ABSTRACT
The scientific evidence supporting the gut microbiome in relation to health maintenance and links with various disease states afflicting humans, from metabolic to mental health, has grown dramatically in the last few years. Strategies addressing the positive modulation of microbiome functionality associated with these disorders offer huge potential to the food and pharmaceutical industries to innovate and provide therapeutic solutions to many of the health issues affecting modern society. Such strategies may involve the use of probiotics and prebiotics as nutritional adjunct therapies. Probiotics are generally recognized to be a good form of therapy to keep harmful, intestinal microorganisms in check, aid digestion and nutrient absorption, and contribute to immune function. Probiotics are reported to improve microbial balance in the intestinal tract and promote the return to a baseline microbial community following a perturbing event (dysbiosis) such as antibiotic therapy. Prebiotics are selectively fermented ingredients that allow specific changes, both in the composition and/or activity in the gastrointestinal microflora, which confers benefits upon host well-being and health.
KEYWORDS: gut bacteria, health, microbiota, microbiome, probiotic
General acceptance and interest
Not many years ago, bacteria in our body were medically seen as foreign “invaders,” and thus would be a concern due to their potential to cause infection and other problems. In line with this, research on microbiology was mainly focused on how to kill bacteria with disinfectants and antibiotics. However, over the last few years, we have begun to appreciate the symbiotic relationship we have with the microorganisms cohabiting our bodies. While some bacteria can cause disease, others play beneficial roles in human health. Although the concept of probiotics -which are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” according to the WHO/FAO- is not new, public awareness and interest in probiotics and gut health is now at an all-time high, driven in part by new science supporting the health benefits for gut microbiota and new evidence of health benefits of probiotic-containing products and food supplements emerging in the marketplace.1,2,3 Given the increasingly widespread use of probiotics in both community and healthcare settings, and prompted by market requirements, research into the association of the gut microbiota with health and disease is constantly expanding.4 This is evidenced by the continuous upward trend in the number of probiotic-related scientific studies over the last 20 y (Fig. 1). In the PubMed database, the term ‘probiotic’ appeared about a dozen times per year in the 1990s, increasing hundreds of times per year in the 2000s and is now showing up in the thousands range. Indeed, in 2014 PubMed has indexed 1800 research articles that use the term ‘probiotic’. This number is twice as high as that reported in 2007 (820 indexed articles) and 10 times higher than that in 1999 (172 indexed articles). The probiotic field, which until recently concerned primarily microbiologists, food and agricultural scientists, has undergone a diversification over the past few years toward other disciplines such as dentistry, immunology, gastroenterology, veterinary or pharmacology.5
Figure 1.
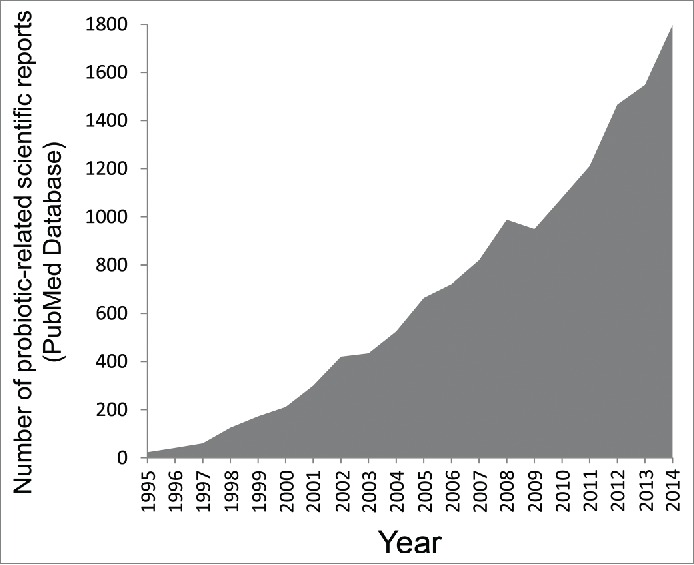
Trend in the number of scientific studies referencing the term “probiotic” indexed in the PubMed database over the last 20 y.
Increased commercial interest in exploiting the proposed health attributes of probiotics has contributed significantly to the rapid growth and expansion of this sector of the market.6,7,8 Sales of probiotic products have experienced significant growth between 2010 and 2014, increasing globally by 35% from 23.1 billion USD to 31.3 billion USD,9 and it is predicted to surge by 6.8% a year until 2018, Europe being among the dominant markets.10
The microbiota in numbers
We have coevolved with microbes in and on our body, with each individual having a unique set of microorganisms (microbiota).11 The most abundant and well-studied microbiota is found in the gut, where the bacterial density reaches 1011–1012 cells/g in the distal human colon.12 It has been estimated that the number of bacteria in the human gut may outnumber the somatic cells in the body by an order of magnitude and the biomass of the gut microbiota may reach up to 1.5 kg12,13 (Fig. 2). Thus, one may consider the gut microbiota as a multicellular organ similar in size to the liver.14,37 Indeed, it is sometimes referred to as our “forgotten organ”.14,15 Furthermore, the combined genomes of the gut microbiota -the microbiome- contain a number of genes ~150 times larger than the human genome,16 and these genes complement the human genome and contribute significantly to human physiology and metabolism.16 The total number of bacterial species inhabiting the normal healthy bowel has been estimated to exceed 1,000, and at least 160 species are shared among individuals.16,17
Figure 2.
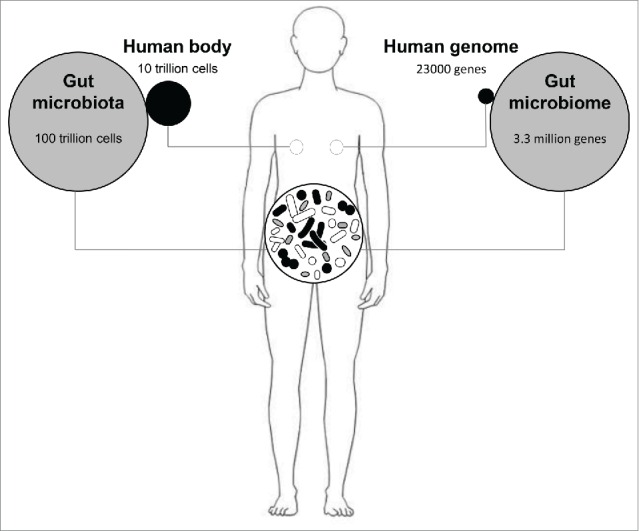
The gut microbiome in numbers. On average, the number of bacterial cells living the human gut is 10 times higher than the number of eukaryotic cells that shape the human body, which means that only 10% of the total number of cells in the human body consists of human cells, with the rest coming from symbiotic bacterial cells. Similarly, the combined genomes of the gut microbiota -the microbiome- contain a number of genes ~150 times larger than the human genome (23000 genes).
Generally, the microbiota within a given body habitat can be defined as the diversity and abundance distribution of distinct types of microorganisms. This microbial composition is highly influenced by individual factors such as diet, age, lifestyle, ethnicity, and host health, among others.18 The differential combination of these multiple factors in each person is the main cause of the strong variation in microbiota composition observed between individuals. Recent surveys have revealed that some of this variation is stable over time, leading to speculation that individuals might possess unique microbial “fingerprints” that distinguish them from the population.19 However, although no taxa are observed to be universally present among all individuals, some microbial patterns demonstrate broad prevalence.20 The four dominant phyla resident in the human gut are Firmicutes (which contains lactobacilli), Bacteroidetes, Actinobacteria (which contains bifidobacteria), and Proteobacteria.21,22 Most bacteria belong to the genera Bacteroides, Clostridium, Fusobacterium, Eubacterium, Ruminococcus, Peptococcus, Peptostreptococcus, and Bifidobacterium.23,24,25 Other genera, such as Escherichia and Lactobacillus are present to a much lesser extent.23 Species from the genus Bacteroides alone constitute about 30% of all bacteria in the human gut, suggesting that this genus is especially important in the functioning of the host.
The evolution of microbiota during life
Recent research suggests early in utero microbial exposure during pregnancy.26 Following birth, the newborn's digestive tract is quickly colonized by microorganisms from the mother (vaginal, faecal, skin, breast milk, etc.) and the environment in which the delivery takes place. Following birth, the microbiota that enters and evolves in the infant gut is dependent upon a number of factors, with delivery mode and feeding regime (breastfeeding vs infant formula feeding) of prime importance in the early days and weeks of life. Generally, the infant’ gastrointestinal tract is firstly colonized by facultative anaerobic bacteria, i.e., enterobacteria, staphylococci, and streptococci. As time after birth progresses, the amount of available oxygen in the gut decreases, thus allowing strictly anaerobic bacteria such as Bifidobacterium and Bacteroides to become established in the intestine and outnumber facultative anaerobes.27 The infant-type gut microbiota shifts toward a more adult-type microbiota during weaning, with the introduction of solid food. During this period, the microbiota composition goes from a bifidobacteria-enriched community to one dominated by Firmicutes and Bacteroidetes, resembling that of an adult microbiota, characterized by increased functionality and stability.28 By the age of 2 to 3 years, the microbiota becomes essentially established, having reached a steady state, and remains relatively stable throughout life. However, the gut microbiota continuously changes reconfiguring its metagenomic layout in response to daily variations in diet, lifestyle, age and host physiological and immunological health.18,27 Interestingly, the species-level gut microbiota composition varies dramatically among people, and each subject harbours a unique subset of microorganisms. Indeed,29 found that on average 40% of the microbial strains harbored in an adult's intestine was variable in a 5-year sampling period. The microbiota of older people displayed greater inter-individual variation and was significantly less diverse than that of younger adults (18The ELDERMET Consortium).
Health benefits of the microbiota
On the basis of the currently available literature, the gut microbiota is known to contribute to a number of important functions in the host, from protective, immunomodulatory, metabolic to trophic roles,30 as discussed below. These are promoted via a number of mechanisms. For example, members of the gut microbiota can produce anti-inflammatory factors, pain-relieving compounds, antioxidants and vitamins to protect and nurture the body. Additionally, they may prevent attachment and action of harmful bacteria that can produce toxins causing chronic disease. This close and specific contact with human cells, exchanging nutrients and metabolic wastes, makes symbiotic bacteria essentially a human organ.13
Gastrointestinal infection prevention
The indigenous intestinal microbiota serve as a line of resistance to colonization by exogenous microbes such as Clostridium difficile and Helicobacter pylori (Fig. 3), and thus assists in competitive exclusion of pathogens preventing the potential invasion, termed colonisation resistance.31 Indeed, antibiotic-associated diarrhea occurs when antibiotic treatment disturbs the natural balance of the gut microbiota causing harmful bacteria (i.e., Clostridium difficile) to proliferate and multiply. Probiotics may reduce antibiotic-associated diarrhea by up to 60%, when compared with a placebo.32 Controlled trials have shown that Lactobacillus GG can shorten the course of infectious diarrhea in infants and children.33 This effect may due to the ability of probiotics to restore the natural balance of bacteria in the gastrointestinal tract.
Figure 3.
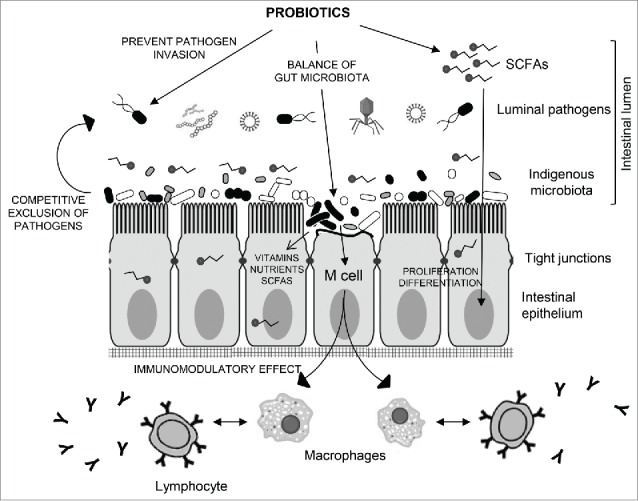
Schematic representation of the cross-talk interaction of indigenous microbiota and probiotics with the intestinal epithelium. Intestinal microbiota protects the mucosa from adherence and invasion by exogenous pathogens and thus assists in balance microbiota maintenance and prevention of dysbiosis. These probiotic bacteria may also allow beneficial effects through release of nutrients (vitamins, SCFAs, sugars). Intestinal absorption of SCFAs translates into reinforcement of the intestinal epithelial cells. Indigenous microbes and probiotics would also interact M cells and consequently modulate innate and adaptive immunity by activating release of macrophages and cytokines including IL-4, TGF-β, IL-5, IL-6, and IL-10. M cells in Peyer's patches may contribute to present microbial antigens to naive T cells, allowing IgA antibody-mediated mucosal response.
Immunomodulatory effects
Commensal bacteria are capable of interacting with the host immune system in ways that modulate the hosts immune response and counteracts the development of disease.34 The complex interactions that may occur between ingested probiotic bacteria, commensals and the mucosal surface is possible because of the mucosa-associated immune system, typically organized into lymphoid aggregates (Peyer's patches). This cross-talk interaction (Fig. 3) enhances cellular immune response characterized by activation of macrophages, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines.35 Lb. salivarius and B. breve have been attributed to play important immunomodulatory roles supporting a healthy immune system.36 Furthermore, some probiotics such as Lb. plantarum, B. infantis, or Lb. rhamnosus may be effective in the prevention and/or alleviation of allergies and auto-immune diseases like irritable bowel syndrome and inflammatory bowel diseases (Crohn's disease and ulcerative colitis).37,38 Although the etiology of these diseases is still unclear, the main hypothesis is that they are a result of an excessive immune response to endogenous bacteria, which occurs in genetically predisposed individuals.39
Nutritional and trophic benefits
The metabolic activity of the gut microbiota makes an important contribution to nutritional status of the host, via its ability to synthesize certain vitamins and various bioactive metabolites, such as short chain fatty acids (SCFA) that then become bioavailable to the host. The importance of the symbiotic relationship between microbiota and host has been demonstrated in germfree mice, who continuously require supplementation with vitamin K and some B vitamins (i.e., folate, B12, and biotin) since these vitamins are microbially derived products and thus absent in the germ free gut.40,41 Indeed, these vitamins are synthesized by several intestinal genera, including Bacteroides, Eubacterium, Propionibacterium, and Fusobacterium. Other small molecules from the human microbiome can play a relevant role in microbiota-host interaction and contribute to the stability and dynamics of microbial communities. Examples include bacteriocins, oligosaccharides, glycolipids and terpenoids.41,42
It has been reported that consumption of yogurt containing Lactobacillus bulgaricus, delbrüeckii or acidophilus could alleviate lactose intolerance during gastric passage through their enzyme lactase.44 However, the major metabolic function of the colonic microflora is the fermentation of nondigestible carbohydrates, which are key sources of energy in the colon. These carbohydrates also include large polysaccharides (i.e., resistant starches, pectins, and cellulose) and some oligosaccharides that escape digestion, as well as unabsorbed sugars and alcohols. The primary metabolic endpoint of this fermentation is the generation of SCFA (acetate, proprionate, and butyrate), which play fundamental trophic roles in growth, proliferation and differentiation of the intestinal epithelium.45
Other benefits of the gut microbiome on human health, such as a role in supporting the health of the reproductive tract, oral cavity, lungs, skin and gut–brain axis are currently under investigation. The influence of microbiota on the brain function, i.e. psychobiotics46,47 has been (at least partially) linked with their ability to microbially biosynthesize neuroactive metabolites that can be absorbed and distributed to the central nervous system, where they affect mood, emotions and behavior.48 However evidence has not yet been linked to a broad enough cross-section to consider these effects to be shared across the whole class of probiotics.49 When the normal composition of the microbiome is thrown off balance there is a pontential risk of disease.50 In general, a decrease in microbiota diversity has been linked to several human diseases. Several studies have documented an imbalance of gut microbiota in patients with a wide range of diseases including cancer, asthma, Parkinson, obesity, Alzheimer, type-2 diabetes, cardiovascular disease and possibly even autism in comparison to healthy subjects,51,52,31,53 but whether this is a cause or a consequence of the disease remains to be elucidated.
Requirements for probiotic strains
The most widely accepted definition of probiotics i.e., “live microorganisms which when administered in adequate amounts confer a health benefit on the host” published by the Food and Agriculture Organization of the United Nations in 200154 highlights the importance of health benefits of probiotics, while safety considerations are similarly of paramount importance to their successful implementation and routine use in clinical practices and everyday use, as recently further elaborated by ISAPP.49 A recent review by8 summarizes the regulatory challenges associated with the marketing of probiotics in different geographical locations worldwide.
Generally, these microorganisms have a long safety history, and a probiotic bacterium must lack potential toxicity and pathogenicity as well as antibiotic resistance.55 In this regard, the availability of complete genome sequences of probiotic strains can help to predict bacterial safety.
In order to arrive alive to their workplace (i.e. the gastrointestinal tract), orally administered probiotics must be able to resist stomach acid, bile and the effects of digestive enzymes. Certain mechanisms of action (such as delivery of certain enzymes to the intestine) may not require live cells to play a physiologic benefit. However, under a strict definition, dead microbes are not considered probiotics.11 Hence, a probiotic must contain as many live bacteria as claimed on the label. Generally, probiotic effects have a dosage threshold. The minimum effective dose, which affects the intestinal environment and provides beneficial effects on human health, is considered to be 106-109 live microbial cells per day. The minimum dose depends on the particular strain and the type of foodstuffs.57,58,56 In addition to survive the stomach and arrive to the intestine in optimal numbers, probiotic strains must be able to adhere to intestinal epithelium and/or mucus, persist and multiply in the gut to maintain its metabolic activity and confer their probiotic properties in the human body.
Probiotic strains
Current probiotics for human use belong almost exclusively to the genera Lactobacillus and Bifidobacterium as they are considered to have the added advantage of a long history of safe use. Most of the probiotic bacterial strains described belong to the species Bifidobacterium (adolescentis, animalis, bifidum, breve and longum) and Lactobacillus (acidophilus, brevis, casei, fermentum, gasseri, johnsonii, paracasei, plantarum, delbrueckii, rhamnosus, reuteri and salivarius).49 Besides Lactobacillus and Bifidobacterium, strains belonging to the genera Propionibacterium and Streptococcus (in particular thermophilus) were observed to possess favorable atributes and are also in vogue as probiotic microorganisms.59,60 Dairy propionibacteria influence gut microbial balance, exclusion of pathogens and immunomodulation60 whereas S. thermophilus produces large quantities of the enzyme lactase, making it effective in the prevention of lactose intolerance.61 It is commonly admitted that most effects of probiotic are strain-specific and cannot be extended to other probiotics of the same genus or species.62
Nowadays, the use of probiotic preparations as food supplements has become widespread. The number of probiotic brands on supermarket and grocery store shelves is getting higher. These probiotic preparations can consist of one single strain (e.g., Yakult, Japan – L. casei Shirota) or are mixed cultures of 2 or even more strains.63 Table 1 summaries the probiotic bacterial species primarily used in the food industry. The strains Lactobacillus rhamnosus GG (Valio), Lactobacillus paracasei Shirota (Yakult) and Bifidobacterium lactis BB12 (Chr. Hansen), are the world's most documented probiotics reported to have the strongest human health efficacy against some or all of the following infections and imbalances: lactose intolerance, immune response modulation, protection against Clostridium difficile infection, protection against Helicobacter pylori infection, rotaviral diarrhea, antibiotic-associated diarrhea, Travelers' diarrhea, as well as some other bacterial diarrheas.64,65 The main physiological mechanisms by which these strains could promote microbiota balance and protect the host against intestinal infection are i) production of inhibitory substances such as organic acids, hydrogen peroxide or bacteriocins which are inhibitory to both gram-positive and gram-negative bacteria, ii) competition with pathogenic bacteria to adhere to the intestinal epithelial surface and blocking of adhesion sites, iii) competition for nutrients required by pathogen bacteria, and iv) stimulating of immunity against pathogens.63
Table 1.
Some bacterial strains used as commercial probiotic cultures for which there is published peer-reviewed clinical evidence of probiotic effect.
| Strain | Company | Rotaviral diarrhea | Antibiotic-associated diarrhea | Protection against Clostridium difficile | Travelers´ diarrhea | Other acute bacterial diarrhea | Lactose intolerance | Atopic ezcema and food allergy | Cholesterol lowering | Chronic constipation | Irritable bowel syndrome | Protection against Helicobacter pylori | Immune response modulation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactobacillus rhamnosus GG | Valio | • | • | • | • | • | • | • | • | ||||
| Lactobacillus paracasei Shirota | Yakult | • | • | • | • | • | |||||||
| Bifidobacterium animalis BB12 | Ch. Hansen | • | • | • | • | • | • | • | • | ||||
| Lactobacillus reuterii | BioGaia | • | • | • | |||||||||
| Lactobacillus johnsonii La1 | Nestle | • | • | • | • | ||||||||
| Lactobacillus acidophilus La5* | Ch. Hansen | • | • | • | • | • | |||||||
| Bifidobacterium longum BB536 | Morinaga | • | • | • | • | ||||||||
| Bifidobacterium breve | Yakult | • | • | ||||||||||
| Lactobacillus acidophilus NFCM | Rhodia | • | • | • | |||||||||
| Lactobacillus plantarum 299v | ProViva | • | • | ||||||||||
| Lactobacillus casei DN-114.001 | Danone | • | • | • | |||||||||
| Bifidobacterium lactis DR10 | Danisco | • | • | • |
Data for this strain uncertain, as it was usually co-administered with B. lactis BB12
Prebiotics
The gut microbiota can be also influenced by prebiotics. Prebiotics are defined as non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of probiotic bacteria (in particular Bifidobacteria) in the colon, which can improve host health.8 Today, only bifidogenic, non-digestible oligosaccharides (particularly inulin, its hydrolysis product oligofructose, and trans-galactooligosaccharides), fulfill all the criteria for prebiotic classification. Although prebiotics are fibers that occur naturally in some foods, diet supplementation may contribute to increase the levels of probiotic bacteria in the human intestines (bifidogenic effect). For example, in the last years successful attempts have been reported to make infant formula more breast milk-like by the addition of fructo- and (primarily) galactooligosaccharides.66
Future research
The gut microbiota is increasingly being accepted as an environmental factor that is central to host health, and that disturbances can affect host metabolism and contribute to associated pathological conditions. Although new insights are emerging rapidly, the link between these microbes and human health remains challenging and is the focus of a growing number of research initiatives. Concerning this, animal models could be useful to test dietary interventions and to manipulate the gut microbiota to improve health and prevent disease and ultimately establish solid scientific evidence for probiotic mechanisms. Beside this, gut microbiota studies can be used to obtain robust predictive biomarkers for health and disease. Nowadays, the definition of a baseline for a healthy adult microbiota remains unanswered. This issue has been hampered by interpersonal variations of the gut microbiota or even intraindividual variations. Another key but challenging consideration when profiling gut microbiota, would be to differentiate live microorganisms habiting the gut from those transient ingested with food or drinks which only travel through the intestine but do not survive the harsh conditions and/or do not inhabit it. For this purpose, metagenomic approaches should be complemented by metatranscriptomics and metabolomics to correlate microbial genes with specific microbial functions and investigate which bacterial genes are active in a person and not just at which bacteria are there. This would allow establishing causal relationships between the microbiome and disease. Overall, the field of probiotics still remains in its infancy and a huge expansion of knowledge and development of clinical potential is expected in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the Alimentary Pharmabiotic Center, which is a Center for Science and Technology (CSET) funded by the Science Foundation Ireland (SFI), through the Irish Government's National Development Plan (Grant no. 02/CE/B124 and 07/CE/B1368).
References
- 1.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr 2006; 83(6):1256-1264; PMID:16762934 [DOI] [PubMed] [Google Scholar]
- 2.Sanders ME. Probiotics: Definition, sources, selection, and uses. Clin Infect Dis 2008; 46(2):S58-S61; PMID:18181724; http://dx.doi.org/ 10.1086/523341 [DOI] [PubMed] [Google Scholar]
- 3.Ouwehand AC, DongLian C, Weijian X, Stewart M, Ni J, Stewart T, Miller LE. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine 2014; 32(4):458-463; PMID:24291194; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 4.Ray K. Gut microbiota: Married to our gut microbiota. Nat Rev Gastroenterol Hepatol 2012; 9:555; PMID:23034427; http://dx.doi.org/ 10.1038/nrgastro.2012.165 [DOI] [PubMed] [Google Scholar]
- 5.Olle B. Medicines from microbiota. Nat Biotech 2013; 31(4):309-315; http://dx.doi.org/ 10.1038/nbt.2548 [DOI] [PubMed] [Google Scholar]
- 6.Stanton C, Gardiner G, Meehan H, Collins K, Fitzgerald G, Lynch PB, Ross RP. Market potential for probiotics. Am J Clin Nutr 2001; 73(2):476S-483S. Review; PMID:11157361 [DOI] [PubMed] [Google Scholar]
- 7.Reardon S. Microbiome therapy gains market traction. Nature 2014. 509(7500):269-70; PMID:24828169; http://dx.doi.org/ 10.1038/509269a [DOI] [PubMed] [Google Scholar]
- 8.Kumar H, Salminen S, Verhagen H, Rowland I, Heimbach J, Bañares S, Young T, Nomoto K, Lalonde M. Novel probiotics and prebiotics: road to the market. Curr Opin Biotechnol 2015; 32:99-103; PMID:25499742; http://dx.doi.org/ 10.1016/j.copbio.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 9.Statista 2014. Probiotic market: global sales by region 2010-2015 (fee-based). Retrieved 12 December 2014 [Google Scholar]
- 10.Transparency Market Research 2013. Probiotics market (dietary supplements, animal feed, foods & beverages)—Global industry analysis, market size, share, trends, analysis, growth and forecast 2012-2018 [Google Scholar]
- 11.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009; 326:1694-1697; PMID:19892944; http://dx.doi.org/ 10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Hostbacterial mutualism in the human intestine. Science 2005; 307:1915-1920; http://dx.doi.org/ 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- 13.Zhao L. Genomics: The tale of our other genome. Nature 2010; 465:879-880; PMID:20559375; http://dx.doi.org/ 10.1038/465879a [DOI] [PubMed] [Google Scholar]
- 14.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006; 7:688-693; http://dx.doi.org/ 10.1038/sj.embor.7400731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes 2013; 62(10):3341-9. Review; PMID:24065795; http://dx.doi.org/ 10.2337/db13-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al.. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464(7285):59-65; PMID:20203603; http://dx.doi.org/ 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandal RS, Saha S, Das S. Metagenomic surveys of gut microbiota. Genomics Proteomics Bioinformatics 2015; S1672-0229(15):00054-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al.. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488(7410):178-184; PMID:22797518; http://dx.doi.org/ 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- 19.Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannan BJ, Huttenhower C. Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci U S A 2015; 112(22):E2930-8; PMID:25964341; http://dx.doi.org/ 10.1073/pnas.1423854112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207-214; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012; 489:242-249; PMID:22972297; http://dx.doi.org/ 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 22.Khanna S, Tosh PK. “A clinician's primer on the role of the microbiome in human health and disease. Mayo Clin Proc 2014; 89(1):107-114; PMID:24388028; http://dx.doi.org/ 10.1016/j.mayocp.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 23.Guarner F, Malagelada J. Gut flora in health and disease. Lancet 2003; 361(9356): 512-519; http://dx.doi.org/ 10.1016/S0140-6736(03)12489-0 [DOI] [PubMed] [Google Scholar]
- 24.Beaugerie L, Petit JC. Antibiotic-associated diarrhoea. Best Practice Res Clin Gastroenterol 2004; 18(2):337-352; PMID:15123074; http://dx.doi.org/ 10.1016/j.bpg.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 25.Vedantam G, Hecht DW. Antibiotics and anaerobes of gut origin. Curr Opin Microbiol 2003; 6(5):457-461; PMID:14572537; http://dx.doi.org/ 10.1016/j.mib.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 26.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 2012; 9(10):565-76; PMID:22890113; http://dx.doi.org/ 10.1038/nrgastro.2012.144 [DOI] [PubMed] [Google Scholar]
- 27.Quercia S, Candela M, Giuliani C, Turroni S, Luiselli D, Rampelli S, Brigidi P, Franceschi C, Bacalini MG, Garagnani P, et al.. From lifetime to evolution: timescales of human gut microbiota adaptation. Front Microbiol 2014; 5:587; PMID:25408692; http://dx.doi.org/ 10.3389/fmicb.2014.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci 2011; 108(1):4578-4585; PMID:20668239; http://dx.doi.org/ 10.1073/pnas.1000081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman A L, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science 2013; 341:1237439; PMID:23828941; http://dx.doi.org/ 10.1126/science.1237439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res 2014; 76(1):2-10; PMID:24732106; http://dx.doi.org/ 10.1038/pr.2014.49 [DOI] [PubMed] [Google Scholar]
- 31.Canny GO, McCormick BA. Bacteria in the intestine, helpful residents or enemies from within? Infect Immun 2008; 76(8):3360-3373; PMID:18474643; http://dx.doi.org/ 10.1128/IAI.00187-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea (Review). The Cochrane Collaboration. 2011. Published by John Wiley & Sons, Ltd [DOI] [PubMed] [Google Scholar]
- 33.Casburn-Jones AC, Farthing ML. Management of infectious diarrhoea. Gut 2004; 53(2):296-305; PMID:14724167; http://dx.doi.org/ 10.1136/gut.2003.022103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell 2012; 148(6):1258-1270; PMID:22424233; http://dx.doi.org/ 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashrafa R, Shaha NP. Immune system stimulation by probiotic microorganisms. Critical Rev Food Sci Nutri 2014; 54 (7):938-956; http://dx.doi.org/ 10.1080/10408398.2011.619671 [DOI] [PubMed] [Google Scholar]
- 36.Drago L, De Vecchi E, Gabrieli A, De Grandi R, Toscano M. Immunomodulatory effects of Lactobacillus salivarius LS01 and Bifidobacterium breve BR03, alone and in combination, on peripheral blood mononuclear cells of allergic asthmatics. Allergy Asthma Immunol Res 2015; 7(4):409-413; PMID:25749784; http://dx.doi.org/ 10.4168/aair.2015.7.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elian SD, Souza EL, Vieira AT, Teixeira MM, Arantes RM, Nicoli JR, Martins FS. Bifidobacterium longum subsp. infantis BB-02 attenuates acute murine experimental model of inflammatory bowel disease. Benef Microbes 2015; 6(3):277-286; PMID:25391346; http://dx.doi.org/ 10.3920/BM2014.0070 [DOI] [PubMed] [Google Scholar]
- 38.Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One 2009; 4(9):e7056; PMID:19756155; http://dx.doi.org/ 10.1371/journal.pone.0007056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, Bruno G, Petito V, Laterza L, Cammarota G, et al.. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int 2013; 435268; PMID:23991417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev 1982; 46:241-280; PMID:6127606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wostmann BS. The germfree animal in nutritional studies. Annu Rev Nutr 1981; 1:257-279; PMID:6764717; http://dx.doi.org/ 10.1146/annurev.nu.01.070181.001353 [DOI] [PubMed] [Google Scholar]
- 42.Cotter PD, Ross PR, Hill C. Bacteriocins - a viable alternative to antibiotics? Nat Rev Microbiol 2013; 11:95-105; PMID:23268227; http://dx.doi.org/ 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- 43.Donia MS, Fischbach MA. Human Microbiota: Small molecules from the human microbiota. Science 2015; 349(6246):1254766; PMID:26206939; http://dx.doi.org/ 10.1126/science.1254766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vonk RJ, Reckman GA, Harmsen HJ, Priebe MG. Probiotics and lactose intolerance. In: Probiotics. 2012. Edited by Everlon Cid Rigobelo. Intech. Chapter 7 [Google Scholar]
- 45.Frankel WL, Zhang W, Singh A, Klurfeld DM, Don S, Sakata T, Modlin I, Rombeau JL. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology 1994; 106:375-380; PMID:8299904 [DOI] [PubMed] [Google Scholar]
- 46.Dinan TG, Stanton C, Cryan JF. Psychobiotics, a novel class of psychotropic. Biol Psychiatry 2013; 74(10):720-6. Review; PMID:23759244; http://dx.doi.org/ 10.1016/j.biopsych.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 47.Schmidt C. Mental health: thinking from the gut. Nature. 2015. 518(7540):S12-5; PMID:25715275; http://dx.doi.org/ 10.1038/518S13a [DOI] [PubMed] [Google Scholar]
- 48.Patterson E, Cryan JF, Fitzgerald GF, Ross RP, Dinan TG, Stanton C. Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc. 2014; 73(4):477-89. Review; PMID:25196939; http://dx.doi.org/ 10.1017/S0029665114001426 [DOI] [PubMed] [Google Scholar]
- 49.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al.. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11(8):506-514; PMID:24912386; http://dx.doi.org/ 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 50.Balter M. Taking stock of the human microbiome and disease. Science 2012; 336:1246-1247; PMID:22674333; http://dx.doi.org/ 10.1126/science.336.6086.1246 [DOI] [PubMed] [Google Scholar]
- 51.Couzin-Frankel J. Bacteria and asthma: untangling the links. Science 2010; 330:1168-1169; PMID:21109643; http://dx.doi.org/ 10.1126/science.330.6008.1168 [DOI] [PubMed] [Google Scholar]
- 52.Pennisi E. Girth and the gut (bacteria). Science 2011; 332:32-33; PMID:21454769; http://dx.doi.org/ 10.1126/science.332.6025.32 [DOI] [PubMed] [Google Scholar]
- 53.de Weerdt S. Microbiome: Microbial mystery. Nature. 2015; 521(7551):S10-1; PMID:25970451; http://dx.doi.org/ 10.1038/521S10a [DOI] [PubMed] [Google Scholar]
- 54.Food and Agricultural Organization of the United Nations and World Health Organization Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. World Health Organization 2001 [Google Scholar]
- 55.Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, Huys G, Levy DD, Lutgendorff F, Mack D, et al.. Safety assessment of probiotics for human use. Gut Microbes 2010; 1(3):164-85; PMID:21327023; http://dx.doi.org/ 10.4161/gmic.1.3.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Champagne CP, Ross RP, Saarela M, Hansen KF, Charalampopoulos D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int J Food Microbiol 2011; 149:185-193; PMID:21803436; http://dx.doi.org/ 10.1016/j.ijfoodmicro.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 57.Reid G. The Importance of Guidelines in the Development and Application of Probiotics. Curr Pharm Des 2005; 11:11-16; PMID:15638748; http://dx.doi.org/ 10.2174/1381612053382395 [DOI] [PubMed] [Google Scholar]
- 58.Williams NT. Probiotics. Am J Health Syst Pharm 2010; 67:449-458; PMID:20208051; http://dx.doi.org/ 10.2146/ajhp090168 [DOI] [PubMed] [Google Scholar]
- 59.Lyera R, Tomara SK, Kapilab S, Manic J, Singha R. Probiotic properties of folate producing Streptococcus thermophilus strains. Food Res Int 2010; 43(1):103-110; http://dx.doi.org/ 10.1016/j.foodres.2009.09.011 [DOI] [Google Scholar]
- 60.Zárate G. Dairy Propionibacteria: Less conventional probiotics to improve the human and animal health. In: Probiotic in Animals. Intech 2012. Chapter 8. Pag 153-202 [Google Scholar]
- 61.Rul F, Ben-Yahia L, Chegdani F, Wrzosek L, Thomas S, Noordine ML, Gitton C, Cherbuy C, Langella P, Thomas M. Impact of the metabolic activity of Streptococcus thermophilus on the colon epithelium of gnotobiotic rats. J Biol Chem 2011; 286(12):10288-96; PMID:21239485; http://dx.doi.org/ 10.1074/jbc.M110.-168666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rea MC, Alemayehu D, Ross RP, Hill C. Gut solutions to a gut problem: bacteriocins, probiotics and bacteriophage for control of Clostridium difficile infection. J Med Microbiol 2013, 62(9):1369-1378; PMID:23699066; http://dx.doi.org/ 10.1099/jmm.0.058933-0 [DOI] [PubMed] [Google Scholar]
- 63.Otles S. CRC Press; 2013. Probiotics and prebiotics in food, nutrition and health. [Google Scholar]
- 64.Hui YH. Handbook of food science, technology, and engineering 2006. Edited by Hi YH. Taylor and Francis. CRC Press, volume 4 [Google Scholar]
- 65.Shah NP. Probiotics. In: Biotechnology in functional foods and nutraceuticals. 2010. Chapter 26 Edited by Bagchi D, Lau FC, Ghosh DK. CRC Press [Google Scholar]
- 66.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 2008; 111:1-66; PMID:18461293 [DOI] [PubMed] [Google Scholar]


