA and MCFA participation in energy-dependent mitochondrial processes have already been briefly reviewed by us previously (23).
DEFINITION AND PHYSICOCHEMICAL PROPERTIES
SCFAs and MCFAs, being monocarboxylic acids with a hydrocarbon chain length of 1 to 12 total carbon atoms, are abundant in nature, although they are present in plant and animal material at much smaller quantities than LCFAs (24, 25). Fatty acids of total carbon atom numbers from 1 to 6 are usually classified as SCFAs, whereas those of 7 to 12 carbon atoms are defined as MCFAs. Fatty acids with shorter chains, up to 9 total carbon atoms, are liquid at room temperature (Table 1) (26). The odor of the first members is pungent, whereas that of the higher members is rancid or none. The lipophilicity of SCFAs and MCFAs, measured as partition of the free acid between water and heptane, gradually increases with increasing carbon atom chain length so that MCFAs become comparable, in this aspect, to LCFAs (27–30). Due to their lower lipophilicity, as compared with LCFAs, SCFAs do not form micellar structures and do not participate in the formation of biological membranes (31). SCFAs and MCFAs are weak acids, with pKa values around 4.8, except for formic acid whose pKa is about one unit lower (Table 1). Thus, their alkali metal salts are considerably hydrolyzed in aqueous solutions. Water-soluble members of the family have a high tendency to form bimolecular associates in water solution. Interestingly, incorporation of SCFAs and MCFAs into bilayer membranes is known to increase their pKa values similarly, as in case of LCFAs (32, 33).
TABLE 1.
Nomenclature and basic physical properties of SCFAs and MCFAs
| Number of Carbon Atoms | Systematic Name | Common Name | Formula | Melting Temperature (°C) | Boiling Temperature (°C) | pKa |
| 1 | Methanoic acid | Formic acid | HCOOH | 8.6 | 100.8 | 3.75 |
| 2 | Ethanoic acid | Acetic acid | CH3COOH | 16.5 | 118.1 | 4.75 |
| 3 | Propanoic acid | Propionic acid | CH3CH2COOH | −22.0 | 140.9 | 4.88 |
| 4 | Butanoic acid | Butyric acid | CH3(CH2)2COOH | −7.9 | 162.5 | 4.81 |
| 5 | Pentanoic acid | Valeric acid | CH3(CH2)3COOH | −34.5 | 186.4 | 4.80 |
| 6 | Hexanoic acid | Caproic acid | CH3(CH2)4COOH | −3.9 | 205 | 4.84 |
| 7 | Heptanoic acid | Oenanthic acid | CH3(CH2)5COOH | −7.5 | 223 | 4.84 |
| 8 | Octanoic acid | Caprylic acid | CH3(CH2)6COOH | 16.3 | 239 | — |
| 9 | Nonanoic acid | Pelargonic acid | CH3(CH2)7COOH | 12.3 | 254 | — |
| 10 | Decanoic acid | Capric acid | CH3(CH2)8COOH | 31.3 | 269 | — |
| 11 | Undecanoic acid | Undecylic acid | CH3(CH2)9COOH | 29.3 | 228a | — |
| 12 | Dodecanoic acid | Lauric acid | CH3(CH2)10COOH | 44 | 225a | — |
Data from (26).
At reduced pressure of 100 mm Hg.
ORIGIN OF SCFAs AND MCFAs
In humans, the major source of SCFAs is the fermentation of dietary fiber and undigested saccharides in the gut by colonic anaerobic bacteria (2) [reviewed in (3, 4, 7)]. Acetate is mainly formed by reductive methylation of CO2 (34). There are two main routes producing propionate by colonic bacteria. In the methylmalonic-CoA pathway (also called the dicarboxylic pathway), propionate is generated from lactate that is supplied by lactate fermenting bacteria. In short, lactate is taken up by propionic bacteria and thereafter dehydrogenated to pyruvate, which becomes carboxylated by methylmalonyl-CoA-carboxyl transferase to oxaloacetate. Subsequently, the latter is converted to propionate through a four-carbon pathway consisting of the intermediates, malate, fumarate, succinate, and methylmalonyl-CoA (34). It should be noted that this pathway generates, in addition to propionate, one acetate molecule per two molecules of propionate. Other bacteria, such as Clostridium propionicum and Megasphaera elsdenii, produce propionate easily from lactate (35, 36). In this route, the CoA ester of lactate (lactoyl-CoA) is converted via acryloyl-CoA to propionyl-CoA, which subsequently becomes hydrolyzed to propionic acid (the acrylate pathway) (34). Butyrate is formed by the condensation of two acetyl-CoA molecules to form acetoacetyl-CoA, followed by the reductive conversion of acetoacetyl-CoA to butyryl-CoA (37). According to an estimate, acetate, propionate, and butyrate are formed in the human colon at a ratio of about 3:1:1 (38, 39). In an in vivo study, the rate of SCFA release by the gut to the circulatory system amounted to about 35 μmol/kg body weight per hour (40). The highest concentrations (70–140 mM) were found in the proximal colon (2). SCFAs may contribute to approximately 10% of the total human caloric uptake (4).
For newborn mammals, including human babies, mother’s milk constitutes an important source of MCFAs and SCFAs that are present mainly in the form of triglycerides and phospholipids. For example, the content of MCFAs (C7:0–C12:0) amounts to 6–17% and to 9–28% of all fatty acids in bovine (1) and human (41, 42) milk, respectively. Cow milk and milk products remain the main dietary source of SCFAs, mainly butyric acid, in adult humans. Other natural sources of MCFAs and SCFAs are coconut oil and palm kernel oil [(1) and references therein]. In comparison to triglycerides containing LCFAs, those containing MCFAs are more rapidly hydrolyzed in the intestinal tract and do not become incorporated into chylomicrons. SCFAs and MCFAs are transported by portal bloodstream to the liver, where they are readily metabolized (21).
SCFAs and MCFAs can also be formed in mammalian and human tissues, mainly liver. Thus, the peroxisomal β-oxidation of LCFAs produces chain-shortened acyl-CoAs (43) that can be hydrolyzed inside peroxisomes by distinct acyl-CoA thioesterases and released into the cytosol. In addition, peroxisomes are also equipped with carnitine-acetyl and carnitine-octanoyl transferases, and thus shortened acyl-CoAs are converted into carnitine esters for the supply to mitochondria [reviewed in (44)].
Under pathological conditions (for example, in inborn medium-chain acyl-CoA dehydrogenase deficiency), octanoate and decanoate accumulate to considerable amounts in tissues (45), resulting in an impairment of functioning of the mitochondrial respiratory complexes (46). These disorders are often accompanied by elevated urinary excretion of dicarboxylic MCFAs (mainly adipic, suberic, and sebacic acids) that apparently originate from microsomal ω-oxidation of corresponding medium-chain acyl-CoAs (47, 48). Based on their rapid absorption, triglycerides of MCFAs were introduced as a quickly available energy source in clinical nutrition in the middle of the last century. Emulsions enriched with MCFA-containing triglycerides were applied to patients suffering from various forms of impaired digestion of normal LCFA-containing triglycerides (1). In addition, due to the rapid transport of MCFAs from the gut to the liver, breath tests were developed for noninvasive clinical diagnosis using 13C-labeled octanoate (49, 50). Thereby, specific hepatic pathways as well as the speed of gastric emptying can be measured [reviewed in (51)].
PRINCIPLES OF METABOLISM IN ANIMAL TISSUES
Transport from the gut to the liver
Butyrate is taken up by enterocytes, presumably by means of the monocarboxylate transporter 1 (MCT-1) and the sodium-coupled monocarboxylate transporter 1 (SMCT-1) [reviewed in (52, 53)]. Butyrate is used by these cells mostly as fuel. According to other authors, SCFAs are also absorbed from the intestinal lumen by an exchange with Cl− (54) and/or HCO3− (55). More recently, however, the nonionic diffusion of protonated SCFAs and MCFAs across the colon epithelium has been favored (56). The latter mechanism is also supported by studies in model systems (30). According to this mechanism, the intestinal absorption of SCFAs depends on pH, while slight acidification of luminal pH, possibly by bacterial metabolic activity, increases the prevalence of the protonated form of SCFAs. Otherwise, the transport of SCFAs from enterocytes into the blood might be driven by anion exchange. Thus, it seems likely that the transport across the basolateral membrane is based on the anionic form of SCFAs against HCO3− (57). Butyrate, as well as other SCFAs and MCFAs, that has not been utilized by enterocytes is transported by the portal vein to the liver (40, 58) and metabolized by hepatocytes.
In contrast to LCFAs, which are esterified to triglycerides in enterocytes, incorporated into chylomicrons, and then enter the lymphatic system, SCFAs and MCFAs from the intestinal tract enter the portal vein as free acids. There, MCFAs become partly bound to plasma albumin. The proportion between albumin-bound and free MCFAs increases with increasing chain length, so that the first equilibrium constant (i.e., for the strongest binding site) between the albumin-bound and the free forms increases from 1.5 × 104 for hexanoate through 3.4 × 104 for octanoate and 105 for decanoate, up to 2.4 × 106 for laureate (59, 60). It has to be remembered that LCFAs are present in circulating blood practically totally bound to plasma albumin, with the first equilibrium constant of the order of 107 to 109 (60). The subsequent uptake of SCFAs and MCFAs, at least the lower members of that group, by liver and muscle cells, as well as other tissues, is independent of fatty acid binding proteins (1). Similarly, their uptake by cells requires neither fatty acid transport proteins, nor plasma membrane-embedded fatty acid translocase, nor cytosolic fatty acid-binding proteins. These observations provide a likely explanation of why octanoate oxidation by isolated hepatocytes is about five times faster than that of oleate (61). Moreover, the intracellular metabolism of SCFAs and MCFAs seems to require no, or much fewer, fatty acid-binding proteins (62, 63). In contrast, LCFAs require fatty acid-binding proteins for their cellular uptake, intracellular transport, regulatory functions, and metabolism [reviewed in (63)]. Binding of free LCFAs or LCFA-CoA esters to fatty acid binding proteins also minimizes their toxic effects, such as the lytic property or enzyme inhibition (63). Interestingly, in rats deficient in one of the fatty acid transport proteins (CD36 protein), feeding with a SCFA- and MCFA-rich diet eliminated increased glucose uptake, hyperinsulinemia, and heart hypertrophy (64). Similarly, in CD36-deficient mice, octanoate alleviated poor heart ischemic tolerance (65). These observations may have important implications to human medicine.
Fueling the tissue energy metabolism
The utilization by different tissues of acetate formed by intestinal bacteria greatly differs between ruminants and nonruminants [(4) and references therein]. Acetate is also endogenously generated in adult humans by ethanol oxidation, which operates mainly in the liver (66). Thus, it has been shown that ethanol oxidation could result in a 20-fold increase of the acetate level in peripheral blood (67). In addition, net acetate generation has been found during fatty acid oxidation in perfused rat liver (68). Formed acetate results mainly from the operation of acetyl-CoA hydrolase (acetyl-CoA deacylase), which, in rat liver, has been found predominantly in the mitochondrial matrix (69, 70). Because acetyl-CoA hydrolase is inhibited by free CoASH (Ki = 17 μM), the level of free CoASH has to be strongly lowered before hydrolase can produce free acetate from acetyl-CoA (70). On the other hand, free acetate produced in the liver by oxidation of ethanol or as a byproduct of ketogenesis is barely oxidized in this organ. This is because of the high Km of hepatic mitochondrial acetyl-CoA synthetase (71) or its absence in these organelles [(72), see also the next paragraph]. However, acetate can be transported by circulation to other organs, e.g., the heart and skeletal muscles, where the Km of mitochondrial acetyl-CoA synthetase is much lower and where it can be utilized as fuel (70).
Activation of SCFAs occurs in the liver and several other tissues by acyl-CoA synthetases (72). These enzymes are located in the cytosol as well as in the mitochondrial matrix, where they are loosely bound to the inner mitochondrial membrane. In mammals, acetate is activated to acetyl-CoA by two different acetyl-CoA synthetases, of which one (AceCS1) is cytosolic (78 kDa, Km = 0.11 mM) and the other one (AceCS2) is mitochondrial (71 kDa, Km = 0.06) (73). According to these authors (73), AceCS2 is present in a wide range of tissues, with the highest level in heart (bovine and rodent), but essentially absent in the liver. In contrast, earlier investigations (74–76) demonstrated the presence of acetyl-CoA synthetase activity in hepatocytes in both mitochondria (20–50%) and the cytosol (50–80%).
Acetate is an important fuel during fasting, as evidenced by the observation that in skeletal muscles of mice lacking AceCS2, the ATP content declined to 50% in comparison to wild-type mice (77). Interestingly, the activity of cytosolic and mitochondrial acetyl-CoA synthetases is regulated by a reversible acetylation. Furthermore, this process is under the control of NAD+-dependent deacetylases, sirtuin 1 and sirtuin 3 [reviewed in (78)]. Sirtuin 1 is a nuclear and cytoplasmic enzyme, whereas sirtuin 3 is predominantly located within mitochondria. In summary, in mammals, acetate not only plays an important role in energy homeostasis, but also as a substrate for sirtuins; it is also involved in the regulation of gene silencing and the aging processes (78).
In contrast to LCFAs that are activated to acyl-CoAs in the cytosol and must be transferred to the mitochondrial interior via the carnitine shuttle, SCFAs and MCFAs, at least those of carbon atom number up to C8, permeate the inner mitochondrial membrane in the nonesterified form and are activated to their CoA-derivatives in the mitochondrial matrix. Localization of medium-chain acyl-CoA synthetase in the mitochondrial matrix was first described in the late 60s of the past century (79) [for more recent reports see (80, 81)]. The latter property may have important metabolic consequences under specific conditions, as will be discussed further. SCFAs and MCFAs activated inside mitochondria are used as substrates in mitochondrial β-oxidation and the citric acid cycle. Interestingly, it has been demonstrated using the perfusion technique that, in rat liver and heart, octanoate can also undergo peroxisomal β-oxidation, thereby delivering acetyl-CoA to the cytosol (82, 83).
As the energy source for tissue metabolism, triglycerides of MCFAs have several advantages compared with those of LCFAs. First, they are more rapidly digested and the liberated MCFAs are more quickly absorbed in the intestinal lumen (21, 84). Second, tissue metabolism of SCFAs and partly of MCFAs does not depend on proteins for binding, transport, and transmembrane translocation (see the Transport from the gut to the liver section above). Therefore, they can serve as better energy-providing fuel than LCFAs, especially under pathological conditions, as exemplified by severe inflammation (85). Finally, MCFAs, having a slightly lower energy content than LCFAs (8.4 instead of 9.2 kcal/g), reduce body fat mass and enhance the insulin sensitivity of tissues [reviewed in (1, 21, 22, 62)].
As said before, SCFAs and MCFAs are transported by blood from the alimentary tract to the liver where they are metabolized; therefore, they are not stored in the adipose tissue. Nevertheless, by prolonged feeding of rats with portacaval anastomoses (blood circulation overpassing the liver), the group of van Itallie (86) succeeded to significantly enrich the tissue depot lipids in triglycerides containing higher MCFAs (C8, C9, and C10). General features of the whole-body metabolism and physiological functions of MCFAs, in particular octanoate, the most abundant MCFA, have recently been summarized (87, 88). Like other MCFAs and in contrast to LCFAs, octanoate is rapidly degraded and is stored as triglyceride in the adipose or other tissues only to a very low extent. Octanoate, as a fuel for the energy metabolism in mammals, has been studied in high-energy-requiring tissues such as skeletal muscle, heart, liver, and brain (89–94). Concerning the brain, it is important to remember that SCFAs and MCFAs are able to permeate the blood-brain barrier (95).
The effects of SCFAs and MCFAs on hepatic energy metabolism were studied mostly either by the perfusion technique of isolated rat liver (89, 91–93, 96–98) or by incubation experiments with isolated hepatocytes (99–101). In summary, these studies have shown that addition of octanoate and, to a lesser extent, butyrate enhances oxygen consumption compared with incubations with the Krebs-Henseleit buffer supplemented with pyruvate or lactate as energy supplying substrates. Both the stimulation of oxygen consumption and the associated increase of the cellular level of NAD(P)H (102) indicate that these fatty acids effectively supply reducing equivalents (NADH, FADH2) to the mitochondrial respiratory chain. In addition, octanoate raised the mitochondrial energization, an observation based on the in situ measurement of the mitochondrial membrane potential (ΔΨm) (101). Energization by octanoate of hepatocytes oxidizing pyruvate plus lactate was also manifest (100). However, in contrast to LCFAs (e.g., oleate), octanoate significantly raised the AMP level in the tissue (89, 100). It has also been reported that feeding rats with an MCFA-rich diet enhances skeletal muscle mitochondrial oxidative capacity (62, 103), an observation that is partly attributed to increased activity of citrate synthase (62).
Because MCFA-containing triglycerides are rapidly digested in the intestine and taken up by enterocytes, and are not incorporated into chylomicrons, they are ideal energy-delivering nutrients in clinical situations, where the digestion and/or absorption of LCFA-containing triglycerides is impaired or a rapid energy uptake by the body is required. For this reason, MCFA-containing triglycerides have been used for the nutrition of patients with inherited LCFA β-oxidation disorders (104). While increasing evidence indicates that the diseased heart suffers from energy deficiency, fueling the myocardial energy metabolism with MCFAs has been proposed as metabolic therapy for treating patients suffering from certain cardiomyopathies (87). For this treatment, MCFAs with odd carbon-atom numbers appeared superior compared with those with even carbon-atom numbers (105).
Modulation of carbohydrate and lipid metabolism
Contrary to LCFAs, the oxidation of MCFAs is not affected by the carbohydrate content in the diet. Thus, it has been reported that adaptation of adult rats to low-fat high-carbohydrate or high-fat low-carbohydrate diet does not change the rate of octanoate oxidation measured in hepatocytes. In contrast, oleate oxidation declined by 50% in rats adapted to a low-fat high-carbohydrate diet (61). Furthermore, MCFAs derived from digestion of MCFA-containing triglycerides are predominantly degraded by hepatic mitochondrial β-oxidation. An excess of formed acetyl-CoA is used for the synthesis of ketone bodies (mostly acetoacetate and β-hydroxybutyrate), which are delivered as fuel to nonhepatic tissues (21, 22). MCFA-containing triglycerides are preferentially hydrolyzed compared with those containing LCFAs, and liberated MCFAs are also preferentially oxidized in organs, mostly heart, muscles, kidneys, and liver (93, 106). In vitro studies on isolated hepatocytes and perfused rat liver have shown that SCFAs and MCFAs modulate the hepatic metabolism of carbohydrates and lipids. Thus, butyrate and octanoate inhibit glycolysis (107, 108) and thereby exert the “glucose sparing activity”. In contrast, anabolic pathways of glucose formation (100, 109–111) and lipogenesis (107, 112) become stimulated. As an illustration, glucose formation by hepatocytes fed with pyruvate plus lactate as gluconeogenic precursors is about 2-fold stimulated by octanoate (100). In contrast to inhibiting glycolysis in hepatocytes, decanoate, but not octanoate, has been found to stimulate glycolysis in astrocytes, thus resulting in an enhanced release of lactate into the extracellular space (113). Because lactate is considered a key energy source for neurons, the astrocyte/neuron lactate shuttle supplies this substrate to neighboring neurons.
Generally, it has been discussed that mitochondrial matrix enzymes, pyruvate carboxylase and the pyruvate dehydrogenase complex, are regulated by the ratios of acetyl-CoA/CoA, ATP/ADP, and NADH/NAD+ and, in addition, by pyruvate concentration. On the other hand, however, the fatty acid (octanoate, palmitate)-induced increase of pyruvate flux through both enzymes has been explained exclusively by an increased uptake of pyruvate into the mitochondrial matrix compartment (109, 111). It has been argued that the formation of acetoacetate from fatty acids drives pyruvate uptake across the inner mitochondrial membrane. Therefore, there is reason to hypothesize that SCFAs and MCFAs play a supporting role in the utilization of physiological low concentrations of pyruvate or lactate for glucose generation (104). Nevertheless, acceleration of pyruvate uptake is not sufficient to explain the huge stimulation by fatty acids of glucose generation with aspartate plus glycerol as gluconeogenic precursors (110). Such stimulation is generally attributed to the generation of acetyl-CoA (allosteric effector of pyruvate carboxylase) and reducing equivalents (114), the latter promoting formation of glyceraldehyde-3-phosphate. It is also worth remembering that pyruvate carboxylation in isolated rat liver mitochondria is strongly stimulated by L-octanoylcarnitine, whereas nonesterified butyrate and octanoate exert a strong inhibition (100). In addition, octanoate exerts a short-term dual regulatory effect on hepatic fatty acid synthesis, namely stimulation in the low concentration range (up to 1 mM) and inhibition at higher concentrations (107, 112). The stimulation of lipogenesis has been attributed to the activation of acetyl-CoA carboxylase, presumably by a covalent modification of the enzyme. Moreover, studies with digitonin-permeabilized hepatocytes have shown that stimulation of the acetyl-CoA carboxylase activity depends on the chain length of the fatty acid (112). The stimulation magnitude increased from capronic (C6:0) to lauric (C12:0) acids, but decreased with fatty acids of longer chain length. Malonyl-CoA, the product of the cytosolic acetyl-CoA-carboxylase reaction, acts as a substrate for fatty acid synthesis, but also as an inhibitor of carnitine palmitoyltransferase I.
There is an ongoing discussion that SCFAs and MCFAs activate hepatic AMP-dependent kinase (AMPK) [reviewed in (115) and references therein]. Generally, AMPK activation inhibits ATP-utilizing processes in the cell and stimulates those that produce ATP. Being a cytosolic enzyme, AMPK is activated by elevation of cytosolic AMP. Consequently, the mechanism underlying the SCFA/MCFA activation of AMPK is not clear because the activation of SCFAs and MCFAs to their acyl-CoA esters raises the intramitochondrial AMP level. Interestingly, a recent study with mouse L6 myotubes suggests that AMPK can also be activated without alteration of the cytosolic AMP/ATP ratio. According to this suggestion the activation mechanism of AMPK by MCFAs is mediated by extracellular Ca2+-dependent Ca2+/calmodulin-dependent kinase kinase β (116). Other reported effects of SCFAs and MCFAs on the anabolic pathways are the inhibition of triglyceride synthesis in adipocytes (117) and a sparing effect on hepatic glycogen storage (118). The latter activity is attributed to the competition between fatty acid and glucose oxidation.
The fact that MCFAs with odd-chain and even-chain hydrocarbon skeletons exert different effects on cell energy metabolism is of particular interest and practical importance (119, 120). In contrast to even-chain MCFAs, β-oxidation of odd-chain MCFAs generates acetyl-CoA and, in addition, propionyl-CoA, which is anaplerotic for the citric acid cycle. Propionyl-CoA can enter the citric acid cycle after its conversion into succinate. The anaplerotic function of odd-chain MCFAs seems to be crucial for maintenance of the level of citric acid cycle metabolites in various tissues. This biochemical background explains the proposed use of the MCFA-derived triheptanoin (glycerol triheptanoate) as an anaplerotic drug (121, 122) for the treatment of cardiomyopathies in long-chain fat oxidation disorders (105) and pyruvate carboxylase deficiency (123). This anaplerotic function of odd-chain MCFAs is also important during episodes of epilepsy, when the neurons become excessively excited and thereby release increased amounts of glutamate (124, 125). It is assumed that glutamate release is likely to decrease the level of citric acid cycle metabolites and thereby declines the oxidation of acetyl-CoA by mitochondria. Indeed, it has recently been shown that triheptanoin partially restores the level of citric acid cycle metabolites in an epileptic animal model (126). Triheptanoin is also able to attenuate harmful side effects associated with ischemic stroke (127). As an illustration, when mice were exposed to transient ischemia, triheptanoin reduced the extracellular level of glutamate released in the mouse striatum, maintained the cellular ATP content at the desired level, and prevented a decline of the respiratory activity of isolated brain mitochondria. The latter findings strongly suggest that mitochondrial ATP regeneration is a target of triheptanoin action (127). It is also worthwhile to mention that, in sharp contrast to butyrate and octanoate, the odd-chain SCFA, propionate, has no inhibitory effect on glycolysis and does not stimulate ketogenesis (108). Similarly to propionyl-CoA formed by the cellular degradation of odd-chain fatty acids, external propionate supplies the gluconeogenic pathway with its hydrocarbon skeleton, an activity that is mostly observed in ruminants [reviewed in (128)].
POTENTIAL ADVERSE EFFECTS
Energy coupling
LCFAs are long known as mild uncouplers of oxidative phosphorylation due to their protonophoric effect on the inner mitochondrial membrane [reviewed in (23)]. The mechanism of this effect has been comprehensively explained by Skulachev (129, 130), who showed that LCFA anions can be transferred across the inner mitochondrial membrane by adenine nucleotide translocase, whereas the nondissociated fatty acid molecules can move across the membrane by a flip-flop mechanism. As an effect of this, a net proton transfer occurs. Subsequent studies have shown that LCFA anions can also be transferred across the mitochondrial inner membrane by a number of other mitochondrial inner membrane anion carriers [reviewed in (131, 132)]. This protonophoric effect decreases the electrochemical proton gradient across the inner membrane, thereby decreasing the efficiency of oxidative phosphorylation. Such activity of LCFAs has been repeatedly reported in vitro with isolated mitochondria and there is evidence that protonophoric uncoupling can also operate in vivo after hypoxia/reperfusion or high-fat diet (133, 134). With isolated mitochondria, uncoupling by LCFAs can easily be quantified as an increase in the resting state respiration by micromolar concentrations of these acids. In contrast to LCFAs, the ability to stimulate the resting state respiration by SCFAs or MCFAs is either oligomycin-sensitive (C4 to C8) or weaker, even when applied at millimolar concentrations (100, 132, 135, 136). Furthermore, addition of octanoate or decanoate (at 100 or 300 μM concentration) to cultured neurons or astrocytes did not stimulate their respiration (113).
Based on the fatty acid cycling hypothesis, this difference between LCFAs on one side and MCFAs and SCFAs on the other side can be discussed in two aspects, namely in terms of: i) varying permeation rate of fatty acids across the mitochondrial inner membrane; and ii) various affinities of fatty acid anions to mitochondrial anionic carriers depending on the fatty acid chain length. In this context, it can be expected that SCFAs and MCFAs exhibit lower solubility in the lipid core of the inner mitochondrial membrane because of their lower lipophilicity. In fact, it has been shown (30) that the permeation of fatty acids across phosphatidylethanolamine bilayers depends on their partition coefficient between hexadecane and water, and the latter decreases with decreasing hydrocarbon chain length (137). In addition, there is reason to speculate that the binding of the anionic forms of SCFAs and MCFAs to the mitochondrial anion carriers is lower than the binding of LCFA anions. Such a view is supported by the observation that inhibition of the adenine nucleotide carrier by acyl-CoA thioesters declines with their hydrocarbon chain length (138). Nevertheless, there is evidence, mostly from studies on isolated liver cells and perfused liver, that SCFAs and MCFAs can initiate ATP wastage (89, 96, 97, 99, 100). It has been speculated (100) that this effect may be associated with a futile cycling between the esterified forms of SCFAs or MCFAs and their acyl-CoA thioesters. This would result in a net ATP utilization. Thus, a high rate of acetyl-CoA hydrolysis in rat hepatocytes, its stimulation by increasing acetate concentrations, and substrate cycling between acetate and acetyl-CoA have been suggested (139–141). In the case of acetate, this cycling is likely to occur in the cytoplasm of hepatocytes and may account for as much as 1% of the total heat production (141).
Butyrate and octanoate are known to stimulate oxygen uptake in perfused liver and isolated hepatocytes, to raise the energization of mitochondria, and to support ATP-dependent glucose generation (100, 101, 109). However, it has been repeatedly observed that these two fatty acids increase oxygen uptake and dramatically lower the ATP/O ratio when added to the incubation or perfusion media supplemented with pyruvate plus lactate or lactate alone (89, 96, 97, 100), thus pointing to an impairment of the energy generation. The mechanism of this effect could not be explained by protonophoric uncoupling of oxidative phosphorylation by these SCFAs and MCFAs, as this mechanism is typical for LCFAs (see above). The main argument against such a mechanism was the observation that oligomycin, a well-known inhibitor of mitochondrial ATPase/ATP synthase, abolished most of the enhanced hepatic oxygen consumption (89, 99) as well as the octanoate-stimulated oxygen uptake by isolated rat liver mitochondria (100). Other authors attributed the impairment of energy metabolism by butyrate and octanoate to the stimulation of Na+/K+-ATPase (99) or to an increase in the FADH2/NADH ratio due to β-oxidation (96). However, these explanations seem unlikely because a similarly enhanced oxygen uptake in the presence of LCFAs was not sensitive to oligomycin. A further clue seemed to be a stationary elevated AMP level, which was not observed with LCFAs (89, 100, 142–144). This pointed to an increased turnover of ATP within mitochondria rather than to its impaired production. In addition, this putative ATP turnover competed with intramitochondrial ATP-dependent reactions, i.e., pyruvate carboxylation (100) and citrulline synthesis (144). We have shown (100) that this phenomenon is due to enhanced activation of octanoate within the inner mitochondrial compartment, accompanied by utilization of two high-energy bonds per each molecule of octanoyl-CoA formed. Because both octanoyl-AMP and octanoyl-CoA could be partly hydrolyzed within the mitochondrial matrix, a futile cycle appeared that was responsible for the increased intramitochondrial ATP consumption that resulted in lowering the mitochondrial membrane potential and thus increasing oxygen uptake (Fig. 1). This mechanism may prevent a drastic depletion of intramitochondrial free CoA under high supply of SCFAs and MCFAs with the portal vein. In addition, the octanoate activation-associated increased AMP level decreases the intramitochondrial pool of exchangeable adenine nucleotides, ATP and ADP, an event that slows down the operation rate of the adenine nucleotide translocase and thereby enhances the control strength of this transporter on the total flux of oxidative ATP generation (145).
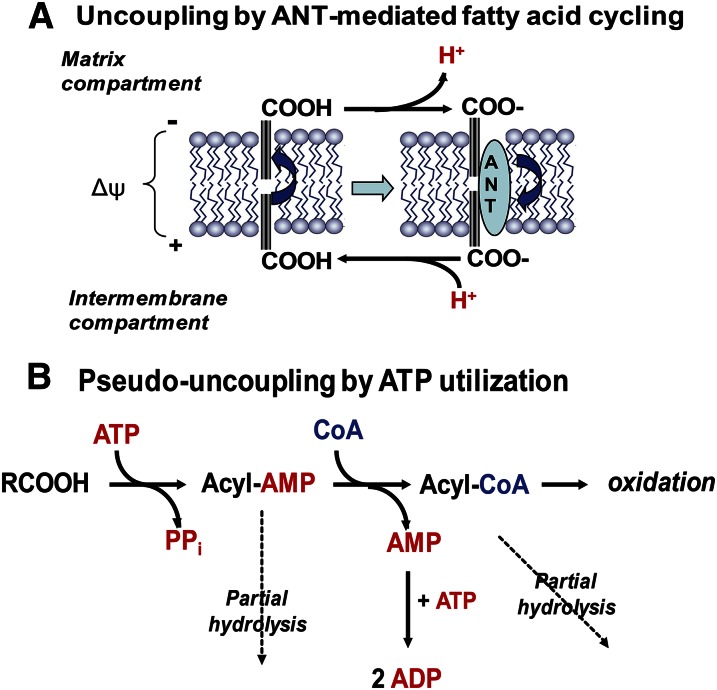
Fig. 1.
Uncoupling by LCFAs and pseudo-uncoupling by SCFAs and MCFAs of energized mitochondria. A: Real protonophoric uncoupling by LCFAs. Undissociated LCFAs undergo spontaneous flip-flop movements across the inner mitochondrial membrane. In the alkaline environment at the inner (matrix) side of the membrane, they undergo dissociation to proton (H+) and the fatty acid anion (RCOO−), which is subsequently transported by the adenine nucleotide transporter (ANT) and other mitochondrial anion carriers back to the external side of the membrane. Here, the LCFA anion becomes reprotonated and can undergo another flip-flop transfer. B: Pseudo-uncoupling by SCFAs and MCFAs. SCFAs and MCFAs are activated to their CoA thioesters in the mitochondrial matrix compartment. This process utilizes ATP and releases AMP and pyrophosphate (PPi). AMP can subsequently react with ATP yielding two molecules of ADP that are rephosphorylated at the expense of the mitochondrial transmembrane potential (ΔΨm), thus producing an uncoupling-like effect. In addition, both acyl-AMP and acyl-CoA are subject to slow hydrolysis, thus increasing AMP production and futile energy utilization.
These specific properties of SCFAs and MCFAs may explain the well-known fact that diets rich in these fatty acids increase energy expenditure and decrease adiposity (146–148). It has been reported (103, 149) that MCFAs, in contrast to LCFAs, contribute to maintain a high sensitivity of muscle cells to insulin. This view has, however, not been confirmed by other authors (150). It is also worthy to note that CoA esters of SCFAs and MCFAs accumulate in tissues in various pathological situations, such as the Reye syndrome (151). Furthermore, it has been reported (152) that octanoyl-CoA at low millimolar concentrations exerts a strong inhibition on complex III activity of the respiratory chain.
Generation of reactive oxygen species
Oxygen consumption by mitochondria is accompanied by the generation of reactive oxygen species (ROS), of which β-oxidation is the most important source (153–156). Theoretically, a one-electron transfer to molecular oxygen, thereby forming superoxide, can take place from complex I of the respiratory chain as well as from acyl-CoA dehydrogenase, the electron transfer flavoprotein (ETF), the ETF-ubiquinone oxidoreductase, and complex III (Fig. 2, more details are given in the figure legend). Indeed, it has been demonstrated for skeletal muscle mitochondria that several sites are involved in the β-oxidation-linked superoxide generation (157). H2O2 release by mitochondria from (rat) skeletal muscle, heart, and liver was measured with carnitine derivatives of palmitate, octanoate, and butyrate as substrates (156, 158). These studies have shown that the β-oxidation-associated ROS generation is similar with LCFAs and MCFAs (156, 158). In contrast, it has been reported that C2C12 myotubes treated with capric (C10) or lauric (C12) acid generate less ROS than those treated with the LCFAs, myristic or palmitic acid; whereas, the oxygen consumption is higher with MCFAs than with LCFAs (149). These authors speculate that this decrease in ROS production might be attributed to an increased expression of uncoupling protein-3 by MCFAs. However, other authors (160) did not observe increased expression of uncoupling protein-3 in the hearts of rats fed a MCFA-rich diet.
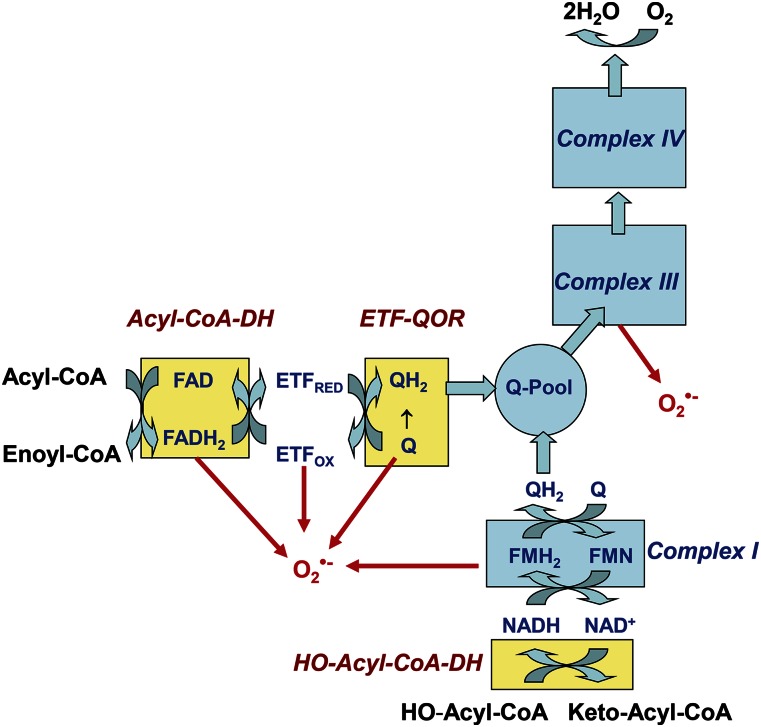
Fig. 2.
Electron transfer from fatty acids to complex IV during β-oxidation and possible sites of superoxide generation. Shown is a simplified scheme summarizing the sites of superoxide generation supported by the mitochondrial degradation of fatty acid thioesters. Electrons are donated from the first enzyme of the β-oxidation pathway, acyl-CoA dehydrogenase (Acyl-CoA-DH), and are transmitted via the ETF to electron-transferring ubiquinone oxidoreductase (ETF-QOR). ETF-QOR reduces ubiquinone (Q) to ubiquinole (QH2). Finally, ubiquinole becomes oxidized to ubiquinone and subsequently electrons move to complex III. The 3-hydroxyacyl-CoA dehydrogenase (HO-CoA-DH), the third enzyme of the β-oxidation pathway, which oxidizes HO-acyl-CoA to keto-acyl-CoA, donates electrons directly to complex I. Sites of superoxide generation are indicated in red.
Along with their direct role in ROS generation as electron donors to the respiratory chain in β-oxidation, fatty acids also have an indirect effect on superoxide production due to modifying both the rate of electron flux along the respiratory chain and the degree of energy coupling. As discussed by us in detail elsewhere (160), LCFAs potentiate ROS generation due to their weak inhibition of the electron flow at the levels of complexes I and III, most likely by interaction within the complex subunit structure, and between complexes III and IV due to the release of cytochrome c from the inner membrane. These effects occur in ROS generation accompanying the so-called forward mode of electron transport. On the other hand, due to the protonophoric action of LCFAs on the inner mitochondrial membrane (“mild uncoupling effect”), they strongly decrease ROS generation in the reverse mode of electron transport (160). Contrary to this, SCFAs and MCFAs, at least lower members of the latter, at low physiological levels, neither affect functioning of the electron transport chain nor exert a protonophoric effect on the inner mitochondrial membrane. On the other side, however, the excessive accumulation of MCFAs that occurs under inborn medium-chain acyl-CoA dehydrogenase deficiency (45) and is connected with an impairment of the mitochondrial respiratory chain complexes (46) may lead to increased ROS production. This results in increased lipid peroxidation, generation of protein carbonyls (as peroxidation products), and a decrease in the nonenzymatic antioxidant defense (161).
CONCLUDING REMARKS
Although SCFAs and MCFAs, compared with LCFAs, constitute a minor component of human and most mammalian diets, they play important roles both as nutrients and metabolic regulators. In addition to their content in food, a large proportion of SCFAs is contributed by the intestinal microflora by fermentation of otherwise undecomposed food constituents, mostly undigested carbohydrates. In conclusion, proper maintenance of gut microflora is important both for better utilization of food constituents and as a source of molecules important as metabolic regulators.
During the past three or four decades, multiple roles of SCFAs and MCFAs have been uncovered within the cellular and whole-body metabolism. Along with their function as “fuels” for the oxidative generation of ATP, SCFAs and MCFAs supply anabolic pathways (gluconeogenesis and lipogenesis) with carbon-containing precursor molecules and contribute to the regulation of cell metabolism by triggering signaling pathways. Thus, MCFAs and, in particular, SCFAs play an important role in a proper balance between lipogenesis and oxidative degradation of fatty acids. Many of these multiple mechanisms of SCFAs and MCFAs still await full elucidation.
Footnotes
Abbreviations:
- AMPK
- AMP-dependent kinase
- ETF
- electron transfer flavoprotein
- LCFA
- long-chain fatty acid
- MCFA
- medium-chain fatty acid
- ROS
- reactive oxygen species
- SCFA
- short-chain fatty acid
REFERENCES
- 1.Marten B., Pfeuffer M., and Schrezenmeir J.. 2006. Medium-chain triglycerides. Int. Dairy J. 16: 1374–1382. [Google Scholar]
- 2.Cummings J. H., Pomare E. W. J., Branch H. W. J., Naylor C. P. E., and MacFarlane G. T.. 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 28: 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong J. M., de Souza R., Kendall C. W., Emam A., and Jenkins D. J.. 2006. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40: 235–243. [DOI] [PubMed] [Google Scholar]
- 4.Bergman E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70: 567–590. [DOI] [PubMed] [Google Scholar]
- 5.Trock B., Lanza E., and Greenwald P.. 1990. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J. Natl. Cancer Inst. 82: 650–661. [DOI] [PubMed] [Google Scholar]
- 6.Hague A., Elder D. J., Hicks D. J., and Paraskeva C.. 1995. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer. 60: 400–406. [DOI] [PubMed] [Google Scholar]
- 7.Tan J., McKenzie C., Potamitis M., Thorburn A. N., Mackay C. R., and Macia L.. 2014. The role of short-chain fatty acids in health and disease. Adv. Immunol. 121: 91–119. [DOI] [PubMed] [Google Scholar]
- 8.Liberato M. V., Nascimento A. S., Ayers S. D., Lin J. Z., Cvoro A., Silveira R. L., Martínez L., Souza P. C., Saidemberg D., Deng T., et al. 2012. Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR)γ activators and pan-PPAR partial agonists. PLoS One. 7: e36297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne C. S., Chambers E. S., Morrison D. J., and Frost G.. 2015. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. (Lond). 39: 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bäckhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A., Semenkovich C. F., and Gordon J. L.. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishna B. S. 2013. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 28(Suppl. 4): 9–17. [DOI] [PubMed] [Google Scholar]
- 12.Georgiadi A., and Kersten S.. 2012. Mechanism of gene regulation by fatty acids. Adv. Nutr. 3: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen L., Siersbæk M., and Mandrup S.. 2012. PPARs: fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 23: 631–639. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura M. T., Yudell B. E., and Loor J. J.. 2014. Regulation of the energy metabolism by long-chain fatty acid. Prog. Lipid Res. 53: 124–144. [DOI] [PubMed] [Google Scholar]
- 15.Hara T., Kimura I., Inoue D., Ichimura A., and Hirasawa A.. 2013. Free fatty acid receptors and their role in regulation of energy metabolism. Rev. Physiol. Biochem. Pharmacol. 164: 77–116. [DOI] [PubMed] [Google Scholar]
- 16.Hara T., Kashihara D., Ichimura A., Kimura I., Tsujimoto G., and Hirasawa A.. 2014. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim. Biophys. Acta. 1841: 1292–1300. [DOI] [PubMed] [Google Scholar]
- 17.Chen J. S., Faller D. V., and Spanjaard R. A.. 2003. Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics? Curr. Cancer Drug Targets. 3: 219–236. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y., Chen Y., Jiang H., and Nie D.. 2011. Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ. 18: 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauser J. K., Matthew G. M., Cummins A. G., and Howart G. S.. 2013. Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy. 59: 214–224. [DOI] [PubMed] [Google Scholar]
- 20.Walsh M. E., Bhattacharya A., Sataranataran K., Qaisar R., Sloane L., Rahman M. M., Kinter M., and van Remmen H.. 2015. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 14: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach A. C., and Babayan V. K.. 1982. Medium-chain triglycerides: an update. Am. J. Clin. Nutr. 36: 950–962. [DOI] [PubMed] [Google Scholar]
- 22.Papamandjaris A. A., MacDougall D. E., and Jones P. J. H.. 1998. Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci. 62: 1203–1215. [DOI] [PubMed] [Google Scholar]
- 23.Wojtczak L., and Schönfeld P.. 1993. Effect of fatty acids on the energy coupling processes in mitochondria. Biochim. Biophys. Acta. 1183: 41–57. [DOI] [PubMed] [Google Scholar]
- 24.Spector A. A., and Yorek M. A.. 1985. Membrane lipid composition and cellular function. J. Lipid Res. 26: 1015–1035. [PubMed] [Google Scholar]
- 25.Seidelin K. N. 1995. Fatty acid composition of adipose tissue in humans. Implications for the dietary fat-serum cholesterol-CHD issue. Prog. Lipid Res. 34: 199–217. [DOI] [PubMed] [Google Scholar]
- 26.Rauen H. M. 1964. Biochemisches Taschenbuch. Springer, Berlin-Göttingen-Heidelberg. [Google Scholar]
- 27.Mukerjee P. 1965. Dimerization of anions of long-chain fatty acids in aqueous solutions and the hydrophobic properties of the acids. J. Phys. Chem. 69: 2821–2827. [Google Scholar]
- 28.Smith R., and Tanford C.. 1973. Hydrophobicity of long chain n-alkyl carboxylic acids as measured by their distribution between heptane and aqueous solutions. Proc. Natl. Acad. Sci. USA. 70: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evtodienko V. Y., Kovbasnjuk O. N., Antonenko Y. N., and Yaguzhinsky L. S.. 1996. Effect of the alkyl chain length of monocarboxylic acid on the permeation through bilayer lipid membranes. Biochim. Biophys. Acta. 1281: 245–251. [DOI] [PubMed] [Google Scholar]
- 30.Kamp F., and Hamilton J. A.. 2006. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot. Essent. Fatty Acids. 75: 149–159. [DOI] [PubMed] [Google Scholar]
- 31.Apel C. L., Deamer D. W., and Mautner M. N.. 2002. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for encapsulation of biopolymers. Biochim. Biophys. Acta. 1559: 1–9. [DOI] [PubMed] [Google Scholar]
- 32.Small D. M., Cabral D. J., Cistola D. P., Parks J. S., and Hamilton J. A.. 1984. The ionization behavior of fatty acids and bile acids in micelles and membranes. Hepatology. 4(Suppl): 77S–79S. [DOI] [PubMed] [Google Scholar]
- 33.Lieckfeldt R., Villalaín J., Gómez-Fernández J. C., and Lee G.. 1995. Apparent pKa of the fatty acids within ordered mixtures of model human stratum corneum lipids. Pharm. Res. 12: 1614–1617. [DOI] [PubMed] [Google Scholar]
- 34.Miller T. L., and Wolin M. J.. 1996. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 62: 1589–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweiger G., and Buckel W.. 1984. On the dehydration of (R)-lactate in the fermentation of alanine to propionate by Clostridium propionicum. FEBS Lett. 171: 79–84. [DOI] [PubMed] [Google Scholar]
- 36.Counotte G. H., Prins R. A., Janssen R. H. A. M., and Debie M. J. A.. 1981. Role of Megasphaera elsdenii in the fermentation of dl-[2-C]lactate in the rumen of dairy cattle. Appl. Environ. Microbiol. 42: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pryde S. E., Duncan S. H., Hold G. L., Stewart C. S., and Flint H. J.. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 217: 133–139. [DOI] [PubMed] [Google Scholar]
- 38.Cummings J. H., Hill M. J., Bone E. S., Branch W. J., and Jenkins D. J.. 1979. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 32: 2094–2101. [DOI] [PubMed] [Google Scholar]
- 39.Tazoe H., Otomo Y., Kaji I., Tanaka R., Karaki S. I., and Kuwahara A.. 2008. Roles of short-chain fatty acid receptors, GPR41 and GPR43, on colonic functions. J. Physiol. Pharmacol. 59(Suppl. 2): 251–262. [PubMed] [Google Scholar]
- 40.Bloemen J. G., Venema K., van de Poll M. C., Olde Damink S. W., Buurman W. A., and Dejong C. H.. 2009. Short chain fatty acids exchange across the gut and the liver in humans measured at surgery. Clin. Nutr. 28: 657–661. [DOI] [PubMed] [Google Scholar]
- 41.van Beusekom C., Martini I. A., Rutgers H. M., Boersma E. R., and Muskiet F. A.. 1990. A carbohydrate-rich diet not only leads to incorporation of medium-chain fatty acids (6:0-14:0) in milk triglycerides but also in each milk-phospholipid subclass. Am. J. Clin. Nutr. 52: 326–334. [DOI] [PubMed] [Google Scholar]
- 42.Bahrami G., and Rahimi Z.. 2005. Fatty acid composition of human milk in Western Iran. Eur. J. Clin. Nutr. 59: 494–497. [DOI] [PubMed] [Google Scholar]
- 43.Masters C., and Crane D.. 1996. The peroxisome. In Principles of Medical Biology. Vol. 3. E. E. Bittar and N. Bittar, editors. Elsevier, Amsterdam. 39–58. [Google Scholar]
- 44.Hunt M. C., Siponen M. I., and Alexson S. H. E.. 2012. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim. Biophys. Acta. 1822: 1397–1410. [DOI] [PubMed] [Google Scholar]
- 45.Onkenhout W., Venizelos V., van der Poel P. F. H., van den Heuvel M. P. M., and Poorthuis B. J. H. M.. 1995. Identification and quantification of intermediates of unsaturated fatty acid metabolism in plasma of patients with fatty acid oxidation disorders. Clin. Chem. 41: 1467–1474. [PubMed] [Google Scholar]
- 46.Scaini G., Simon K. R., Tonin A. M., Busanello E. N., Moura A. O., Ferreira G. C., Wajner M., Streck E. L., and Schuck P. F.. 2012. Toxicity of octanoate and decanoate in rat peripheral tissues: evidence of bioenergetic dysfunction and oxidative damage induction in liver and skeletal muscle. Mol. Cell. Biochem. 361: 329–335. [DOI] [PubMed] [Google Scholar]
- 47.Gregersen N., Mortensen P. B., and Kølvraa S.. 1983. On the biologic origin of C6-C10-dicarboxylic C6-C10-ω-1-hydroxy monocarboxylic acids in human and rat with acyl-CoA dehydrogenation deficiencies: in vitro studies on the ω- and ω-1-oxidation of medium-chain (C6-C12) fatty acids in human and rat liver. Pediatr. Res. 17: 828–834. [DOI] [PubMed] [Google Scholar]
- 48.Gregersen N. 1985. The acyl-CoA dehydrogenation deficiencies. Recent advances in the enzymic characterization and understanding of the metabolic and pathophysiological disturbances in patients with acyl-CoA dehydrogenation deficiencies. Scand. J. Clin. Lab. Invest. Suppl. 174: 1–60. [PubMed] [Google Scholar]
- 49.Grattagliano I., Lautenburg B. H., Palasciano G., and Portincasa P.. 2010. 13C-Breath tests for clinical investigation of liver mitochondrial function. Eur. J. Clin. Invest. 40: 843–850. [DOI] [PubMed] [Google Scholar]
- 50.Bruno G., Lopetuso L. R., Ianiro G., Laterza L., Gerardi V., Petito V., Poscia A., Gasbarrini A., Ojetti V., and Scaldafferri F.. 2013. 13C-Octanoic acid breath test to study gastric emptying time. Eur. Rev. Med. Pharmacol. Sci. 17(Suppl. 2): 59–64. [PubMed] [Google Scholar]
- 51.Bonfrate L., Grattagliano I., Palsciano G., and Portincasa P.. 2015. Dynamic carbon 13 breath tests for the study of liver function and gastric emptying. Gastroenterol. Rep. (Oxf.). 3: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enerson B. E., and Drewes L. R.. 2003. Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J. Pharm. Sci. 92: 1531–1544. [DOI] [PubMed] [Google Scholar]
- 53.Gonçalves P., and Martel F.. 2013. Butyrate and colorectal cancer: the role of butyrate transport. Curr. Drug Metab. 14: 994–1008. [DOI] [PubMed] [Google Scholar]
- 54.Rajendran V. M., and Binder H. J.. 1994. Apical membrane Cl-butyrate exchange mechanism of short chain fatty acid stimulation of active chloride absorption in rat distal colon. J. Membr. Biol. 141: 51–58. [DOI] [PubMed] [Google Scholar]
- 55.Mascolo N., Rajendran V. M., and Binder H. J.. 1991. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 101: 331–338. [DOI] [PubMed] [Google Scholar]
- 56.Charney A. N., Micic L., and Egnor R. W.. 1998. Nonionic diffusion of short-chain fatty acids across rat colon. Am. J. Physiol. 274: G518–G524. [DOI] [PubMed] [Google Scholar]
- 57.Sellin J. H., and DeSoignie R.. 1990. Short-chain fatty acid absorption in the human colon in vitro. Gastroenterology. 99: 676–683. [DOI] [PubMed] [Google Scholar]
- 58.Demigné C., Yacoub C., and Rémésy C.. 1986. Effects of absorption of large amounts of volatile fatty acids on rat liver metabolism. J. Nutr. 116: 77–86. [DOI] [PubMed] [Google Scholar]
- 59.Ashbrook J. D., Spector A. A., and Fletcher J. E.. 1972. Medium chain fatty acid binding to human plasma albumin. J. Biol. Chem. 247: 7038–7042. [PubMed] [Google Scholar]
- 60.Ashbrook J. D., Spector A. A., Santos E. C., and Fletcher J. E.. 1975. Long chain fatty acid binding to human plasma albumin. J. Biol. Chem. 250: 2333–2338. [PubMed] [Google Scholar]
- 61.Pégorier J. P., Duée P. H., Herbin C., Laulan P. Y., Bladée C., Peret J., and Girard J.. 1988. Fatty acid metabolism in hepatocytes isolated from rats adapted to high-fat diets containing long- or medium-chain triacylglycerols. Biochem. J. 249: 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishizawa R., Masuda K., Sakata S., and Nakatani A.. 2015. Effects of different fatty acid chain lengths on fatty acid oxidation-related protein expression levels in rat skeletal muscles. J. Oleo Sci. 64: 415–421. [DOI] [PubMed] [Google Scholar]
- 63.Atshaves B. P., Martin G. G., Hostetler H. A., McIntosh A. L., Kier A. B., and Schroeder F.. 2010. Liver fatty acid-binding proteins and obesity. J. Nutr. Biochem. 21: 1015–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hajri T., Ibrahimi A., Coburn C. T., Knapp F. F. Jr., Kurtz T., Praveneci M., and Abumrad N. A.. 2001. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J. Biol. Chem. 276: 23661–23666. [DOI] [PubMed] [Google Scholar]
- 65.Irie H., Krukenkamp I. B., Brinkmann J. F. F., Gaudette G. R., Saltman A. E., Jou W., Glatz J. F. C., Abumrad N. A., and Ibrahimi A.. 2003. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc. Natl. Acad. Sci. USA. 100: 6819–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lundquist F., Tygstrup N., Winkler K., Mellemgaard K., and Munck-Petersen S.. 1962. Ethanol metabolism and production of free acetate in the human liver. J. Clin. Invest. 41: 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lundquist F. 1960. The concentration of acetate in blood during alcohol metabolism in man. Acta Physiol. Scand. 175: 97–105. [Google Scholar]
- 68.Seufert C. D., Graf M., Janson G., Kuhn A., and Söling H. D.. 1974. Formation of free acetate by isolated perfused livers from normal, starved and diabetic rats. Biochem. Biophys. Res. Commun. 57: 901–909. [DOI] [PubMed] [Google Scholar]
- 69.Grigat K. P., Koppe K., Seufert C. D., and Söling H. D.. 1979. Acetyl-coenzyme A deacylase activity in liver is not an artefact. Subcellular distribution and substrate specificity of acetyl-coenzyme A deacylase activities in rat liver. Biochem. J. 177: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamashita H., Kaneyuki T., and Tagawa K.. 2001. Production of acetate in the liver and its utilization in peripheral tissues. Biochim. Biophys. Acta. 1532: 79–87. [DOI] [PubMed] [Google Scholar]
- 71.Groot P. H. E. 1975. The activation of short-chain fatty acids by the soluble fraction of guinea-pig heart and liver mitochondria. The search for distinct propionyl-CoA synthase. Biochim. Biophys. Acta. 380: 12–20. [DOI] [PubMed] [Google Scholar]
- 72.Scholte H. R., and Groot P. H. E.. 1975. Organ and intracellular localization of short-chain acyl-CoA synthetases in rat and guinea-pig. Biochim. Biophys. Acta. 409: 283–296. [DOI] [PubMed] [Google Scholar]
- 73.Fujino T., Kondo J., Ishikawa M., Morikawa K., and Yamamoto T. T.. 2001. Acetyl-CoA synthase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 276: 11420–11426. [DOI] [PubMed] [Google Scholar]
- 74.Barth C., Sladek M., and Decker K.. 1971. The subcellular distribution of short-chain acyl-CoA synthetase activity in rat tissues. Biochim. Biophys. Acta. 248: 24–33. [DOI] [PubMed] [Google Scholar]
- 75.Goldberg R. P., and Brunengraber H.. 1980. Contribution of cytosolic and mitochondrial acetyl-CoA synthetases to the activation of lipogenic acetate in rat liver. Adv. Exp. Med. Biol. 132: 413–418. [DOI] [PubMed] [Google Scholar]
- 76.Endemann G., Goetz P. G., Tomera J. F., Rand W. M., Desrochers S., and Brunengraber H.. 1987. Lipogenesis from ketone bodies in the perfused rat liver: effects of acetate and ethanol. Biochem. Cell Biol. 65: 989–996. [DOI] [PubMed] [Google Scholar]
- 77.Sakakibara I., Fujino T., Ishii M., Tanaka T., Shimosawa T., Miura S., Zhang W., Tokutake Y., Yamamoto J., Awano M., et al. 2009. Fasting-induced hypothermia and reduced energy production in mice lacking acetyl-CoA synthetase 2. Cell Metab. 9: 191–202. [DOI] [PubMed] [Google Scholar]
- 78.Shimazu T., Hirschey M. D., Huang J-Y., Ho L. T. Y., and Verdin E.. 2010. Acetate metabolism and aging: An emerging connection. Mech. Ageing Dev. 131: 511–516. [DOI] [PubMed] [Google Scholar]
- 79.Aas M., and Bremer J.. 1968. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim. Biophys. Acta. 164: 157–166. [DOI] [PubMed] [Google Scholar]
- 80.Vessey D. A., Kelley M., and Warren R. S.. 1999. Characterization of the CoA ligases of human liver mitochondria catalyzing the activation of short- and medium-chain fatty acids and xenobiotic carboxylic acids. Biochim. Biophys. Acta. 1428: 455–462. [DOI] [PubMed] [Google Scholar]
- 81.Boomgaarden I., Vock C., Klapper M., and Döring F.. 2009. Comparative analyses of disease risk genes belonging to the acyl-CoA synthetase medium-chain (ACSM) family in human liver and cell lines. Biochem. Genet. 47: 739–748. [DOI] [PubMed] [Google Scholar]
- 82.Kasumov T., Adamas J. E., Bian F., David F., Thomas K. R., Jobbins K. A., Minkler P. E., Hoppel C. L., and Brunengraber H.. 2005. Probing peroxisomal β-oxidation and the labelling of acetyl-CoA proxies with [1-13C]octanoate and [3-13C]octanoate in the perfused rat liver. Biochem. J. 389: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bian F., Kasumov T., Thomas K. R., Jobbins K. A., David F., Minkler P. E., Hoppel C. L., and Brunengraber H.. 2005. Peroxisomal and mitochondrial oxidation of fatty acids in the heart, assessed from the 13C labeling of malonyl-CoA and the acetyl moiety of citrate. J. Biol. Chem. 280: 9265–9271. [DOI] [PubMed] [Google Scholar]
- 84.Jeppesen P. B., and Mortensen P. B.. 1999. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. JPEN J. Parenter. Enteral Nutr. 23: S101–S105. [DOI] [PubMed] [Google Scholar]
- 85.Hecker M., Sommer N., Voigtmann H., Pal O., Mor A., Wolf M., Vadasz I., Herold S., Weissmann N., Morty R. E., et al. 2014. Impact of short- and medium-chain fatty acids on mitochondrial function in severe inflammation. JPEN J. Parenter. Enteral Nutr. 38: 587–594. [DOI] [PubMed] [Google Scholar]
- 86.Zurier R. B., Campbell R. G., Hashim S. A., and van Itallie T. B.. 1967. Enrichment of depot fat with odd and even numbered medium chain fatty acids. Am. J. Physiol. 212: 291–294. [DOI] [PubMed] [Google Scholar]
- 87.Labarthe F., Gélinas R., and Des Rosiers C.. 2008. Medium-chain fatty acids as metabolic therapy in cardiac disease. Cardiovasc. Drugs Ther. 22: 97–106. [DOI] [PubMed] [Google Scholar]
- 88.Lemarié F., Beauchamp E., Legrand P., and Rioux V.. 2016. Revisiting the metabolism and physiological functions of caprylic acid (C8:0) with special focus on ghrelin octanoylation. Biochimie. 120: 40–48. [DOI] [PubMed] [Google Scholar]
- 89.Debeer L. J., Mannaerts G., and de Scheppe P. J.. 1974. Effects of octanoate and oleate on energy metabolism in the perfused rat liver. Eur. J. Biochem. 47: 591–600. [DOI] [PubMed] [Google Scholar]
- 90.Kingsley-Hickman P. B., Sako E. Y., Uğurbil K., From A. H. L., and Foker J. E.. 1990. 31P NMR measurement of mitochondrial uncoupling in isolated rat hearts. J. Biol. Chem. 265: 1545–1550. [PubMed] [Google Scholar]
- 91.Scholz R., Schwabe U., and Soboll S.. 1984. Influence of fatty acids on energy metabolism. 1. Stimulation of oxygen consumption, ketogenesis and CO2 production following addition of octanoate and oleate in perfused rat liver. Eur. J. Biochem. 141: 223–230. [DOI] [PubMed] [Google Scholar]
- 92.Soboll S., Gründel S., and Scholz R.. 1984. Influence of fatty acids on energy metabolism. 2. Kinetics of changes in metabolic rates and changes in subcellular adenine nucleotide contents and pH gradients following addition of octanoate and oleate in perfused rat liver. Eur. J. Biochem. 141: 231–236. [DOI] [PubMed] [Google Scholar]
- 93.Walton M. E., Ebert D., and Haller R. G.. 2003. Octanoate oxidation measured by 13C-NMR spectroscopy in rat skeletal muscle, heart, and liver. J. Appl. Physiol. 95: 1908–1916. [DOI] [PubMed] [Google Scholar]
- 94.Ebert D., Haller R. G., and Walton M. E.. 2003. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 23: 5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spector R. 1988. Fatty acid transport through the blood-brain barrier. J. Neurochem. 50: 639–643. [DOI] [PubMed] [Google Scholar]
- 96.Beauvieux M-C., Tissier P., Gin H., Canioni P., and Gallis J-L.. 2001. Butyrate impairs energy metabolism in isolated perfused liver of fed rats. J. Nutr. 131: 1986–1992. [DOI] [PubMed] [Google Scholar]
- 97.Gallis J-L., Tissier P., Gin H., and Beauvieux M-C.. 2007. Decrease in oxidative phosphorylation yield in presence of butyrate in perfused liver isolated from fed rats. BMC Physiol. 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gallis J-L., Gin H., Roumes H., and Beauvieux M-C.. 2011. A metabolic link between mitochondrial ATP synthesis and liver glycogen metabolism: NMR study in rats re-fed with butyrate and/or glucose. Nutr. Metab. (Lond). 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Plomp P. J. A. M., van Roermund C. W. T., Groon A. K., Meijer A. J., and Tager J. M.. 1985. Mechanism of the stimulation of respiration by fatty acids in rat liver. FEBS Lett. 193: 243–246. [DOI] [PubMed] [Google Scholar]
- 100.Schönfeld P., Wojtczak A. B., Geelen M. J. H., Kunz W., and Wojtczak L.. 1988. On the mechanism of the so-called uncoupling effect of medium- and short-chain fatty acids. Biochim. Biophys. Acta. 936: 280–288. [DOI] [PubMed] [Google Scholar]
- 101.Nobes C. D., Hay W. W. Jr., and Brand M. D.. 1990. The mechanism of stimulation of respiration by fatty acids in isolated hepatocytes. J. Biol. Chem. 265: 12910–12915. [PubMed] [Google Scholar]
- 102.Hassinen I., Ito K., Nioka S., and Chance B.. 1990. Mechanism of fatty acid effect on myocardial oxygen consumption. A phosphorus NMR study. Biochim. Biophys. Acta. 1019: 73–80. [DOI] [PubMed] [Google Scholar]
- 103.Turner N., Hariharan K., TidAng J., Frangioudakis G., Beale S. M., Wright L. E., Zeng X. Y., Leslie S. J., Li J. Y., Kraegen E. W., et al. 2009. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: potent tissue-specific effects of medium-chain fatty acids. Diabetes. 58: 2547–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saudubray J. M., Martin D. E., de Lonlay P., Touati G., Poggi-Travert F., Bonnet D., Jouvet P., Boutron M., Slama A., Vianey-Saban C., et al. 1999. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J. Inherit. Metab. Dis. 22: 488–502. [DOI] [PubMed] [Google Scholar]
- 105.Roe C. R., Sweetman L., Roe D. S., David F., and Brunengraber H.. 2002. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J. Clin. Invest. 110: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Metges C. C., and Wolfram G.. 1991. Medium- and long-chain triglycerides labelled with 13C: A comparison of oxidation after oral or parenteral administration in humans. J. Nutr. 121: 31–36. [DOI] [PubMed] [Google Scholar]
- 107.Nomura T., Iguchi A., Sakamoto N., and Harris R. A.. 1983. Effects of octanoate and acetate upon hepatic glycolysis and lipogenesis. Biochim. Biophys. Acta. 754: 315–320. [PubMed] [Google Scholar]
- 108.Morand C., Besson C., Demigne C., and Remesy C.. 1994. Importance of the modulation of glycolysis in the control of lactate metabolism by fatty acids in isolated hepatocytes from fed rats. Arch. Biochem. Biophys. 309: 254–260. [DOI] [PubMed] [Google Scholar]
- 109.González-Manchón C., Ayuso M. S., and Parrilla R.. 1989. Control of hepatic gluconeogenesis: Role of fatty acid oxidation. Arch. Biochem. Biophys. 271: 1–9. [DOI] [PubMed] [Google Scholar]
- 110.Winiarska K., Drożak J., Węgrzynowicz M., Jagielski A. K., and Bryła J.. 2003. Relationship between gluconeogenesis and glutathione redox state in rabbit kidney-cortex tubules. Metabolism. 52: 739–746. [DOI] [PubMed] [Google Scholar]
- 111.Agius L., and Alberti G. M. M.. 1985. Regulation of flux through pyruvate dehydrogenase and pyruvate carboxylase in rat hepatocytes. Eur. J. Biochem. 152: 699–707. [DOI] [PubMed] [Google Scholar]
- 112.Geelen M. J. 1994. Medium-chain fatty acids as short-term regulators of hepatic lipogenesis. Biochem. J. 302: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thevenet J., DeMarchi U., Domingo J. S., Christinat N., Bultot L., Lefebvre G., Sakamoto K., Descombes P., Masoodi M., and Wiederkehr A.. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J. Epub ahead of print. February 2, 2016; doi:10.1096/fj.201500182. [DOI] [PubMed] [Google Scholar]
- 114.Williamson J. R., Browing E. T., and Olson M. S.. 1968. Interrelationships between fatty acid oxidation and the control of gluconeogenesis in perfused rat liver. Adv. Enzyme Regul. 6: 67–100. [DOI] [PubMed] [Google Scholar]
- 115.Hu G-X., Chen G-R., Xu H., Ge R-S., and Lin J.. 2010. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypotheses. 74: 123–126. [DOI] [PubMed] [Google Scholar]
- 116.Takikawa M., Kumagai A., Hirata H., Soga M., Yamashita Y., Ueda M., Ashida H., and Tsuda T.. 2013. 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5′-AMP-activated protein kinase in L6 myotubes and mice. Mol. Nutr. Food Res. 57: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 117.Guo W., Lei T., Corkey B. E., and Han J.. 2003. Octanoate inhibits triglyceride synthesis in 3T3-LI and human adipocytes. J. Nutr. 133: 2512–2518. [DOI] [PubMed] [Google Scholar]
- 118.Beauvieux M. C., Roumes H., Robert N., Gin H., Rigalleau V., and Gallis J. L.. 2008. Butyrate ingestion improves hepatic glycogen storage in the re-fed rat. BMC Physiol. 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okere I. C., McElfresh T. A., Brunengraber D. Z., Martini W., Sterk J. P., Huang H., Chandler M. P., Brunengraber H., and Stanley W. C.. 2006. Differential effects of heptanoate and hexanoate on myocardial citric acid cycle intermediates following ischemia-reperfusion. J. Appl. Physiol. 100: 76–82. [DOI] [PubMed] [Google Scholar]
- 120.Kajimoto M., Ledee D. R., Olson A. K., Isern N. G., Des Rosiers C., and Portman M. P.. 2015. Differential effects of octanoate and heptanoate on myocardial metabolism during extracorporal membrane oxygenation in infant swine model. Am. J. Physiol. Heart Circ. Physiol. 309: H1157–H1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brunengraber H., and Roe C. R.. 2006. Anaplerotic molecules: current and Future. J. Inherit. Metab. Dis. 29: 327–331. [DOI] [PubMed] [Google Scholar]
- 122.Marin-Valencia I., Good L. B., Ma Q., Malloy C. R., and Pascual J. M.. 2013. Heptanoate as neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J. Cereb. Blood Flow Metab. 33: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mochel F., DeLonlay P., Touati G., Brunengraber H., Kinman R. P., Rabier D., Roe C. R., and Saudubray J. M.. 2005. Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol. Genet. Metab. 84: 305–312. [DOI] [PubMed] [Google Scholar]
- 124.Willis S., Stoll J., Sweetman L., and Borges K.. 2010. Anticonvulsant effects of triheptanoin diet in two mouse chronic seizure models. Neurobiol. Dis. 40: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borges K., and Sonnewald U.. 2012. Triheptanoin–a medium-chain triglyceride with odd chain fatty acids: a new anaperotic anticonvulsant treatment. Epilepsy Res. 100: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hadera M. G., Smeland O. B., McDonald T. S., Ni Tan K., Sonnewald U., and Borges K.. 2014. Triheptanoin partially restores levels of tricarboxylic cycle intermediates in the mouse pilocarpine model of epilepsy. J. Neurochem. 129: 107–119. [DOI] [PubMed] [Google Scholar]
- 127.Schwarzkopf T. M., Koch K., and Klein J.. 2015. Reduced severity of ischemic stroke and improvement of mitochondrial function after dietary treatment with the anaplerotic substance triheptanoin. Neuroscience. 300: 201–209. [DOI] [PubMed] [Google Scholar]
- 128.Al-Lahham S. H., Peppelenbosch M. P., Roelofsen H., Vonk R. J., and Venema K.. 2010. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta. 1801: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 129.Skulachev V. P. 1991. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 294: 158–162. [DOI] [PubMed] [Google Scholar]
- 130.Skulachev V. P. 1999. Anion carriers in fatty acid-mediated physiological uncoupling. J. Bioenerg. Biomembr. 31: 431–445. [DOI] [PubMed] [Google Scholar]
- 131.Wojtczak L., and Więckowski M. R.. 1999. The mechanisms of fatty acid-induced proton permeability of the inner mitochondrial membrane. J. Bioenerg. Biomembr. 31: 447–455. [DOI] [PubMed] [Google Scholar]
- 132.Bernardi P., Penzo D., and Wojtczak L.. 2002. Mitochondrial energy dissipation by fatty acids: Mechanisms and implications for cell death. Vitam. Horm. 65: 97–126. [DOI] [PubMed] [Google Scholar]
- 133.Feldkamp T., Weinberg J. M., Hörbelt M., von Kropff C., Witzke O., Nürnberger J., and Kribben A.. 2009. Evidence for involvement of nonesterified fatty acid protonophoric uncoupling during mitochondrial dysfunction caused by hypoxia and reoxygenation. Nephrol. Dial. Transplant. 24: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cole M. A., Murray A. J., Cochlin L. E., Heather L. C., McAleese S., Knight N. S., Sutton E., Jamil A. A., Parassol N., and Clarke K.. 2011. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res. Cardiol. 106: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schönfeld P., Schild L., and Kunz W.. 1989. Long-chain fatty acids act as protonophoric uncouplers of oxidative phosphorylation in rat liver mitochondria. Biochim. Biophys. Acta. 977: 266–272. [DOI] [PubMed] [Google Scholar]
- 136.Shabalina I. G., Backlund W. C., Bar-Tana J., Cannon B., and Nedergaard J.. 2008. Within brown-fat cells, UCP1-mediated fatty acid-induced uncoupling is independent of fatty acid metabolism. Biochim. Biophys. Acta. 1777: 642–650. [DOI] [PubMed] [Google Scholar]
- 137.Walter A., and Gutknecht J.. 1984. Monocarboxylic permeation through lipid bilayer membrane. J. Membr. Biol. 77: 255–264. [DOI] [PubMed] [Google Scholar]
- 138.Woldegiorgis G., Shrago E., Gipp J., and Yatvin M.. 1981. Fatty acyl coenzyme A-sensitive adenine nucleotide transport in a reconstituted liposome system. J. Biol. Chem. 256: 12297–12300. [PubMed] [Google Scholar]
- 139.Rabkin M., and Blum J. J.. 1985. Quantitative analysis of intermediary metabolism in hepatocytes incubated in the presence and absence of glucagon with a substrate mixture containing glucose, ribose, fructose, alanine and acetate. Biochem. J. 225: 761–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crabtree B., Souter M. J., and Anderson S. E.. 1989. Evidence that the production of acetate in rat hepatocytes is a predominantly cytoplasmic process. Biochem. J. 257: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crabtree B., Gordon M. J., and Christie S. L.. 1990. Measurement of the rates of acetyl-CoA hydrolysis and synthesis from acetate in rat hepatocytes and the role of these fluxes in substrate cycling. Biochem. J. 270: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akerboom T. P. M., Bookelman H., Zuurendonk P. F., van den Meer R., and Tager J. M.. 1978. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur. J. Biochem. 84: 413–420. [DOI] [PubMed] [Google Scholar]
- 143.Otto D. A. 1984. Relationship of the ATP/ADP ratio to the site of octanoate activation. J. Biol. Chem. 259: 5490–5494. [PubMed] [Google Scholar]
- 144.Lutz W. H., Geisbuhler T. P., Pollack J. D., McClung H. J., and Merola A. J.. 1985. Inhibition of citrulline synthesis by octanoate and its modulation by adenine nucleotides. Biochem. Med. 34: 1–10. [DOI] [PubMed] [Google Scholar]
- 145.Schönfeld P., and Bohnensack R.. 1991. Intramitochondrial fatty acid activation enhances control strength of the adenine nucleotide translocase. Biomed. Biochim. Acta. 50: 841–849. [PubMed] [Google Scholar]
- 146.Baba N., Bracco E. F., and Hashim S. A.. 1982. Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with diet containing medium chain triglyceride. Am. J. Clin. Nutr. 35: 678–682. [DOI] [PubMed] [Google Scholar]
- 147.St-Onge M. P., Ross R., Parsons W. D., and Jones P. J. H.. 2003. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes. Res. 11: 395–402. [DOI] [PubMed] [Google Scholar]
- 148.St-Onge M. P., and Bosarge A.. 2008. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am. J. Clin. Nutr. 87: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Montgomery M. K., Osborne B., Brown S. H., Small L., Mitchell T. W., Cooney G. J., and Turner N.. 2013. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J. Lipid Res. 54: 3322–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hoeks J., Mensink M., Hesselink M. K. C., Ekroos K., and Schrauwen P.. 2012. Long- and medium-chain fatty acids induce insulin resistance to a similar extent in humans despite marked differences in muscle fat accumulation. J. Clin. Endocrinol. Metab. 97: 208–216. [DOI] [PubMed] [Google Scholar]
- 151.Corkey B. E., Hale D. E., Glennon M. C., Kelley R. I., Coates P. M., Kilpatrick L., and Stanley C. A.. 1988. Relationship between unusual hepatic acyl coenzyme A profiles and the pathogenesis of Reye syndrome. J. Clin. Invest. 82: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sauer S. W., Okub J. G., Hoffmann G. F., Koelker S., and Morath M. A.. 2008. Impact of short- and medium-chain organic acids, acylcarnitines, and acyl-CoAs on mitochondrial energy metabolism. Biochim. Biophys. Acta. 1777: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 153.St-Pierre J., Buckingham J. A., Roebuck S. J., and Brand M. D.. 2002. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277: 44784–44790. [DOI] [PubMed] [Google Scholar]
- 154.Hoffman D. L., and Brookes P. S.. 2009. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J. Biol. Chem. 284: 16236–16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seifert E. L., Estey C., Xuan J. Y., and Harper M. E.. 2010. Electron transport chain-dependent and independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J. Biol. Chem. 285: 5748–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schönfeld P., Więckowski M. R., Lebiedzińska M., and Wojtczak L.. 2010. Mitochondrial fatty acid oxidation and oxidative stress: Lack of reverse electron transfer-associated production of reactive oxygen species. Biochim. Biophys. Acta. 1797: 929–938. [DOI] [PubMed] [Google Scholar]
- 157.Perevoshchikova I. V., Quinlan C. L., Orr A. L., Gerencser A. A., and Brand M. D.. 2013. Sites of superoxide and hydrogen peroxide production during fatty acid oxidation in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 61: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schönfeld P., and Wojtczak L.. 2012. Brown adipose tissue mitochondria oxidizing fatty acids generate high levels of reactive oxygen species irrespective of the uncoupling protein-1 activity state. Biochim. Biophys. Acta. 1817: 410–418. [DOI] [PubMed] [Google Scholar]
- 159.Murray A. J., Knight N. S., Little S. E., Cochlin L. E., Clements M., and Clarke K.. 2011. Dietary long-chain, but not medium-chain triglycerides, impair exercise performance and uncouple cardiac mitochondria in rats. Nutr. Metab. (Lond). 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schönfeld P., and Wojtczak L.. 2008. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 45: 231–241. [DOI] [PubMed] [Google Scholar]
- 161.Schuck P. F., Ferreira G. C., Moura A. P., Busanello E. N. B., Tonin A. M., Dutra-Filho C. S., and Wajner M.. 2009. Medium-chain fatty acids accumulating in MCAD deficiency elicit lipid and protein oxidative damage and decrease non-enzymatic antioxidant defenses in rat brain. Neurochem. Int. 54: 519–525. [DOI] [PubMed] [Google Scholar]