Abstract
For selected patients with type 1 diabetes, β-cell replacement is the treatment of choice, either by islet transplantation (ITX) or whole pancreas transplantation (PTX). When either modality fails, current practice is to consider retransplantation, or return to exogenous insulin. We investigate outcomes with PTX after failed ITX (PAI), and ITX after failed PTX (IAP). All patients receiving PAI or IAP at a single institution were identified. Donor and recipient variables were documented, including transplant outcomes analyzed for insulin requirement and metabolic control. Five subjects were listed for PAI, and 2 received transplants. Of the 4 listed for IAP, 3 have received transplants. The mean waitlist time was 4.5 ± 4.1 y for PAI and 0.35 ±0 .4 y for IAP (p = 0.08). Metabolic control was excellent after PAI, with 2/2 insulin-independent. After IAP, 1/2 achieved insulin independence and good metabolic control after 2 islet infusions. The third could not receive 2nd infusion and presented c-peptide levels < 0.1 nmol/L. Both strategies are feasible. The outcomes after PAI in our center must be offset by much longer waitlist time due to the sensitization status of these patients. Data from multicentre experience will allow more robust comparative outcomes to be made, the current observations being restricted to a limited patient set.
Keywords: islet transplantation, pancreas transplantation, transplantation failure
Abbreviations
- ITX
islet transplantation
- PTX
pancreas transplantation
- IEQ
islet equivalent
- PAI
pancreas-after-failed islet transplantation
- IAP
islet-after-failed pancreas transplantation
- cPRA
calculated panel reactive antibodies
- T1DM
type 1 diabetes mellitus
- CNI
calcineurin inhibitor
Introduction
The prevalence of type I diabetes mellitus (T1DM) is increasing and is associated with a high economic and human cost.1,2 Although intensive exogenous insulin significantly delays or slows the progression of chronic complications associated with T1DM, this treatment can lead to severe hypoglycaemia.3 For selected patients presenting with glycaemic lability and recurrent hypoglycaemia an alternative is β cell replacement, either by islet transplantation (ITX) or whole pancreas transplantation (PTX).4 Both approaches provide excellent metabolic control and can eliminate hypoglycaemia, irrespective of insulin-independent status.5-7 Indications, risk and outcomes differ between ITX and PTX, although indications should be tailored to a particular patient. Compared to ITX, inclusion and exclusion criteria for PTX are much more restrictive in many centers with high islet transplant case load such as Edmonton, but PTX has shown more durable long-term insulin-independence,8,9 although ITX outcomes have improved over time. Indeed, 6 centers with important experience in islet transplantation recently reported 5-year insulin independence rates of >50 % for islet-alone transplantation, which are similar to the long term outcomes of pancreas-alone transplantation from registry data.10-12
After PTX or ITX failure, potential options include return to exogenous insulin, or consideration of re-transplantation. Pancreas re-transplantation may offer similar graft survival to primary transplantation but in highly selected patients13 and likewise, ITX re-transplantation may restore insulin-independence after graft dysfunction in >80% for selected patients.14 However, recent large series demonstrated significant decrease in graft survival after second compared to primary PTX,15,16 and similarly ITX outcomes may also be associated with dismal graft function after re-transplantation. The common pathway of autoimmune recurrence, or re-exposure to sensitized allo-antigens pose an identical potential challenge in either setting.
We herein summarize our limited single center experience with ITX after failed PTX (IAP) and PTX alone after failed ITX (PAI) as alternative rescue therapies.
Results
Between April 2002 and August 2014, a total of 5 subjects were listed for PAI, and 2 underwent transplantation. Of the 4 listed for IAP, 3 have received transplants (2 patients received 2 infusion and 1 patient 1 infusion) and 1 remains listed. Patient demographics PAI and IAP groups are shown in Tables 1 and 2 respectively.
Table 1.
Characteristics of patients in the group pancreas-after-failed islet transplantation, and patients with failed islet transplantation on the waitlist for pancreas transplantation.
| ID # | Gender | Age at 1st ITX (years) | Age at PTX (years) | # of ITX | Total IEQ/Kg received | Period of insulin independance after ITX (days) | cPRA before PTX (%) | PTX induction | Wait time listing for PTX-PTX (days) |
|---|---|---|---|---|---|---|---|---|---|
| PAI-1 | F | 30 | 39 | 2 | 15,709 | 747 | 89 | ATG | 1036 |
| PAI-2 | M | 29 | 41 | 4 | 25,666 | 300 | 39 | Basiliximab | 296 |
| PAI-3 | F | 24 | WL | 2 | 11,741 | 168 | 100 (WL) | WL | 3575† |
| PAI-4 | F | 30 | WL | 3 | 19,074 | 75 | 100 (WL) | WL | 2899† |
| PAI-5 | F | 34 | WL | 4 | 14,450 | 71 | 99 (WL) | WL | 463† |
PAI: Pancreas after Islet; PTX: pancreas transplant; ITX: islet transplant; IEQ: islet equivalent; cPRA: calculated Panel Reactive Antibodies; †: at the time of writing of the manuscript; ATG: anti-thymocytes globulin; WL: on the waitlist for PTX, not transplanted yet
Table 2.
Characteristics of patients in the group islet-after-failed pancreas transplantation, and patient with failed pancreas transplantation on the waitlist for islet transplantation.
| ID # | Gender | Age atthe time of the 1st ITX | Age at the time of the PTX | Type ofPTX | # of ITX | Total IEQ/Kg received | Period of insulin independance after ITX (days) | Cause of PTX failure | Interval PTX to pancreas failure (days) | Interval pancreas failure to ITX (days) | cPRA pre first ITX | HbA1C pre-first ITX (%) | HbA1C at last FU (%) | Insulin requirement pre-first ITX (units/kg/day) | Insulin requirement at last FU (units/kg/day) | Wait time listing for ITX-ITX (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAP-1 | F | 31 | 31 | SPK | 2 | 14,476 | Off insulin | SVT | 4 | 24 | 0 | 7.7 | 5.3 | 0.69 | 0 | 10 |
| IAP-2 | M | 52 | 49 | PAK | 2 | 10,831 | 0 | NPanc | 111 | 1014 | 70 | 10.5 | 12.1 | 0.67 | 0.43 | 278 |
| IAP-3 | M | 36 | 35 | SPK | 1 | 5,033 | 0 | SVT | 1 | 106 | 0 | 6.4 | 5.0 | 0.62 | 0.67 | 101 |
| IAP-4 | F | WL | 41 | SPK | WL | WL | WL | NPanc | 8 | WL | 95 (WL) | 8.6 † | WL | 0.33 † | WL | 10† |
IAP: Islet after Pancreas; PTX: pancreas transplant; ITX: islet transplant; SPK: Simultaneous pancreas/kidney transplant; PAK: Pancreas after kidney transplant; KTX: kidney transplant; IEQ: Islet equivalent; SVT: splenic vein thrombosis; NPanc: Necrotizing pancreatitis; cPRA: calculated Panel Reactive Antibodies; WL: on the waitlist for ITX, not transplanted yet; †: at the time of writing of the manuscript
PTX after failed ITX: The 2 patients who received a PAI previously achieved periods of insulin independence after islet transplantation, then developed graft loss. The first patient (PAI-1) became insulin-independent shortly after the second ITX and was listed for PAI after losing islet graft function 44 months post first infusion. The second patient (PAI-2) also became insulin-independent after the second ITX but received 2 additional ITX after losing transplanted islet graft function 62 months after first ITX. Renal function deteriorated progressively in patient 1 but creatinine stabilized at 162µM/L after pancreas transplantation. Renal function in patient 2 was not affected by PTX and was 79µM/L at time of most recent follow up (Fig. 1). At time of PTX, both had elevated HbA1C (7.6% and 9.3% for patients 1 and 2 respectively). Both were sensitized to previous islet donor HLA antigens at time of listing for PTX (Table 1), and waited 2.8 and 0.8 y respectively (mean time on wait list 1.8 years, SD. One.4) to receive PTX. Both continue to have a full functioning transplanted pancreas with HbA1C of 4.8% and 5.3% (on no exogenous insulin) at time of most recent follow-up (40 months and 5 months post-PTX respectively, Fig. 1). A further 3 subjects await PAI currently, and have been listed for a mean of 6.3 ±4 .5 y All are sensitized to previous donor HLA, and represent subjects transplanted in the original Edmonton Protocol series (sirolimus-based immunosuppression with low dose tacrolimus). The risk of HLA sensitization in islet induction and maintenance protocols in place since 2002, using alemtuzumab induction, and higher dose tacrolimus maintenance therapy is low, and have not been associated with risk of HLA sensitization in our center.
Figure 1.

Patients who received a pancreas alone transplant after failed islet transplant. Evolution of c-peptide, HbA1C and creatinine since the first islet transplant. Islet transplants (ITX) and pancreas transplant (PTX) are indicated with an arrow. Patient PAI-1 had one determination of c-peptide after the PTX, patient PAI-2 had none. The creatinine of patient PAI-1 culminated at 363 [μmol/L], although the vertical scale was caped at 250 [μmol/L].
Both patients presented a similar creatinine at the time of the first ITX and at the time of PTX (78 and 94 for PAI-1; 66 and 65 for PAI-2). One patient (PAI-1) had a temporary rise of the creatinine before losing islets function, when higher therapeutic immunosuppression levels were targeted. The same patient presented an acute kidney injury after PTX, secondary to a combination of dehydration and increased dose of tacrolimus in the context of PTX. Creatinine returned to lower values after adequate rehydration but did not reach basal levels (Fig. 1). Of note, patient PAI-1 also presented neurogenic bladder, which likely contributed to renal dysfunction.
ITX after failed PTX: Three subjects have received ITX after failed PTX, and one remains listed. In contrast to PAI, all in the IAP group had previous end-stage renal disease (Table 2). Two developed pancreatic necrosis secondary to splenic vein thrombosis in the early post-operative period (both pancreata had been perfused with Histidine Ketoglutarate Solution (HTK), associated with poorer pancreas transplant survival in a large retrospective analysis17). The third also lost transplanted pancreas function at 3.6 months post transplant, secondary to necrotizing pancreatitis requiring graft explantation. One of the 3 developed HLA sensitization associated with the failed pancreas transplant. The mean time on the waitlist was 0.36 ± 0.4 y Of the 2 subjects receiving 2 ITX and more than 10,000 IEQ/kg islet mass, one became insulin-independent 74 d after the second infusion and remains insulin-free at most recent last follow up (5 y post second ITX). The second patient did not reach insulin-independence despite robust levels of c-peptide, initially around 0.9 nmol/L, and had elevated HbA1C (Fig. 2, IAP-2). Since insulin resistance was a predominant factor, likely related to obesity (BMI 30.8) combined with the effect of prednisone given for the renal transplant, a further islet transplant was not felt to be beneficial. The third patient awaits a second ITX to reach 10,000 IEQ/kg, and remains on insulin at time of the last follow up. Extensive condylomata acuminate infection, requiring recurrent surgical resection, prevented a second ITX and the patient returned to dialysis secondary to acute kidney rejection 22.5 months post-simultaneous kidney-PTX while his tacrolimus trough levels were kept in a low range to treat the condyloma infection.
Figure 2.
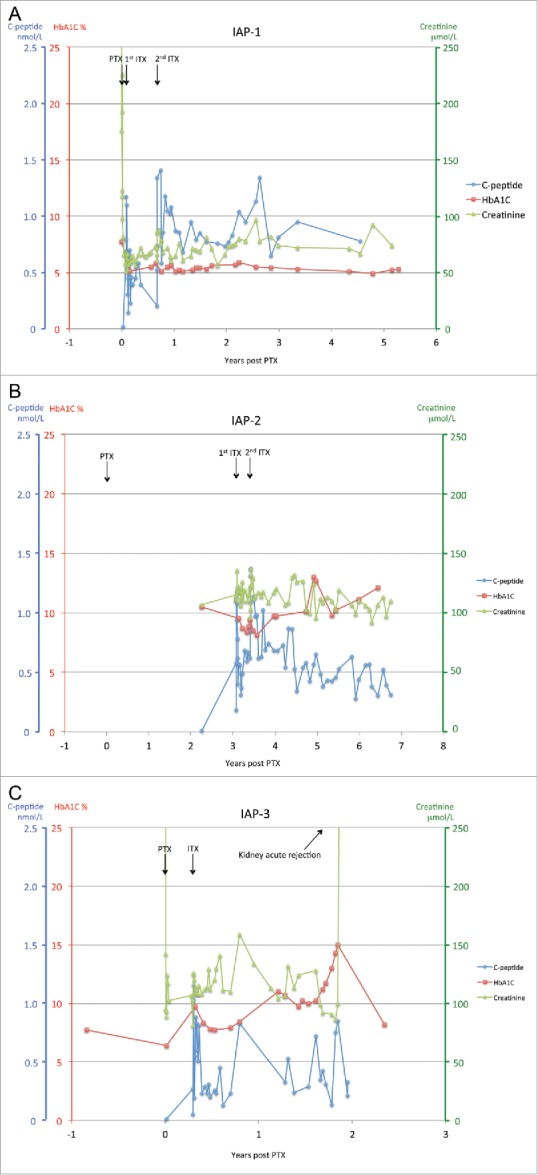
Patients who received an islet transplant after failed pancreas transplant. Evolution of c-peptide, HbA1C and creatinine since the pancreas transplant. The timing of pancreas graft failure is indicated in Table 2. Islet transplants (ITX) and pancreas transplant (PTX) are indicated with an arrow. Patient IAP-2 did not receive the PTX in our center and started his follow-up in our institution more than 2 y after-PTX. The patient IAP-3 presented an acute kidney rejection indicated by an arrow. His follow-up was managed in a distant center and the measurement of his c-peptide was discontinued after re-starting his dialysis.
C-peptide, HbA1C and creatinine for the 3 IAP patients are summarized in Figure 2.
Compared to PAI, IAP spent a shorter waitlist period to transplant (0.36 versus 1.8 y for IAP and PAI transplanted patients respectively, p = 0.17). This difference was more pronounced when considered by waitlist only, with 0.35 ± 0.3 years, vs. 4.5 ± 4.1 years, for IAP and PAI respectively (p = 0.08), but short of statistical significance certainly due to the small number of subjects.
The analysis of the renal function is more difficult in IAP group as 2/3 patients received a simultaneous pancreas-kidney transplant and therefore had high creatinine levels at the time of pancreas transplantation. Additionally, the waiting time between PTX failure and ITX is relatively short making the renal function difficult to analyze during this period. One patient (IAP-3) developed acute rejection of the previous transplanted kidney 1.6 y after one islet infusion, unlikely related to the ITX.
Discussion
This case series demonstrates both feasibility and safety of PAI and IAP in the setting of primary graft failure. Both modalities provide a more effective alternative to exogenous insulin in patients that are already receiving immunosuppression. Although a series of 6 cases of PAI have been presented in the 2015 American Transplant Congress,18 no prospective study has been published on this topic. Furthermore, this work is, to our knowledge, the first to describe islet transplantation as a rescue therapy after failed pancreas transplantation.
In PAI group, the 2 transplants were successful, no technical complications were reported and patients remain insulin-independent with excellent metabolic control at most recent follow-up. Although graft survival of pancreas alone transplants is inferior to simultaneous kidney-pancreas transplant,19 it may still provide good outcomes. In this limited series, the most striking difference between IAP and PAI is the timing of subsequent transplantation. In our local experience, we have been able to list and find suitable donors for IAP candidates within weeks of failed pancreas transplantation, largely due to access to a much larger national pool of potential donors, and for IAP recipients that are largely non-sensitized. In contrast, those few patients with failed multiple islet infusions that become highly sensitized from our original islet protocols and may sit on the pancreas transplant waiting list for years. The waiting time is certainly highly reflective of the sensitization status. Indeed, a few of these cases may never receive PAI due to lack of suitable donors and limited data regarding the safety or outcomes of desensitization in this population. It should be noted that risk of HLA sensitization after islet transplantation in the current era of T-depletional induction with alemtuzumab, combined anti-inflammatory therapies and high dose tacrolimus and mycophenolate mofetil maintenance is exceedingly rare as long as immunosuppression is maintained.
By contrast, only one of the 2 subjects receiving IAP and >10,000 IEQ achieved good glycaemic control, reflected by insulin independence and/or prolonged HbA1C below 6.5%. Of note, the patient who did not achieve insulin independence (IAP-2) had evidence of persistent islet function reflected by c-peptide levels but presented a probable insulin resistance that contraindicated a further infusion. Diabetic nephropathy is associated with insulin resistance and features of the metabolic syndrome. The third subject (IAP-3) has not yet received full islet transplantation (>10,000 IEQ).
Our report raises several questions that should be addressed in future studies: 1) Which potential candidates benefit most from PAI or IAP; 2) How should immunosuppression be managed while waiting for the next transplant after a failed graft? Handling of immunosuppression after a failed transplant is driven by concern for recipient sensitization; 3) Does recurrent autoimmunity play a role in future graft outcomes?
Pancreas re-transplantation has shown similar graft and overall survival compared to primary transplants in highly selected patients, but requires invasive surgery in a non-virgin abdomen,13 although previous surgery is not an absolute contra-indication to pancreas transplantation. IAP is a much simpler and safer procedure than pancreas re-transplantation.
Sensitization post islet transplant was previous reported and has been associated with graft failure. In particular broad sensitization was seen after discontinuation of immunosuppression.20 Current practice is to maintain immunosuppression (at least with mycophenolate mofetil) after failed islet transplantation with the goal to limit development of de novo HLA-antibodies.20 As the IAP recipients still had functioning kidney transplants they were already on maintenance immunosuppression and had not developed DSA post pancreas failure. We therefore recommend maintaining low level of immunosuppression if PAI or IAP is contemplated at the time of failed primary graft. In this case, we recommend mycophenolate mofetil maintenance monotherapy as a means to minimize calcineurin inhibitor (CNI) exposure to protect renal function. With this approach, we did not observe a long standing worsening of the renal function on the waitlist for the 5 patients. This is particularly relevant for PAI patients for whom the maintenance of the immunosuppression is only indicated to avoid an HLA sensitization. One subject (PAI-1) experienced a worsening of renal function after PTX, secondary to the rise of CNI levels and a prolonged dehydration. However, this acute kidney failure could have been enhanced by the previous low-dose tacrolimus treatment, as well as diabetic neurogenic bladder dysfunction and underlying diabetic nephropathy. We emphasize the need to follow closely the renal function of patients on the waitlist for PAI. In IAP, all our patients had a previous kidney transplant that required full immunosuppression.
In summary, we have presented herein our preliminary early experience in patients receiving PAI or IAP for failed prior modality transplantation. Our results show that PAI is associated with a good metabolic control but is a difficult goal to achieve as most of the early islet candidates are sensitized and experience a long waiting time. Current islet induction and maintenance protocols are much less susceptible to recipient sensitization. In contrast, it is easier to access IAP, which represents a minor and safe procedure, especially for patients already taking immunosuppression for previous kidney transplant, but the results suggest a good metabolic control for a more limited percentage of transplanted patients. These observations are clearly based on a limited number of cases and further experience is required. Meanwhile, we continue to recommend this option be offered in a timely manner where possible to protect renal function and prevent secondary complications of diabetes.
Patients and Methods
All patients receiving ITX after PTX (IAP) or PTX after ITX (PAI) at our institution were identified through retrospective analysis of our prospective electronic registry.
Pancreas transplant: Indication for PAI was loss of transplanted islet function after completion of ≥2 ITX with infusion of >10,000 islet-equivalents (IEQ)/kg. Functional loss was defined as stimulated c-peptide <0.1nmol/L. Immunosuppression was maintained to prevent further sensitization while waiting. HLA antibody specificities were identified using single antigen beads (One Lambda - A Thermo-Fisher brand, CA). Calculated Panel Reactive Antibodies (cPRA) was determined at time of listing: high cPRA was not a contraindication for listing but a negative virtual crossmatch was required prior to organ acceptance. A donor-recipient cell based flow cross match was performed prior to transplant. The principles of whole pancreas procurement are well described previously21 but briefly the entire gland was dissected using a no touch technique leaving the vascular supply intact, and the iliac and carotid arteries were procured as conduits. On the back-table, iliac arteries were anastomosed to the splenic and superior mesenteric arteries in Y-graft configuration. Portal venous drainage via the superior mesenteric vein was carried out in 2 cases, and systemic (caval) drainage PTX in the third case, with enteric exocrine drainage performed via the proximal recipient jejunum in all. Maintenance immunosuppression was based on tacrolimus (blood trough levels 10-12 ng/mL), mycophenolate mofetil (up to 2g per day as tolerated) and tapered prednisone. Graft function was assessed with serial random blood glucose, hemoglobin A1C (HbA1C), amylase and lipase. Post-PTX c-peptide levels were not monitored routinely.
Islet transplant: Methods for islet isolation have been described previously,22 but in brief pancreas weight was documented, the main pancreatic duct cannulated, and cold collagenase was perfused under controlled pressure for 10 min. The cut pancreas was introduced in a Ricordi chamber and warmed to 37°C. After digestion and continuous density gradient purification, islet yield, expressed as islet equivalent (IEQ), purity, and viability were assessed before and after culture, prior to transplantation. Intraportal islet transplantation was performed through percutaneous ultrasound and fluoroscopic-guided access, as described previously.23 Maintenance immunosuppression was based on combined tacrolimus (trough levels 8-12 ng/mL) and mycophenolate mofetil (up to 2g daily in divided dose as tolerated). Therapeutic heparin was initiated by intraportal infusion followed by continuous peripheral intravenous infusion, and transitioned to low molecular weight heparin and aspirin for 2 weeks thereafter. Transplanted islet function was assessed with blood glucose, HbA1C, random and stimulated c-peptide monitoring. Renal function was monitored closely in both groups.
Biographies
Author Contributions
Axel Andres: Research design, data gathering, data analysis, writing of the paper
Scott Livingstone: Data gathering, data analysis, writing of the paper
Tatsuya Kin: Research design, data gathering, writing of the paper
Patricia M. Campbell: Data gathering, data analysis, writing of the paper
Peter Senior: Research design, writing of the paper
Norman Kneteman: Research design, writing of the paper
David Bigam: Research design, writing of the paper
AM James Shapiro: Research design, data analysis, writing of the paper.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
Axel Andres: Research design, data gathering, data analysis, writing of the paper; Scott Livingstone: Data gathering, data analysis, writing of the paper; Tatsuya Kin: Research design, data gathering, writing of the paper; Patricia M. Campbell: Data gathering, data analysis, writing of the paper; Peter Senior: Research design, writing of the paper; Norman Kneteman: Research design, writing of the paper; David Bigam: Research design, writing of the paper; AM James Shapiro: Research design, data analysis, writing of the paper.
Funding
A.M.J.S. is supported through a Canada Research Chair in Transplantation Surgery and Regenerative Medicine, and through a Senior Clinical Scholarship from Alberta Innovates Healthcare Solutions.
References
- 1.Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, et al.. Projections of type 1 and type 2 diabetes burden in the US population aged. Diabetes Care 2012; 35(12):2515-20; PMID:23173134; http://dx.doi.org/ 10.2337/dc12-0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009; 373(9680):2027-33; PMID:19481249; http://dx.doi.org/ 10.1016/S0140-6736(09)60568-7 [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus . The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329(14):977-86; PMID:8366922; http://dx.doi.org/ 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 4.Choudhary P, Rickels MR, Senior PA, Vantyghem MC, Maffi P, Kay TW, Keymeulen B, Inagaki N, Saudek F, Lehmann R, et al.. Evidence-Informed Clinical Practice Recommendations for Treatment of Type 1 Diabetes Complicated by Problematic Hypoglycemia. Diabetes Care 2015; 38(6):1016-29; PMID:25998294; http://dx.doi.org/ 10.2337/dc15-0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54(7):2060-9; PMID:15983207; http://dx.doi.org/ 10.2337/diabetes.54.7.2060 [DOI] [PubMed] [Google Scholar]
- 6.Warnock GL, Thompson DM, Meloche RM, Shapiro RJ, Ao Z, Keown P, Johnson JD, Verchere CB, Partovi N, Begg IS, et al.. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation 2008; 86(12):1762-6; PMID:19104418; http://dx.doi.org/ 10.1097/TP.0b013e318190b052 [DOI] [PubMed] [Google Scholar]
- 7.Gremizzi C, Vergani A, Paloschi V, Secchi A. Impact of pancreas transplantation on type 1 diabetes-related complications. Curr Opin Organ Transplant 2010; 15(1):119-23; PMID:20010104; http://dx.doi.org/ 10.1097/MOT.0b013e32833552bc [DOI] [PubMed] [Google Scholar]
- 8.Ludwig B, Ludwig S, Steffen A, Saeger HD, Bornstein SR. Islet versus pancreas transplantation in type 1 diabetes: competitive or complementary? Curr Diab Rep 2010; 10(6):506-11; PMID:20830612; http://dx.doi.org/ 10.1007/s11892-010-0146-y [DOI] [PubMed] [Google Scholar]
- 9.Vardanyan M, Parkin E, Gruessner C, Rodriguez Rilo HL. Pancreas vs. islet transplantation: a call on the future. Curr Opin Organ Transplant 2010; 15(1):124-30; PMID:20009930; http://dx.doi.org/ 10.1097/MOT.0b013e32833553f8 [DOI] [PubMed] [Google Scholar]
- 10.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012; 12(6):1576-83; PMID:22494609; http://dx.doi.org/ 10.1111/j.1600-6143.2011.03977.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nature biotechnology. 2015;33(5):518-523; PMID:25893782; http://dx.doi.org/ 10.1038/nbt.3211 [DOI] [PubMed] [Google Scholar]
- 12.Shapiro AM, Ricordi C. Textbook of Organ Transplantation In: al Kirk A. D. et al., editors. Vol. 1, Wiley-Blackwell; 2014. [Google Scholar]
- 13.Buron F, Thaunat O, Demuylder-Mischler S, Badet L, Brunet M, Ber CE, Thivolet C, Martin X, Berney T, Morelon E. Pancreas retransplantation: a second chance for diabetic patients? Transplantation 2013; 95(2):347-52; PMID:23222920; http://dx.doi.org/ 10.1097/TP.0b013e318271d795 [DOI] [PubMed] [Google Scholar]
- 14.Koh A, Imes S, Kin T, Dinyari P, Malcolm A, Toso C, Shapiro AM, Senior P. Supplemental islet infusions restore insulin independence after graft dysfunction in islet transplant recipients. Transplantation 2010; 89(3):361-5; PMID:20145529; http://dx.doi.org/ 10.1097/TP.0b013e3181bcdbe8 [DOI] [PubMed] [Google Scholar]
- 15.Rudolph EN, Finger EB, Chandolias N, Kandaswamy R, Sutherland DE, Dunn TB. Outcomes of pancreas retransplantation. Transplantation 2015; 99(2):367-74; PMID:25594555; http://dx.doi.org/ 10.1097/TP.0000000000000566 [DOI] [PubMed] [Google Scholar]
- 16.Siskind E, Maloney C, Jayaschandaran V, Kressel A, Akerman M, Shen A, Amodu L, Platz J, Ricci JP, Bhaskaran M, et al.. Pancreatic retransplantation is associated with poor allograft survival: an update of the united network for organ sharing database. Pancreas 2015; 44(5):769-72; PMID:25931257 [DOI] [PubMed] [Google Scholar]
- 17.Stewart ZA, Cameron AM, Singer AL, Dagher NN, Montgomery RA, Segev DL. Histidine-tryptophan-ketoglutarate (HTK) is associated with reduced graft survival in pancreas transplantation. Am J Transplant 2009; 9(1):217-21; PMID:18986383 [DOI] [PubMed] [Google Scholar]
- 18.Gardner J, Roll G, Wisel S, Harbell J, Hynson B, Kaufman D, Posselt A, Stock P. Pancreas After Failed Islet Transplantation; a Successful Strategy to Maintain Insulin Independence [abstract]. Am J Transplant. 2015; 15 (suppl 3). [Google Scholar]
- 19.Gruessner AC, Sutherland DE, Gruessner RW. Long-term outcome after pancreas transplantation. Curr Opin Organ Transplant 2012; 17(1):100-5; PMID:22186094; http://dx.doi.org/ 10.1097/MOT.0b013e32834ee700 [DOI] [PubMed] [Google Scholar]
- 20.Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, Imes S, Halpin A, Ryan EA, Shapiro AM. High risk of sensitization after failed islet transplantation. Am J Transplant 2007; 7(10):2311-7; PMID:17845564 [DOI] [PubMed] [Google Scholar]
- 21.Fridell JA, Powelson JA, Kubal CA, Burke GW, Sageshima J, Rogers J, Stratta RJ. Retrieval of the pancreas allograft for whole-organ transplantation. Clin Transplant 2014; 28(12):1313-30; PMID:25203627; http://dx.doi.org/ 10.1111/ctr.12459 [DOI] [PubMed] [Google Scholar]
- 22.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988; 37(4):413-20; PMID:3288530; http://dx.doi.org/ 10.2337/diab.37.4.413 [DOI] [PubMed] [Google Scholar]
- 23.Low G, Hussein N, Owen RJ, Toso C, Patel VH, Bhargava R, Shapiro AM. Role of imaging in clinical islet transplantation. Radiographics 2010; 30(2):353-66; PMID:20228322; http://dx.doi.org/ 10.1148/rg.302095741 [DOI] [PubMed] [Google Scholar]


