Abstract
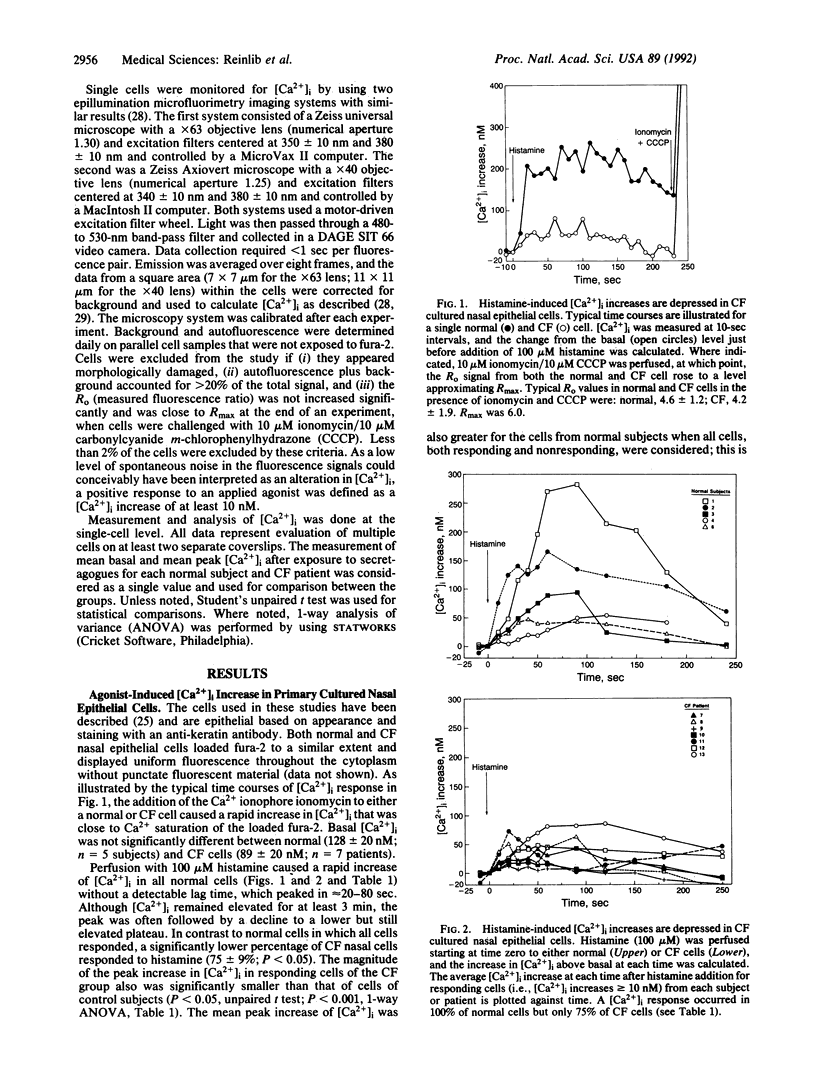
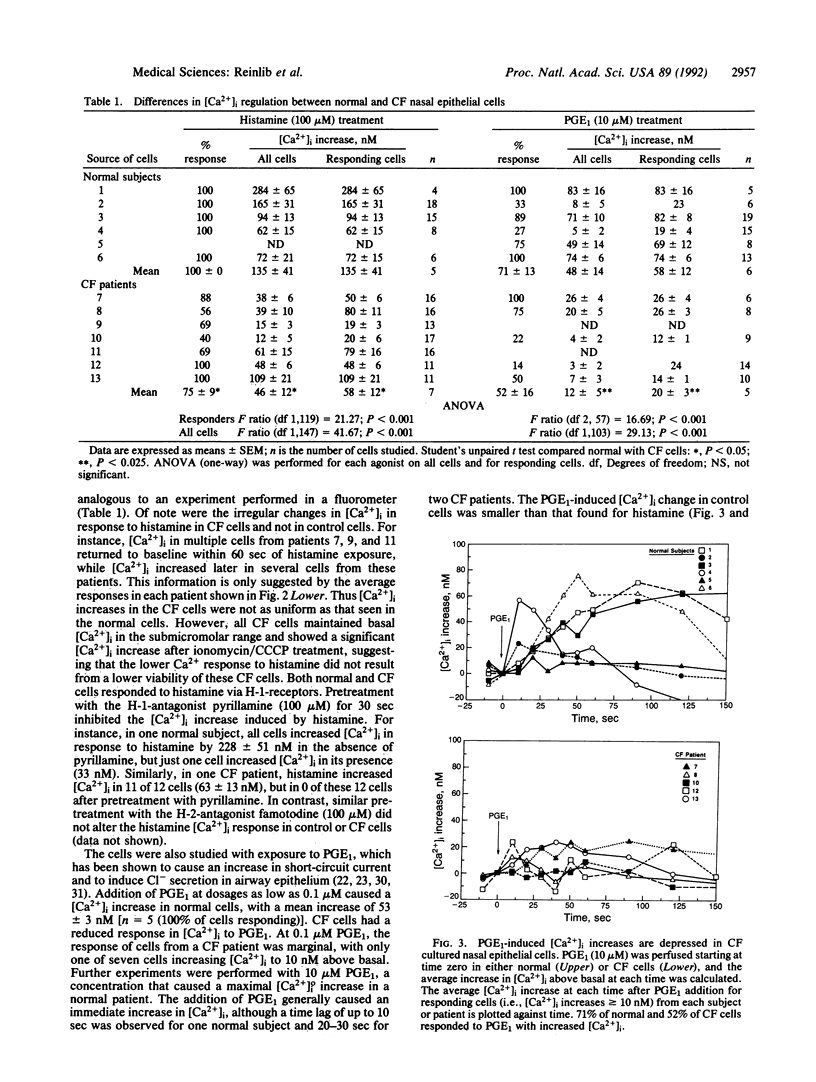
These studies identify a further abnormality in cystic fibrosis (CF). The increase in intracellular free calcium concentration ([Ca2+]i) after exposure to histamine and PGE1 is demonstrated to be abnormally low in nasal cells, studied in short-term culture, from patients with CF compared with control subjects. [Ca2+]i is measured by using the Ca(2+)-sensitive fluorescent dye fura-2 and a fluorescence microscope imaging system. The percentage of CF cells that increase [Ca2+]i in response to histamine is decreased compared with controls, and, even in those CF cells that increase [Ca2+]i, the magnitude of the increase in [Ca2+]i in response to histamine is smaller than in controls. When exposed to PGE1, a similar number of control and CF cells responded with an increase in [Ca2+]i, but again the magnitude of the response was smaller in the CF cells. The mechanism of the PGE1-induced increase in [Ca2+]i is not mediated by cAMP, since 8-bromo-cAMP failed to increase [Ca2+]i in these cells. This abnormality in [Ca2+]i response did not apply to all secretagogues, with the response to carbachol being similar in CF and normal cells. How the abnormal CF gene product accounts for the abnormality in intracellular Ca2+ response to some but not all secretagogues is unknown.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Berschneider H. M., Knowles M. R., Azizkhan R. G., Boucher R. C., Tobey N. A., Orlando R. C., Powell D. W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988 Jul;2(10):2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Cheng E. H., Paradiso A. M., Stutts M. J., Knowles M. R., Earp H. S. Chloride secretory response of cystic fibrosis human airway epithelia. Preservation of calcium but not protein kinase C- and A-dependent mechanisms. J Clin Invest. 1989 Nov;84(5):1424–1431. doi: 10.1172/JCI114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Knowles M. R., Cantley L., Gatzy J. T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986 Nov;78(5):1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrini G., De Togni P. Increased cytosolic calcium in cystic fibrosis neutrophils effect on stimulus-secretion coupling. Life Sci. 1985 Apr 22;36(16):1561–1567. doi: 10.1016/0024-3205(85)90380-7. [DOI] [PubMed] [Google Scholar]
- Cheng P. W., Boat T. F., Cranfill K., Yankaskas J. R., Boucher R. C. Increased sulfation of glycoconjugates by cultured nasal epithelial cells from patients with cystic fibrosis. J Clin Invest. 1989 Jul;84(1):68–72. doi: 10.1172/JCI114171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Corrales R. J., Coleman D. L., Jacoby D. B., Leikauf G. D., Hahn H. L., Nadel J. A., Widdicombe J. H. Ion transport across cat and ferret tracheal epithelia. J Appl Physiol (1985) 1986 Sep;61(3):1065–1070. doi: 10.1152/jappl.1986.61.3.1065. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Nash N. T., al-Bazzaz F., Layden T. J., Rao M. C. Rectum has abnormal ion transport but normal cAMP-binding proteins in cystic fibrosis. Am J Physiol. 1988 May;254(5 Pt 1):C719–C724. doi: 10.1152/ajpcell.1988.254.5.C719. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jefferson D. M., Valentich J. D., Marini F. C., Grubman S. A., Iannuzzi M. C., Dorkin H. L., Li M., Klinger K. W., Welsh M. J. Expression of normal and cystic fibrosis phenotypes by continuous airway epithelial cell lines. Am J Physiol. 1990 Dec;259(6 Pt 1):L496–L505. doi: 10.1152/ajplung.1990.259.6.L496. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Keulemans J., Van Heyningen V., Scholte B. J., Bijman J., van der Kamp A. W., Kansen M., De Jonge H., Galjaard H., Hoogeveen A. T. Cultured epithelial cells from patients with cystic fibrosis have an increased expression of the 14 kDa Ca2(+)-binding protein CFA. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1281–1286. doi: 10.1016/0006-291x(91)91560-y. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Orlando R. C., Powell D. W., Croom R. D., Berschneider H. M., Boucher R. C., Knowles M. R. Colonic and esophageal transepithelial potential difference in cystic fibrosis. Gastroenterology. 1989 Apr;96(4):1041–1048. doi: 10.1016/0016-5085(89)91621-1. [DOI] [PubMed] [Google Scholar]
- Reinlib L., Mikkelsen R., Zahniser D., Dharmsathaphorn K., Donowitz M. Carbachol-induced cytosolic free Ca2+ increases in T84 colonic cells seen by microfluorimetry. Am J Physiol. 1989 Dec;257(6 Pt 1):G950–G960. doi: 10.1152/ajpgi.1989.257.6.G950. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Schöni M. H., Schöni-Affolter F., Jeffery D., Katz S. Intracellular free calcium levels in mononuclear cells of patients with cystic fibrosis and normal controls. Cell Calcium. 1987 Feb;8(1):53–63. doi: 10.1016/0143-4160(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Shapiro B. L. Evidence for a mitochondrial lesion in cystic fibrosis. Life Sci. 1989;44(19):1327–1334. doi: 10.1016/0024-3205(89)90389-5. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Welsh M. J., Stoff J. S., Frizzell R. A. Chloride secretion by canine tracheal epithelium: I. Role of intracellular c AMP levels. J Membr Biol. 1982;70(3):217–226. doi: 10.1007/BF01870564. [DOI] [PubMed] [Google Scholar]
- Suter S., Lew P. D., Ballaman J., Waldvogel F. A. Intracellular calcium handling in cystic fibrosis: normal cytosolic calcium and intracellular calcium stores in neutrophils. Pediatr Res. 1985 Apr;19(4):346–348. doi: 10.1203/00006450-198519040-00006. [DOI] [PubMed] [Google Scholar]
- Tallant E. A., Wallace R. W. Altered binding of 125I-labeled calmodulin to a 46.5-kilodalton protein in skin fibroblasts cultured from patients with cystic fibrosis. J Clin Invest. 1987 Feb;79(2):643–648. doi: 10.1172/JCI112861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Baxter P. S., Hardcastle J., Hardcastle P. T. Absence of secretory response in jejunal biopsy samples from children with cystic fibrosis. Lancet. 1987 Jul 11;2(8550):107–108. doi: 10.1016/s0140-6736(87)92781-4. [DOI] [PubMed] [Google Scholar]
- Taylor C. J., Baxter P. S., Hardcastle J., Hardcastle P. T. Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut. 1988 Jul;29(7):957–962. doi: 10.1136/gut.29.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek E., de Jonge H. R., Bijman J., Keulemans J., Sinaasappel M., van der Kamp A. W., Scholte B. J. Chloride transport in cultured nasal epithelium of cystic fibrosis patients. Pflugers Arch. 1990 Feb;415(5):540–546. doi: 10.1007/BF02583504. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Cystic fibrosis. Acidification indication. Nature. 1991 Jul 4;352(6330):23–24. doi: 10.1038/352023b0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Welsh M. J. Ion transport by dog tracheal epithelium. Fed Proc. 1980 Nov;39(13):3062–3066. [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol. 1989 Feb;256(2 Pt 1):C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]



