Abstract
Background:
Effects of platelet-derived growth factor B (PDGF-B) on peripheral nerve regeneration was studied using a rat sciatic nerve transection model.
Materials and Methods:
Forty-five male, white Wistar rats were divided into three experimental groups (n = 15), randomly: Normal control group (NC), silicon group (SIL), and PDGF-B treated group (SIL/PDGF). In NC group, left sciatic nerve was exposed through a gluteal muscle incision and after homeostasis muscle was sutured. In the SIL group, the left sciatic nerve was exposed in the same way and transected proximal to tibio-peroneal bifurcation leaving a 10-mm gap. Proximal and distal stumps were each inserted into a silicone conduit and filled with 10 μL phosphate buffered solution. In SIL/PDGF group, the silicon conduit was filled with 10 μL PDGF-B (0.5 ng/mL). Each group was subdivided into three subgroups of five and were studied in 4, 8, 12 weeks after surgery.
Results:
Behavioral testing, sciatic nerve functional study, gastrocnemius muscle mass, and histomorphometric studies showed earlier regeneration of axons in SIL/PDGF than in SIL group (P < 0.05).
Conclusion:
Local administration of PDGF-B combined with silicon grafting could accelerate functional recovery and may have clinical implications for the surgical management of patients after facial nerve transection.
Keywords: Conduit, functional recovery, local, nerve repair, platelet-derived growth factor, sciatic
INTRODUCTION
The need to achieve a tension-free repair in peripheral nerve surgery has led to the accepted standard of nerve grafting. However, the associated morbidities and suboptimal clinical results provide a compelling reason to search for alternatives. Inert conduits avoid both the problems inherent in harvesting a nerve for autograft and the potential nerve compression and irritation found with some resorbable synthetic grafts. Allowing to grow through conduits, and thus to be subject to the neurotrophic guidance cues that collect there, may ultimately allow more precise alignment of fascicles and thus better functional recovery in comparison to primary nerve repair or nerve autograft. These conduits appear to offer significant advantages including improved regeneration when compared with traditional repair and are currently available for use clinically. Incorporating neurotropic and neurotrophic factors into conduits are encouraging and likely playing a prominent role in peripheral nerve repair.[1]
Regenerating axons react to a host of molecular cues in the local environment. Several attempts have been made to overcome the length restrictions inherent in other synthetic tubes using tissue engineering to combine the advantages of neurotrophic and neurotropic substances that are known to support or enhance axonal growth with the benefits of tubulization. Combining synthetic tubes with biological factors known to encourage and hasten nerve growth an active part of research. Several neurotrophic factors such as brain-derived neurotrophic factor, nerve growth factor, basic fibroblast growth factors-1 and 2, neurotrophin-3, ciliary neurotrophic factor, insulin like growth factor-1, interlukin-1 and transforming growth factor have demonstrated capability to promote axonal growth in vitro.[2]
Neurotrophic factors are a family of growth factors that support and influence the growth and regenerative capacity of neurons. These substances are produced by a number of tissues during development and direct the formation of the brain and spinal cord and their connections to target organs such as muscle.[3,4,5] It has already been suggested that platelet-derived growth factor (PDGF) plays an important role in both the developing and mature mammalian central nervous system (CNS), although PDGF is thought primarily to be a mitogen for mesenchymally derived cells. PDGF and its receptors have been found to be expressed abundantly in both the central and peripheral nervous system.[6,7,8,9] PDGF is a growth regulatory molecule with diverse functions that play critical roles in the development of kidney and neural crest-derived cells.[10,11] PDGF exerts activity by binding to specific high-affinity cell surface receptors. Two receptor subunits have been identified that can form mature dimeric receptor complexes: α-subunit, which can bind to the A- or B- and C-chain of PDGF; and the β-subunit, which can bind to the B-, C- and D-chain.[12,13,14,15,16,17]
To the best knowledge of the authors, the literature is poor regarding the local effect of PDGF on transected nerve repair in vivo. Aimed to study local effects of PDGF on nerve regeneration, a study was designed to attempt to determine if PDGF do in fact reduce dysfunction after nerve injury in the rat sciatic nerve transection model. Assessment of the nerve regeneration was based on behavioral, functional (walking track analysis) and muscle mass measurement and histopathological criteria within 12 weeks after surgery.
MATERIALS AND METHODS
Experimental design
Forty-five male, white Wistar rats weighing approximately 280 g were divided into three experimental groups (n = 15), randomly: Normal control group (NC), silicone control group (SIL), and PDGF-treated group (SIL/PDGF). Each group was again subdivided into three subgroups of five and surveyed in 4, 8, and 12 weeks. Two weeks before and during the entire experiments, the animals were housed in individual plastic cages with an ambient temperature of 23°C ± 3°C, stable air humidity, and a natural day/night cycle. The rats had free access to standard rodent laboratory food and tap water.
Grafting procedure
Animals were anesthetized by intraperitoneal administration of ketamine-xylazine (ketamine 5%, 90 mg/kg and xylazine 2%, 5 mg/kg). All procedures followed a standard microsurgery technique under a magnifying lenses (BIO-ART EQUIPMENTOS ODONTOLOGICOS LTDA, Sao Carlos/SP- Brasil). The procedures were carried out based on the guidelines of the Ethics Committee of the International Association for the Study of Pain.[18] The University Research Council approved all experiments.
Following surgical preparation in the NC group the left sciatic nerve was exposed through a gluteal muscle incision and after careful hemosthasis, the muscle was sutured with resorbable 4/0 sutures and the skin with 3/0 nylon. In the SIL group, the left sciatic nerve was exposed through a gluteal muscle incision and transected proximal to the tibio-peroneal bifurcation where a 7 mm segment was excised, leaving a gap about 10 mm due to retraction of nerve ends. Proximal and distal stumps were each inserted 2 mm into a silicone tube, and two 10/0 nylon sutures were placed at each end of the cuff to fix the tube in place and to leave a 10-mm gap between the stumps. The conduit was filled with 10 μL the phosphate buffered saline and sterile Vaseline was used to seal the ends of the tubes to avoid leakage. In the SIL/PDGF group, the conduit was filled with 20 μL PDGF (Sigma-Aldrich Chemie GmbH, Germany) solution (0.5 ng/mL) diluted with normal saline. Others used dosage of 5 mg/kg for PDGF in systemic administration. In local application, we used a reduced dosage (0.5 ng/mL) in our study design.[19] The animals were anesthetized (see above) and euthanized with transcardial perfusion of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde buffer (pH 7.4) 4, 8, and 12 weeks after surgery.
Behavioral testing
Functional recovery of the nerve was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale for rat hind limb motor function.[20] Although BBB is widely used to assess functional recovery in spinal cord injured animals; however, it has been demonstrated that it could be most useful in the assessment of never repair processes in peripheral nerve injuries.[21] Scores of 0 and 21 were given when there were no spontaneous movement and normal movement, respectively. Then, BBB recordings were performed by a trained observer who was blinded to the experimental design. The testing was performed in a serene environment. The animals were observed and assessed within a course of a 4-minute exposure to an open area of a mental circular enclosure. After that, BBB scores were recorded once before surgery to establish a baseline control and again weekly thereafter to assess functional recovery during 16 weeks.
Functional assessment of reinnervation
Sciatic functional index
Walking track analysis was performed 4, 8, and 12 weeks after surgery based on the method of others.[22] The lengths of the third toe to its heel (PL), the first to the fifth toe (TS), and the second toe to the fourth toe (IT) were measured on the experimental side (E) and the contralateral normal side (N) in each rat [Figure 1]. The sciatic function index (SFI) of each animal was calculated by the following formula:
Figure 1.
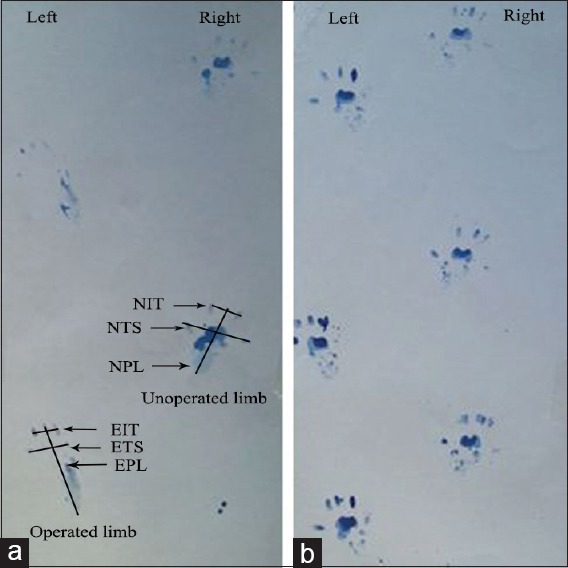
Quantitative analysis of walking the track. The pattern of platelet-derived growth factor treated rats (a) and normal rats (b). Paw prints in platelet-derived growth factor treated rats 8 weeks after surgery with for designated measurements: Toe spread, print length, intermediate toe spread. E: Experimental, N: Normal. Note the elongation of the print length and narrowing of toe spread and intermediate toe spread in operated limb (left).
SFI = −38.3× (EPL-NPL)/NPL + 109.5× (ETS-NTS)/NTS + 13.3× (EIT-NIT)/NIT − 8.8
In general, SFI oscillates around 0 for normal nerve function, whereas around − 100 SFI represents total dysfunction. SFI was assessed in the NC group, and the normal level was considered 0. SFI was a negative value and a higher SFI meant the better function of the sciatic nerve.
Measurement of gastrocnemius muscles mass
Recovery assessment was also indexed using the weight ratio of the gastrocnemius muscles 16 weeks after surgery. Immediately after sacrificing of animals, gastrocnemius muscles were dissected and harvested carefully from intact and injured sides and weighed while still wet, using an electronic balance [Figure 2]. Two independent observers unaware of the analyzed group made all measurements.
Figure 2.
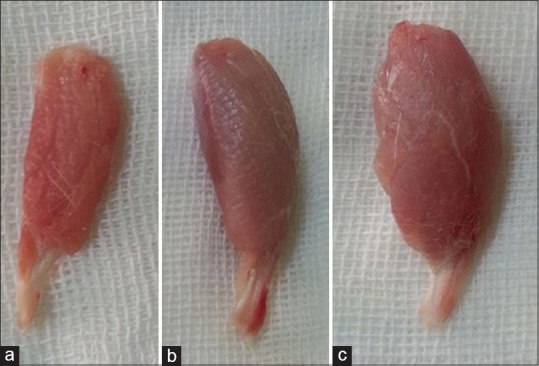
Gastrocnemius muscles dissected and harvested from injured sides. Note to the lesser mass of gastrocnemius muscle of silicon group (a) and silicon group/platelet-derived growth factor (b) groups compared to that of normal control group (c) 12 weeks after surgery.
Histological preparation and morphometric studies
Midpoint of normal sciatic nerve NC group regenerated nerve of SIL group and regenerated nerves of SIL/PDGF group were harvested and fixed with glutaraldehyde 2.5%. The grafts were then embedded in paraplast paraffin, cut in 5 μm and were next stained with toluidine blue. Morphometric analysis was performed using an image analyzing software (Image-Pro Express, version 6.0.0.319, Media Cybernetics, Silver Springs, MD, USA). Morphometric analysis was carried out using an image analyzing software (Image-Pro Express, version 6.0.0.319, Media Cybernetics, Silver Springs, MD, USA). Equal opportunity, systematic random sampling, and two-dimensional dissector rules were followed to cope with sampling-related, fiber-location-related and fiber-size related biases.[23]
Statistical analysis
Experimental results were expressed as means ± standard deviation (SD). Statistical analyses were performed using PASW 18.0 (SPSS Inc., Chicago, IL, USA). Model assumptions were evaluated by examining the residual plot. Results were analyzed using repeated measures and a factorial ANOVA with two between-subjects factors and. Bonferroni test for pairwise comparisons was used to examine the effect of time and treatments. The differences were considered significant when P < 0.05.
RESULTS
Basso, Beattie, and Bresnahan recovery
To assess hind limb recovery the open field locomotor was used. Figure 3 shows BBB scores compared to the baseline. All experimental groups, except for NC, showed the greatest degree of functional deficit 1 week after surgery. The PDGF-treated group showed significant improvement in locomotion of the operated limb compared to the control group during the study (P < 0.05).
Figure 3.
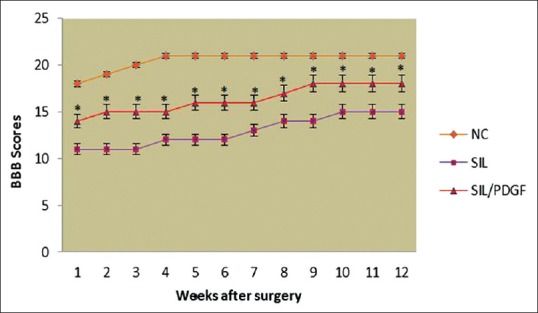
Basso, Beattie, and Bresnahan score for all experimental groups. Local administration of platelet-derived growth factor with silicon grafting gave better scores than in silicon group. Standard error at each data point is shown with bars. *P < 0.05 versus silicon group. Data are presented as mean ± standard deviation.
Recovery of sciatic nerve function and reinnervation
Sciatic function index outcome
Figure 4 shows SFI values in experimental groups. Before surgery, SFI values in both groups were near zero. After the nerve transection, the mean SFI decreased to −100 due to the complete loss of sciatic nerve function in all animals. At the end of the study, animals of SIL/PDGF achieved a mean value for SFI of −38.3 ± −3.25, whereas in control group a mean value of −57.2 ± −3.5 was found. The statistical analyses revealed that the recovery of nerve function was significantly (P < 0.05) different between SIL and SIL/PDGF groups and administration of PDGF improved functional recovery in the course of time.
Figure 4.
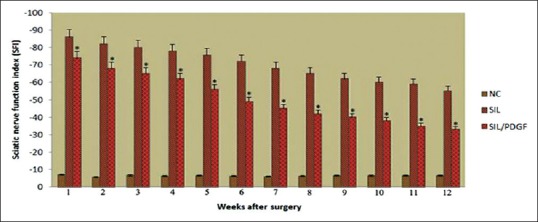
Diagrammatic representation of effects on the sciatic nerve function index in each experimental group during the study period. Statistically significant improvement (P < 0.05) was observed in functional recovery of the sciatic nerve in platelet-derived growth factor treated animals at the end of the study period. *P < 0.05 versus silicon group. Data are presented as mean ± standard deviation.
Gastrocnemius muscles mass measurement
The mean ratios of gastrocnemius muscles weight were measured. There was statistically significant difference between the muscle weight ratios of SIL/PDGF and SIL groups (P < 0.05). The results showed that in SIL/PDGF group muscle weight ratio was bigger than SIL group, and the gastrocnemius muscle weight loss was improved by administration of PDGF [Figure 5].
Figure 5.
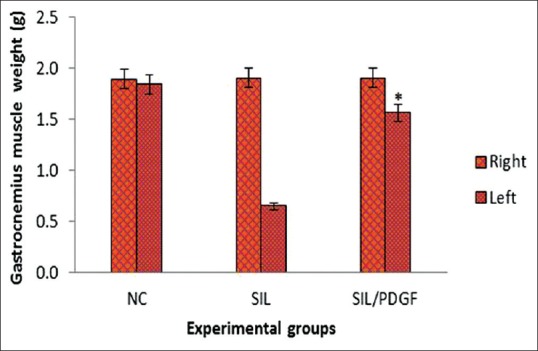
Measurement of gastrocnemius muscle mass. The gastrocnemius muscles of both sides (operated left and unoperated right) are excised and weighed in the experimental groups at 12 weeks after surgery, *P < 0.05 versus silicon group. Data are presented as mean ± standard deviation.
Histological and Morphometric findings
The animals of SIL/PDGF group presented significantly greater nerve fiber, axon diameter, and myelin sheath thickness during the study, compared to SIL animals (P < 0.05). NC group presented significantly greater nerve fiber and axon diameter, and myelin sheath thickness compared to SIL/PDGF and SIL groups animals [Table 1 and Figure 6].
Table 1.
Morphometric analyses of sciatic nerve in each of the experimental groups: Values are given as mean±SE
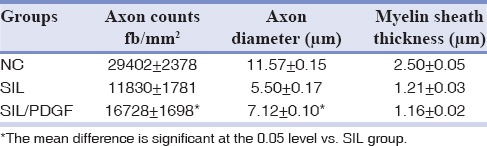
Figure 6.
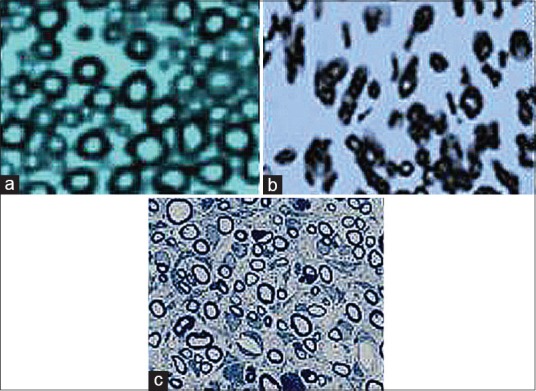
Light micrograph of representative cross section taken from the midpoint of (a) normal sciatic nerve (normal control group), (b) silicon group and (c) silicon group/platelet-derived growth factor groups 12 weeks after surgery (Toluidine blue staining, ×400).
DISCUSSION
The results of this study showed that administration of PDGF resulted in the faster functional recovery of the sciatic nerve during the study. We used silicone tube as a scaffold for keeping the delivered drug in situ and also preventing the surrounding connective tissue migrating into the gap and proliferating there. Selectiing an appropriate method to evaluate functional recovery of nerve regeneration is extremely influential. Although both morphological and functional data have been used to assess neural regeneration after induced crush injuries, the correlation between these two types of assessment is usually poor.[24,25,26] We did not perform nerve conduction tests because electrophysiological studies have poor correlation with functional indices.[27] Nerve conduction velocity and the peak of the action potential amplitude do not evaluate total nerve function but a fraction of nerve fibers population. A compound action potential is derived from extrinsic direct nerve excitation and does not correlate with proper central or peripheral connections.[26] Classical and newly developed methods of assessing nerve recovery, including histomorphometry, retrograde transport of horseradish peroxidase and retrograde fluorescent labeling do not necessarily predict the reestablishment of motor and sensory functions.[25,26,28,29,30] Although such techniques are useful in studying the nerve regeneration process, they generally fail in assessing functional recovery.[25] Therefore, research on peripheral nerve injury needs to focus on functional assessment. Castañeda and Kinne,[30] suggested that arrival of sprouts from the proximal stump at the distal nerve stump does not necessarily imply recovery of nerve function. Information taken from BBB scale may be invaluable in the evaluation of peripheral nerve process. Results of this study showed that the PDGF-treated animals had been improved in locomotion of the operated limb compared to the SIL group during the study. Walking track analysis has frequently been used to reliably determine functional recovery following nerve repair in rat models.[20,22] Left gastrocnemius muscle weight was significantly greater in the SIL/PDGF group than in the SIL group, indicating indirect evidence of successful end organ reinnervation in the PDGF-treated animals.
In the histological studies, quantitative morphometrical indices of regenerated nerve fibers showed a significant difference between SIL/PDGF and SIL groups indicating a beneficial effect of local PDGF on the nerve regeneration. Regarding better functional and morphometric indices in group SIL/PDGF versus group SIL at week four, it could be stated that local administration of PDGF accelerated the process of nerve regeneration, and its local application was time saving.
Schwann cells, the glial cells of the peripheral nervous system, surround all peripheral nerve axons, and in the case of myelinated nerve fibers, elaborate the myelin sheaths necessary for fast impulse conduction. There are many important developmental and functional interactions between Schwann cells and axons.[31] A major rate-limiting step in the induction of nerve regeneration across a gap is the proliferation and migration of Schwann cells between the nerve stumps. Therefore, the formation of a properly aligned extracellular matrix scaffold is essential to enhance Schwann cell proliferation in a conduit, through which blood vessels and other cell types migrate and form primordial assembly for the formation of a new nerve structure.[32]
We used a silicone rubber chamber as a conduit to provide a scaffold for PDGF and to facilitate Schwann cells migration. The silicone as a conduit has been utilized to repair segmental nerve tissue loss which proved to be a supportive conduit for peripheral nerve axonal regeneration and maturity.[2] If the nerve is injured, through mechanical trauma, neurotoxins or demyelinating diseases for example, Schwann cells again proliferate to restore the integrity of the Schwann cell sheath and aid regeneration of functional nerve fibers.[33] Attempts to define the factors involved in Schwann cell proliferation have revealed positive responses by various types of Schwann cell in culture to a range of mitogens including PDGF. It has been established that PDGF, particularly the B isoform, is a potent mitogen for cultured rat Schwann cells following induction of Schwann cell PDGF receptor expression by forskolin.[31,34] PDGF-B is a developmentally regulated Schwann cell paracrine growth factor in rat sciatic nerves.[35] PDGF-B is augmented in peripheral nerve injury and could act on Schwann cells and neuronal components to induce peripheral nerve regeneration after injury. And also its intimate localization to axon and ensheathing Schwann cells both in normal and in restored nerve structure after injury, might suggest that PDGF-B could mediate important signals in the axon-Schwann cell interaction. PDGF-B is widely expressed in CNS neurons and Schwann cells. Its functional analysis should provide further clues to the manner of introducing regenerative responses to the nervous system.[36]
Even though our preliminary study shows the neuroprotective action of local PDGF-B in peripheral nerve injuries, determining the molecular mechanisms leading to the neuroprotective action remains in the need of more investigation. We have not given the histological and molecular evidence for neuroprotective action of PDGF-B. This may be considered as a limitation to our study. Therefore, the authors stress that the aim of the current investigation was to evaluate clinical treatment potential of PDGF-B on nerve regeneration including functional assessments of the nerve repair, a matter not considered in previous studies. The results of this study indicated that administration of PDGF-B combined with silicone entubulization could be of benefit after sciatic nerve transection. Detailed mechanism of neuroprotective action remains to be investigated. The experimental model presented here is reproducible, and the behavioral and functional methods could be effectively used for the study of local effects of neurotrophics combined with guidance conduits.
CONCLUSION
This study demonstrated that local administration of PDGF combined with silicon grafting could accelerate functional recovery after nerve transection in sciatic nerve and may have clinical implications for the surgical management of patients after nerve transection. Thus, dose-response studies should be conducted for PDGF to determine the combination of the graft and the compound that achieve maximal efficacy in facial nerve transection models.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Keyvan Amini, Department of Veterinary Pathology, University of Saskatchewan, for contribution in histomorphometrical analyses, Mr. Jaffary for processing and preparation of histologic samples, Mr. Matin, Mr. Valinezhad and Mr. Ansarinia for their help in care of animals.
REFERENCES
- 1.Meek MF, Coert JH. Clinical use of nerve conduits in peripheral-nerve repair: Review of the literature. J Reconstr Microsurg. 2002;18:97–109. doi: 10.1055/s-2002-19889. [DOI] [PubMed] [Google Scholar]
- 2.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–60. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 3.Feldman EL, Sullivan KA, Kim B, Russell JW. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiol Dis. 1997;4:201–14. doi: 10.1006/nbdi.1997.0156. [DOI] [PubMed] [Google Scholar]
- 4.Kim B, Leventhal PS, Saltiel AR, Feldman EL. Insulin-like growth factor-I-mediated neurite outgrowth in vitro requires mitogen-activated protein kinase activation. J Biol Chem. 1997;272:21268–73. doi: 10.1074/jbc.272.34.21268. [DOI] [PubMed] [Google Scholar]
- 5.Matthews CC, Odeh HM, Feldman EL. Insulin-like growth factor-I is an osmoprotectant in human neuroblastoma cells. Neuroscience. 1997;79:525–34. doi: 10.1016/s0306-4522(96)00611-2. [DOI] [PubMed] [Google Scholar]
- 6.Sasahara M, Fries JW, Raines EW, Gown AM, Westrum LE, Frosch MP, et al. PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell. 1991;64:217–27. doi: 10.1016/0092-8674(91)90223-l. [DOI] [PubMed] [Google Scholar]
- 7.Smits A, Kato M, Westermark B, Nistér M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88:8159–63. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh HJ, Ruit KG, Wang YX, Parks WC, Snider WD, Deuel TF. PDGF A-chain gene is expressed by mammalian neurons during development and in maturity. Cell. 1991;64:209–16. doi: 10.1016/0092-8674(91)90222-k. [DOI] [PubMed] [Google Scholar]
- 9.Eccleston PA, Collarini EJ, Jessen KR, Mirsky R, Richardson WD. Schwann cells secrete a PDGF-like factor: Evidence for an autocrine growth mechanism involving PDGF. Eur J Neurosci. 1990;2:985–992. doi: 10.1111/j.1460-9568.1990.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, et al. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–22. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- 11.Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Boström H, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–67. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- 12.Matsui T, Heidaran M, Miki T, Popescu N, La Rochelle W, Kraus M, et al. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989;243:800–4. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- 13.Heldin CH, Bäckström G, Ostman A, Hammacher A, Rönnstrand L, Rubin K, et al. Binding of different dimeric forms of PDGF to human fibroblasts: Evidence for two separate receptor types. EMBO J. 1988;7:1387–93. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldin CH, Westermark B. Platelet-derived growth factor: Three isoforms and two receptor types. Trends Genet. 1989;5:108–11. doi: 10.1016/0168-9525(89)90040-1. [DOI] [PubMed] [Google Scholar]
- 15.Seifert RA, Hart CE, Phillips PE, Forstrom JW, Ross R, Murray MJ, et al. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989;264:8771–8. [PubMed] [Google Scholar]
- 16.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, et al. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–6. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- 17.Gilbertson DG, Duff ME, West JW, Kelly JD, Sheppard PO, Hofstrand PD, et al. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J Biol Chem. 2001;276:27406–14. doi: 10.1074/jbc.M101056200. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Mitlak BH, Finkelman RD, Hill EL, Li J, Martin B, Smith T, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11:238–47. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 20.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 21.Bervar M. Video analysis of standing - An alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods. 2000;102:109–16. doi: 10.1016/s0165-0270(00)00281-8. [DOI] [PubMed] [Google Scholar]
- 22.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–38. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Geuna S, Gigo-Benato D, Rodrigues Ade C. On sampling and sampling errors in histomorphometry of peripheral nerve fibers. Microsurgery. 2004;24:72–6. doi: 10.1002/micr.10199. [DOI] [PubMed] [Google Scholar]
- 24.Dellon AL, Mackinnon SE. Sciatic nerve regeneration in the rat. Validity of walking track assessment in the presence of chronic contractures. Microsurgery. 1989;10:220–5. doi: 10.1002/micr.1920100316. [DOI] [PubMed] [Google Scholar]
- 25.Shen N, Zhu J. Application of sciatic functional index in nerve functional assessment. Microsurgery. 1995;16:552–5. doi: 10.1002/micr.1920160809. [DOI] [PubMed] [Google Scholar]
- 26.Kanaya F, Firrell JC, Breidenbach WC. Sciatic function index, nerve conduction tests, muscle contraction, and axon morphometry as indicators of regeneration. Plast Reconstr Surg. 1996;98:1264–71. doi: 10.1097/00006534-199612000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Munro CA, Szalai JP, Mackinnon SE, Midha R. Lack of association between outcome measures of nerve regeneration. Muscle Nerve. 1998;21:1095–7. doi: 10.1002/(sici)1097-4598(199808)21:8<1095::aid-mus20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–43. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 29.Varejão AS, Melo-Pinto P, Meek MF, Filipe VM, Bulas-Cruz J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol Res. 2004;26:186–94. doi: 10.1179/016164104225013833. [DOI] [PubMed] [Google Scholar]
- 30.Castañeda F, Kinne RK. Omental graft improves functional recovery of transected peripheral nerve. Muscle Nerve. 2002;26:527–32. doi: 10.1002/mus.10229. [DOI] [PubMed] [Google Scholar]
- 31.Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990;110:1353–60. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams LR, Longo FM, Powell HC, Lundborg G, Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J Comp Neurol. 1983;218:460–70. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 33.Salzer JL, Bunge RP. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, wallerian degeneration, and direct injury. J Cell Biol. 1980;84:739–52. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinmaster G, Lemke G. Cell-specific cyclic AMP-mediated induction of the PDGF receptor. EMBO J. 1990;9:915–20. doi: 10.1002/j.1460-2075.1990.tb08189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy M, Reddy UR, Pleasure D. Platelet-derived growth factor and regulation of Schwann cell proliferation in vivo. J Neurosci Res. 1992;31:254–62. doi: 10.1002/jnr.490310206. [DOI] [PubMed] [Google Scholar]
- 36.Oya T, Zhao YL, Takagawa K, Kawaguchi M, Shirakawa K, Yamauchi T, et al. Platelet-derived growth factor-b expression induced after rat peripheral nerve injuries. Glia. 2002;38:303–12. doi: 10.1002/glia.10074. [DOI] [PubMed] [Google Scholar]


