ABSTRACT
Transplantation of islets into the gastric submucosal space (GSMS) has several advantages (e.g., avoidance of the instant blood-mediated inflammatory response [IBMIR], ability to biopsy). The aim of this study was to determine whether endoscopic biopsy of islet allografts transplanted into the GSMS in diabetic pigs can provide histopathological and immunohistochemical information that correlates with the clinical course (e.g.,, blood glucose level, insulin requirement). Islet allografts (Group1: 10,000 kIEq /kg [n = 4]; Group2: 15,000 kIEq /kg [n = 2]) were transplanted into the GSMS of diabetic pigs under immunosuppression. In Group2, the anti-oxidant, BMX-001 was applied during preservation, isolation, and culture of the islets, and at the time of transplantation. Endoscopic biopsies of the islet grafts were obtained one or 2 weeks after transplantation, and histopathological features were compared with the clinical course (e.g., blood glucose, insulin requirement). In Group1, in the absence of anti-oxidant therapy, most of the islets became fragmented, and there was no reduction in exogenous insulin requirement. In Group2, with an increased number of transplanted islets in the presence of BMX-001, more healthy insulin-positive islet masses were obtained at biopsy and necropsy (4 weeks), and these correlated with reductions in both blood glucose level and insulin requirement. In all cases, inflammatory cell infiltrates were present. After islet transplantation into the GSMS, endoscopic biopsy can provide information on graft rejection, which would be an immense advantage in clinical islet transplantation.
KEYWORDS: anti-oxidant, biopsy, endoscopy, gastric submucosal space, islets, islets, pig, transplantation
Introduction
The main treatment for type 1 diabetes is insulin, but this does not fully prevent complications. Furthermore, intensive insulin treatment is associated with hypoglycemic episodes. The transplantation of islets from deceased human donors into patients with type 1 diabetes is proving increasingly successful, with a reduction in hypoglycemic episodes and diabetes-related complications.1 Fifty percent of recipients remain insulin-independent for 5 y2 However, after islet transplantation into the portal vein, there is a significant loss of islets within minutes associated with exposure of the islets to blood - the instant blood-mediated inflammatory reaction (IBMIR).3 An improvement in early engraftment might contribute to improved long-term graft survival.
Our previous study using the pig islet allotransplantation model indicated that the gastric submucosal space (GSMS) was superior to the portal vein for transplantation in terms of reduced IBMIR.4 In addition, islet transplantation into the GSMS by an endoscopic approach offers several advantages compared to the portal vein in the form of reduced technical complications and potential ability to biopsy.5 Monitoring for islet graft survival and rejection would be an immense advantage. Following intra-portal islet transplantation, histological assessment of the allograft cannot be reliably obtained. A previous report demonstrated that, using ultrasound-directed 18-gauge needle liver biopsies, it was only possible to identify insulin-positive cells within the liver parenchyma in 1 of 6 insulin-independent patients (17%).6 The cause of loss of islet function, e.g., acute rejection, cannot be fully assessed.
We previously established the technique of endoscopic biopsy after islet transplantation into the GSMS in non-diabetic pigs not receiving immunosuppressive therapy.5 Tissue obtained at both biopsy and necropsy 5 d after transplantation showed similar histological features of rejection. These results indicated that endoscopic biopsy of islet allografts in the GSMS is feasible, and provides accurate histopathological data.
To extend the previous study, we carried out endoscopic islet transplantation into the GSMS, and endoscopic biopsy in streptozotocin (STZ)-induced diabetic pigs under immunosuppressive therapy. We hypothesized that endoscopic biopsy can provide evidence of allograft rejection, and this would correlate with blood glucose levels, insulin requirements, and the results of immune assays. The present study is the first to demonstrate that endoscopic biopsy offers information on the graft that correlates with the clinical course after islet transplantation into the GSMS.
Materials and methods
Animals
Yucatan miniature swine (Sinclair, St. Louis, MO) were sources of pancreases (retired breeders, 120 kg) and recipients of islet grafts (at 2–3 months of age) (Table 1).
Table 1.
Summary of individual experiments in STZ-induced diabetic pigs.
| Group | IS | BMX-001 | Pig # | Body weight (kg) | Islets (kIEq /kg) | Islets/ site (kIEq /site) | Insulin before Tx† | Insulin after Tx† | BG before Tx‡ | BG after Tx‡ | Biopsy (day) | Day of euthanasia /necropsy | Reason for euthanasia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | TAC, MP | No | 3780 | 13.6 | 10.3 | 35.0 | 0.86 | 0.98 | 317 | 224* | 14 | 25 | Pulmonary infection |
| 1A | TAC, MP | No | 3786 | 14.0 | 10.0 | 35.0 | 1.04 | 0.96 | 308 | 312 | 7 | 28 | Elective |
| 1B | TAC, MP, MMF | No | 3779 | 13.6 | 10.3 | 35.0 | 1.07 | 0.98 | 384 | 217** | 14 | 28 | Elective |
| 1B | TAC, MP, MMF | No | 3777 | 14.0 | 10.0 | 35.0 | 0.67 | 0.57 | 182 | 215 | 7 | 23 | Pulmonary infection |
| 2 | TAC, MP, MMF | Yes | 5401 | 18.4 | 14.7 | 38.6 | 1.10 | 0.69** | 357 | 161** | 14 | 28 | Elective |
| 2 | TAC, MP, MMF | Yes | 5429 | 17.7 | 15.3 | 38.6 | 1.15 | 0.76** | 344 | 166** | 14 | 28 | Elective |
| 3 | None | No | 3781 | 19.5 | ND | ND | 0.96 | ND | 328 | ND | ND | 46 | Elective |
| 3 | None | No | 3802 | 20.0 | ND | ND | 1.02 | ND | 330 | ND | ND | 46 | Elective |
IS = immunosuppressive therapy. MMF = mycophenolate mofetil (Roche, Indianapolis, IN; 100–200mg/kg p.o. twice daily to maintain trough levels at 2–6µg/mL). MP = methylprednisolone (Pfizer, New York, NY; 2 mg/kg i.v. daily, tapering to 0.25mg/kg/day by day 7). ND = not done. TAC = tacrolimus (Astellas, Northbrook, IL; 0.05mg/kg i.m. twice daily to maintain trough levels at 10–15 ng/mL). Tx = transplantation
Insulin requirement is shown as daily dose per body weight (IU/kg/day)
Average of daily blood glucose (BG) level is shown as mg/dL. In Pig 3780 (Group1A) blood glucose level after transplantation was significantly lower than that during the period between STZ and islet transplantation
(p<0.05). In Pig 3779 (Group1B) blood glucose level after transplantation was significantly lower than that during the period between STZ and islet transplantation
(p<0.01). In Pig 5401 and Pig 5429 (Group2) both blood glucose level and insulin requirement were significantly lower than those during the period between STZ and islet transplantation
(p<0.01). Survival of Group3 pigs is indicated as the number days after the administration of STZ (as no islet transplant was performed).
All animal care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86–23, revised 1985). All protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Islet isolation, culture, and functional assays
Pancreatectomy and islet isolation were carried out as previously described.7 The pancreas was maintained in cold UW solution +/− 17 μM BMX-001 (Biomimetix, Greenwood Village, CO)8 during 1-hour transportation. Isolated islets were cultured +/− 17 μM BMX-001 overnight. Islet viability and in vitro insulin release (Table 2) were assessed.7
Table 2.
In vitro insulin secretion of donor islets.
| Donor # | Group | Recipient pig# | SI (high glucose/basal) | SI (theophylline+high glucose/basal) |
|---|---|---|---|---|
| 8004† | 1A / 1B | 3786 / 3777 | 4.6 | 12.4 |
| 9782† | 1A / 1B | 3780 / 3779 | 3.5 | 14.4 |
| 0906 | 2 | 5401 | 5.7‡ | 19.0‡ |
| 1098 | 2 | 5429 | 8.5‡ | 15.0‡ |
SI = stimulation index of insulin secretion.
Islets obtained from 8004 and 9782 were each transplanted into 2 recipients
Insulin secretion was higher when the islets had been cultured in the presence of BMX-001 (Group2), but no statistical analyses were performed because of the limited number of pigs in each group (n = 2).
Induction and monitoring of diabetes
Diabetes was induced by STZ (Zanosar; Sicor Pharmaceuticals, Irvine, CA; 200 mg/kg i.v.) 10–14 d before islet transplantation.9 Blood glucose levels were checked and controlled with exogenous insulin at least x2 daily.4,9 Porcine C-peptide was measured during an arginine stimulation test before and after STZ, and 2 weeks after islet transplantation (Porcine C-peptide ELISA kit, Mercodia, Uppsala, Sweden).
Endoscopic islet transplantation
Under inhalational anesthesia, islets (in culture medium +/− 17 μM BMX-001) were transplanted into the GSMS at 4 (Group1) or 7 (Group2) sites endoscopically via submucosal injection (GIF-2T160, Olympus Medical Systems, Center Valley, PA).5
Immunosuppressive regimen
Recipients were administered tacrolimus, methylprednisolone +/− mycophenolate mofetil (Table 1).
In vitro human peripheral blood mononuclear cells (PBMC) proliferation
BMX-001 was used to reduce oxidative stress induced during islet isolation. Although it also suppresses the proliferation of mouse splenocytes,10,11 there are no data on its efficacy on pig or human PBMCs. We therefore assessed the effect of BMX-001 in suppressing the proliferation of pig and human PBMCs. PBMCs from Yucatan miniature pigs (n = 4) and humans (n = 3) were isolated as previously described.12 Human participants gave informed consent per the guidelines of the Institutional Review Board of the University of Pittsburgh. PBMCs were stimulated with 10 µg/ml phytohaemagglutinin (PHA) (Roche, Basel, Switzerland) +/− 10 µM BMX-001, and cultured for 3 d at 37°C in 5% CO2. Cell proliferation was expressed as 3H-thymidine incorporation.12
Intracellular cytokine staining
Two hundred microliters (200 uL) of pig (n = 4) or human (n = 3) blood were incubated with Golgistop (BD Biosciences, Franklin Lakes, NJ), 40 ng/ml (for pig) or 10 ng/ml (for human) phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St Louis, MO), and 1 μg/ml Ionomycin (Sigma-Aldrich) for 3 h at 37°C in the presence or absence of 10 µM BMX-001. After washing with lysing buffer (BD) followed by PBS, live/dead staining was carried out using a live/dead fixable staining kit (Invitrogen, Grand Island, NY), according to the manufacturer's instructions. Surface staining was carried out using anti-pig CD3ε (clone BB23-8E6-8C8, BD), CD4a (clone 74-12-4, BD), CD8a (clone 76-2-11, BD), or anti-human CD3 (BD, clone SP34-2), CD4 (BD, clone L200), and CD8 (BD, clone RPA-T8) antibodies.
Intracellular cytokine staining was carried out using a Cytofix/Cytoperm plus Fixation/Permeabilization Kit (BD), according to the manufacturer's instructions. Briefly, samples were fixed and permeabilized with Cytofix/Cytoperm buffer for 20 min at 4°C. Cells were washed with Perm/wash buffer. Intracellular cytokines were stained with anti-pig IFN-γ (BD, clone P2G10) or anti-human IFN-γ (BD, clone 4S.B3), and TNF-α (iCyt, Champaign, IL, clone MAb11) which cross-react with pig antibodies. Surface and intracellular cytokine expression was detected by BD™ LSR II flow cytometer (BD). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Detection of donor-specific antibodies
Binding of alloreactive antibodies to donor pig aortic endothelial cells was measured as previously described.13 Briefly, 20% heat-inactivated recipient pig serum was incubated with 5 × 104 target cells for 30 min at 4°C. To prevent nonspecific binding, 10% goat serum was added after washing twice. IgM or IgG binding was detected by further incubation with FITC-conjugated goat anti-pig IgM (µ chain-specific) and IgG (γ chain-specific) (Bethyl Laboratories, Montgomery, TX) for 30 min at 4°C. Binding of IgM and IgG was assessed by relative geometric mean fluorescence intensity (MFI).
Experimental groups (Table 1)
Group1: Four pigs received islets (10,000 kIEq /kg) in the absence of BMX-001, and received tacrolimus and methylprednisolone (Group1A) with the addition of MMF (Group1B).
Group2: Two pigs received islets (15,000 kIEq /kg) that had been exposed to BMX-001. The immunosuppressive regimen was identical to that in Group1B.
Group3: As a diabetic control, 2 pigs were maintained for 6 weeks after STZ without immunosuppressive therapy or islet transplantation.
In Group1, the islets from a single donor were divided and transplanted into 2 recipients, whereas in Group2 single donor islets were transplanted into a single recipient (Table 1); the Group2 recipients received approximately 1.5 times more islets/kg (body weight) than those in Group1. A comparable number of islets were injected at each site in the GSMS in all groups (Group1 4 sites/recipient; Group2 7 sites/recipient.)
Endoscopic detection and biopsy of transplanted islets
Endoscopic ultrasonography and biopsy of the transplanted islets was carried out as previously described.5 Biopsies of 2 graft sites in each pig were obtained at 7 or 14 d after transplantation, using a modified technique of endoscopic submucosal dissection.5
Euthanasia and necropsy
Except in 2 pigs, in which pulmonary infections necessitated euthanasia on days 23 and 25, respectively, all recipients in Groups 1 and 2 underwent elective euthanasia on day 28 (Table 1). Histopathologic examination of the gastric wall (e.g., hematoxylin and eosin, insulin staining) was performed.5 Control (Group3) pigs were electively euthanized 6 weeks after STZ.
Statistical analysis
Values are presented as mean ± SEM. The statistical significance of differences was determined by Student's t-test using GraphPad Prism version 5 (Graphpad Software, San Diego, CA). Differences were considered to be significant at p < 0.05.
Results
In vitro assessment of donor islets
On the day of transplantation, dual fluorescent staining showed between 85–90% viable islets from each donor pig. Insulin secretion was higher in the islets that were cultured in the presence of BMX-001 (Table 2).
Effect of BMX-001 on pig and human PBMC proliferation and cytokine production in vitro
The in vitro suppressive capacity of BMX-001 on PBMC proliferation and cytokine production of T cells was investigated in pigs (Fig. 1A, B, C) and humans (Fig. 1D, E, F). BMX-001 significantly reduced proliferation of pig (Fig. 1A) and human (Fig. 1D) PBMCs stimulated by PHA. It also reduced both IFN-γ and TNF-α cytokine production from human CD4 and CD8 T cells stimulated by PMA (Fig. 1E, F), although BMX-001 only suppressed IFN-γ production from pig CD8 T cells (Fig. 1C). These results indicated that BMX-001 had an immune modulatory effect in addition to its beneficial effect on islet viability during preservation.
Figure 1.
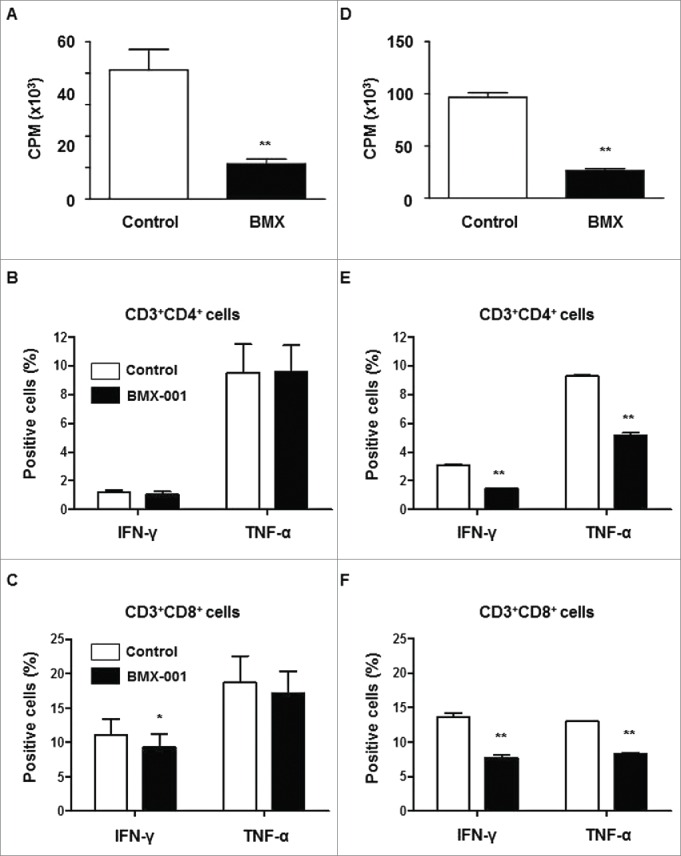
Suppression of T cell proliferation and activation by BMX-001 PBMCs from pigs (n = 4) and humans (n = 3) were stimulated with PHA for 72h with/without 10µM BMX-001. BMX-001 significantly reduced proliferation of both pig (A) and human (D) PBMCs stimulated with PHA (*p<0.01). Results are expressed as 3H-thymidine incorporation (counts per minute). The percentages of T cells in pigs (B,C) and humans (E,F) secreting the inflammatory cytokines, IFN-γ or TNF-α, were measured after intracellular staining of whole blood. In the presence of 10µM BMX-001, the percentages of both IFN-γ- or TNF-α-secreting CD4+ and CD8+ T cells were significantly decreased in humans (*p<0.01) whereas only the percentage of IFN-γ secreting CD8+ T cells was significantly decreased in pigs (*p<0.05). Data are expressed as mean ± SEM.
Clinical course
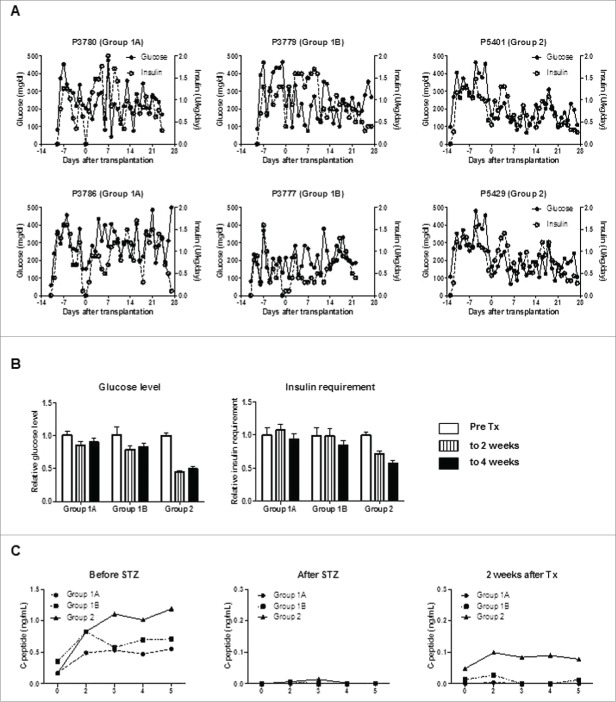
After STZ, all pigs became diabetic without complication. Group3 control diabetic pigs were maintained for 6 weeks (Fig. 2A and B), and diabetic status was confirmed by loss of C-peptide secretion in response to arginine stimulation (Fig. 2C). In Groups 1 and 2, blood glucose was controlled by exogenous insulin (Fig. 3A and B). Daily glucose levels significantly declined in one of the 2 pigs in Group1A (p < 0.05) and one in Group1B (p<0.01), while these received comparable doses of insulin after transplantation. Both glucose levels and insulin requirements declined in both Group2 pigs (p < 0.01) (Table 1). Peak C-peptide levels during arginine stimulation test at 2 weeks were higher in Group2 pigs (Fig. 3C), though they did not approach pre-STZ levels. Body weights remained stable, except Pig 3777 in Group1B (Fig. 4). These results indicated that an increased number of transplanted islets with BMX-001 therapy (in Group2) improved glyco-metabolic control after transplantation.
Figure 2.
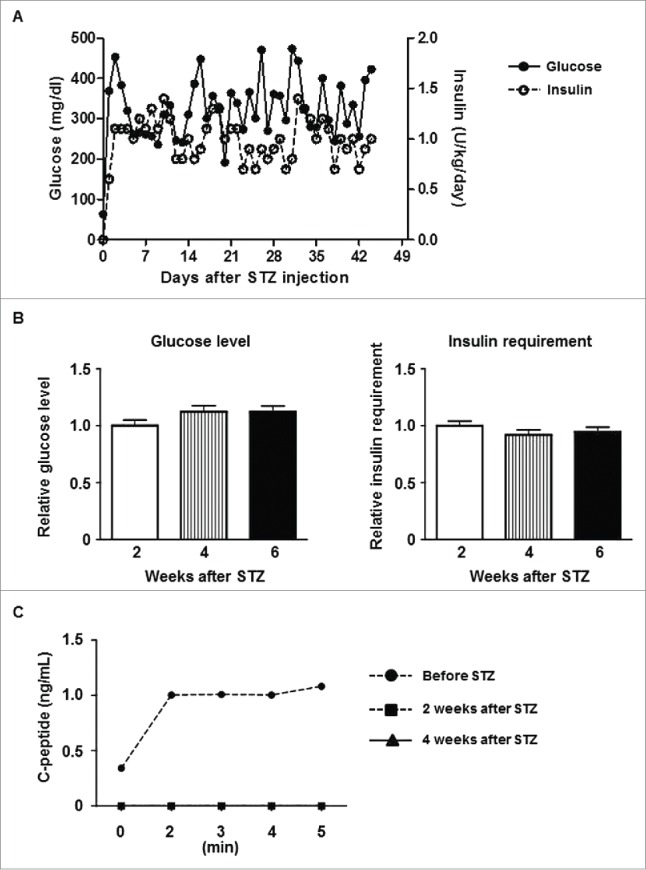
Control data of diabetic pigs (A) Average daily blood glucose levels (left y-axis) and exogenous insulin requirements (right y-axis) in Group3 pigs after STZ. (B) Blood glucose levels (left panel) and exogenous insulin requirements (right panel) during the first 2 weeks after STZ (0–2 weeks, white bar), the next 2 weeks (2–4 weeks, striped bar), and thereafter (to 6 weeks, black bar). The daily values (as documented in A above) were divided by the average of the first 2 weeks, and the ratio of each period is shown. Data are mean ± SEM. Neither glucose level nor insulin requirement showed significant changes in Group3. (C) C-peptide levels in response to arginine stimulation before STZ, and 2 weeks or 4 weeks after STZ. C-peptide secretion was abrogated by STZ.
Figure 3.
An increased number of transplanted islets with BMX-001 treatment of islets lead to improved glyco-metabolic control. (A) Daily blood glucose levels (left y-axis) and exogenous insulin requirements (right y-axis) in each pig in Groups 1 and 2. Blood glucose levels (but not insulin requirements) decreased in one Group1A pig (3780; p<0.05) and in one Group1B pig (3779; p<0.01). Blood glucose levels and insulin requirements decreased significantly in both Group2 pigs (p<0.01). (B) Mean blood glucose levels (left panel) and mean exogenous insulin requirements (right panel) before islet transplantation (pre-transplant, white bar), during the first 2 weeks after transplantation (striped bar), and thereafter (2 to 4 weeks, black bar) in each group were calculated to compare the changes after transplantation without the influence of daily fluctuation. The mean daily values in each group were divided by the mean of the pre-transplant period, and the ratio of this value for each group is shown (n = 2). Both glucose levels and insulin requirements decreased after transplantation in Group2. Data are mean ± SEM. (C) C-peptide levels in response to arginine stimulation before (left panel), after STZ (middle panel), and 2 weeks after transplantation (right panel). Following baseline blood sampling at 0 min, arginine was injected over a 1-min period. Additional blood samples were collected at 2, 3, 4, and 5 min following injection. C-peptide secretion was abrogated by STZ, and partially recovered following islet transplantation (n = 2).
Figure 4.
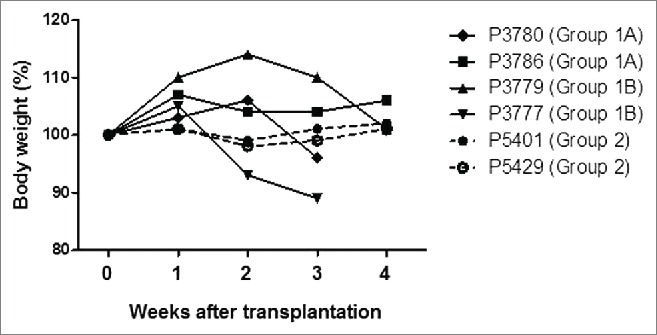
Body weight changes in each recipient pig. The weights of all recipient pigs remained stable after transplantation, except Pig 3777, which lost weight and underwent euthanasia on day 23 due to pulmonary infection.
Detection of anti-donor antibodies
No donor-specific IgM or IgG antibodies were detected in any pig (Fig. 5), except Pig 3780 in Group1A which had a minimal increase of anti-donor IgM antibodies at 2 weeks after transplantation (Fig. 5A).
Figure 5.
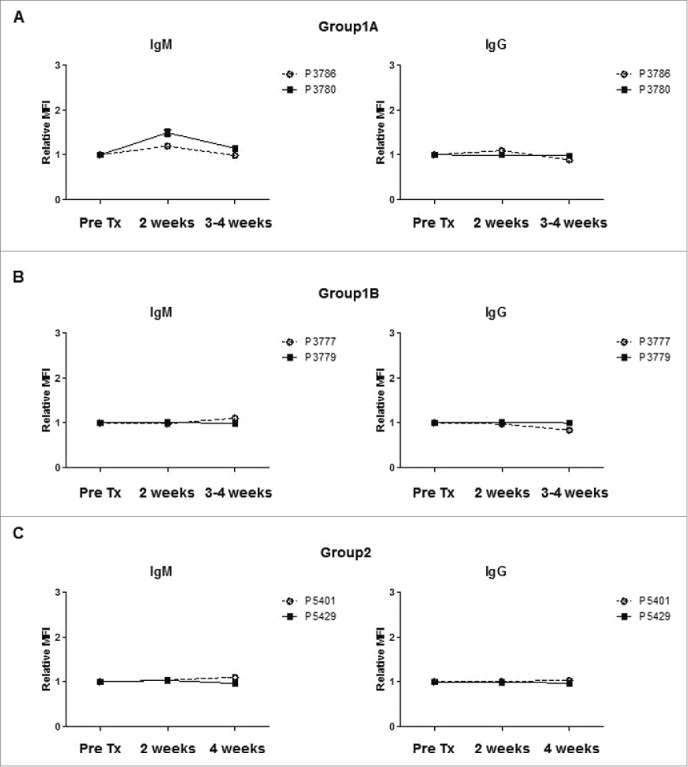
Anti-donor IgM or IgG antibody levels in recipients after islet Tx into the GSMS. To evaluate the sensitization to donor antigens, anti-donor IgM (left panels) and IgG (right panels) antibody levels were determined before, 2 weeks and 4 weeks after transplantation, except when recipient animals (Pig 3780, Pig 3777) were euthanized prior to 4 weeks due to pulmonary infection. Serum was tested at 3 weeks for these animals. IgM antibody levels were slightly elevated, but the level returned to the pre-transplant level in Pig 3780 (Group1A) (A). In contrast, there was no increase in IgM or IgG antibodies in Group1B (B) or Group2 pigs (C).
Histopathology
The islets transplanted into the GSMS could be readily identified and biopsied endoscopically.5 Histological examination of biopsies at 1 or 2 weeks (Fig. 6A) and necropsy at 4 weeks (Fig. 6B) showed fragmented insulin-positive islets in Group1, but significantly preserved islet masses with insulin-positive staining in Group2. A comparable infiltrate of CD3+T cells was seen in all groups (consistent with inadequate immunosuppressive therapy), as was the infiltration of macrophages (although there was possibly a more intensive early infiltrate in the Group1A pigs).
Figure 6.
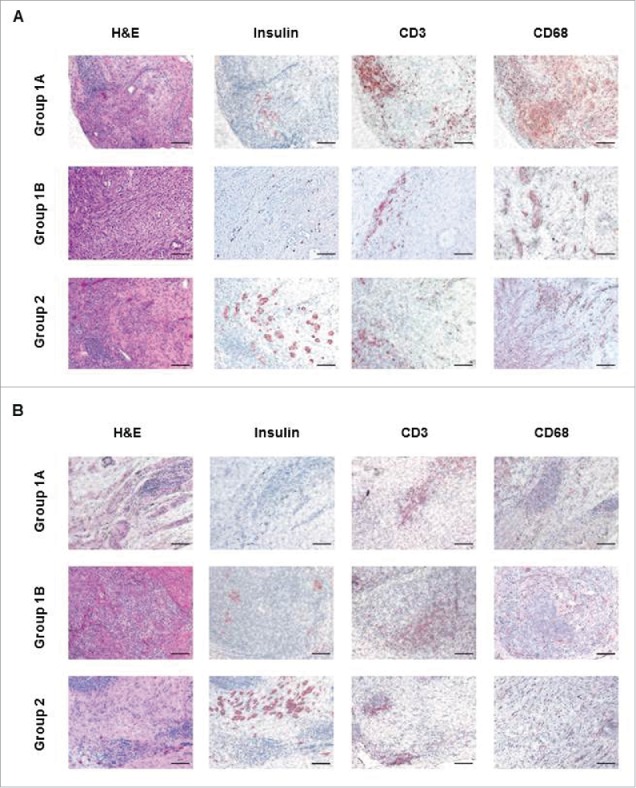
Comparison of histopathological examination in Groups 1 and 2 (A). Histopathological examination of islet allografts at biopsy (2 weeks after transplantation) of sections stained with hematoxylin and eosin (H&E, left columns), immunostained for insulin (second columns panels), CD3 cells (third column), and CD68 cells (right column). While Group1A or Group1B insulin-positive islets were fragmented, Group2 grafts showed preserved islet morphology. Cell infiltration was observed in H&E staining in biopsies from all groups, and most of the infiltrating cells were positive for CD3. CD68-positive cell infiltration was possibly more intensive in the Group1A pigs. (B) Histopathological examination of islet allografts at necropsy was performed 4 weeks after transplantation. While Group1A or Group1B insulin-positive islets were fragmented, Group2 grafts showed preserved islet morphology. Scale bars: 50μm.
Discussion
In clinical islet transplantation, monitoring for islet graft rejection by biopsy would be an immense advantage. This is not possible after islet transplantation into the portal vein, but in the GSMS identification of the islet masses by endoscopic ultrasonography, and biopsy by endoscopic submucosal dissection can be carried out successfully.5 Histopathological examination of biopsies provides valuable information, which will allow steps to be taken to enhance graft survival (e.g., by an increase in immunosuppressive therapy) and/or benefit the recipient (e.g., by indicating the need for retransplantation). The present study demonstrated that clinical observations largely correlated with histopathological findings, e.g., glycemic control was improved in the presence of good islet viability although, even with improved glycemic control, some cellular infiltrate was seen in the grafts.
In contrast to our previous study,4 none of the pigs became euglycemic without exogenous insulin therapy. Possible explanations are (i) the significantly fewer islets transplanted in the present study (mean 11.800 ± 1.000 vs 17.100 ± 1.600 IEQ/kg, p < 0.05), particularly in Group1, (ii) 2 grafts from each pig were removed at biopsy after 1 or 2 weeks (which represented 50% or approximately 30% of the grafts in Groups 1 and 2, respectively), and (iii) haploid-identical donor-recipient pig pairs were selectively used in the previous study (resulting in less cellular infiltration around the grafts), but not in the current study.
Since histological examination obtained from the biopsy and at necropsy showed fewer islets (with considerable fragmentation) in Group1 and, although there was some reduction in glucose level in 2 of 4 pigs, there was no reduction in insulin requirement in Group1, we made modifications in Group2. The number of islets transplanted was increased, and the islets were isolated, preserved, and transplanted in the presence of the antioxidant, BMX-001. Group2 recipient pigs received 1.5-times more islets/kg BW than Group1 recipients.
These modifications affected glyco-metabolic control, as well as C-peptide secretion after arginine stimulation. The histopathology seen on biopsy (and necropsy) indicated that there were well-formed islet masses in Group2, whereas there was fragmentation of the islets in Group1. The difference in histopathological appearance cannot be attributed to variation in the numbers of islets injected at each individual site as these were comparable. These results suggested that increased islet numbers and utilization of BMX-001 improved the clinical course as well as histopathological findings. However, graft infiltration with T cells was similar in all groups, as was the infiltration of macrophages (although there was possibly a more intensive early infiltrate in the Group1A pigs), indicating that all grafts were undergoing cellular rejection.
Islets intrinsically express low levels of antioxidant genes, and are vulnerable to oxidative damage.14,15 Islet isolation is achieved by mechanical and enzymatic digestive processes, followed by density gradient separation7, which results in oxidative stress, leading to proinflammatory cytokine production and islet cell death.16,17 Therefore, preventing oxidative stress (e.g., with an antioxidant) may protect the islets from nonspecific inflammatory events and facilitate islet engraftment. Oxidoreductants reduce the innate and adaptive immune responses by preventing nuclear factor-kappa B (NF-κB)-induced gene expression by preventing NF-κB from binding DNA.10,11,18 NF-κB shapes the innate and adaptive responses19,20 by controlling proinflammatory and proapoptotic genes in multiple cell types, including β-cells.21 When islets were exposed to BMX-010 in vitro, oxidative stress-induced NF-κB activation was inhibited17 and islet grafts in diabetic mice were protected.16,22 We have previously reported on the mechanism of islet viability by BMX agents. BMX agents have been shown to have protective capabilities for islets, but do not induce islet cell proliferation because mature islet cells do not possess proliferative capacity. The catalytic antioxidants (BMX) can scavenge reactive oxygen species, such as superoxide (O2−.), hydrogen peroxide H2O2, as well as nitric oxide. The ability of these agents to behave catalytically as the native superoxide dismutase with a high turnover number for dismutating superoxide allows them to protect islets during isolation-induced oxidative stress and the secondary inflammation that ensues.23-28 This reduction in inflammation is a result of these BMX agents having the ability to also inhibit the binding of NF-κB to DNA. Therefore, the oxidative stress-induced activation of NF-κB, which leads to the release of pro-inflammatory cytokines, is markedly reduced.17,18 A new generation oxidoreductant, BMX-001, which is more lipophilic, affording better bioavailability to target tissues and organelles than conventional BMX-010,8 was available to test in our study.
Improved therapeutic approaches to facilitate islet engraftment are needed,1,29,30 but there are no in vivo data relating to the use of an oxidoreductant in a large animal model. In the present study, we demonstrated the protective effect of BMX-001 on engraftment of pig islets in the GSMS, confirmed by endoscopic biopsy at 1–2 weeks. Repeated injections of BMX-001 by endoscopy at the sites of the islet transplants may enhance early islet engraftment because the oxidoreductant protects islets from apoptosis and reduces the immune response in mice.10,11,18 Both our previous in vivo mouse experiments (systemic injection22) and the present study's in vitro model using human or pig PBMCs demonstrate that BMX could reduce the immune response. Therefore, we suggest that systemic administration of BMX may help reduce the host immune response. A combination of local and systemic administration may prove optimal.
In the present study, graft rejection was not prevented, and therefore improved immunosuppression is required. As some agents are not effective in pigs, e.g., anti-thymocyte globulin, the nonhuman primate model might prove preferable.
Further study is necessary to investigate whether islet transplantation into the GSMS would provide better islet graft survival in patients compared to islet transplantation through portal vein injection into the liver, which is commonly used in the clinic. Although we have shown the beneficial effect of BMX in an animal model, this drug is not currently clinically available. Therefore, further clinical trials of BMX are necessary.
We conclude that the increased number of transplanted islets in combination with use of BMX-001 resulted in improved (though imperfect) glyco-metabolic control, which was associated with improved histological appearances of the islets on biopsies obtained endoscopically. Islet transplantation into the GSMS avoids the IBMIR associated with transplantation into the portal vein and allows the ability to biopsy for histopathological assessment. Importantly, the GSMS is endoscopically accessible and minimally-invasive. The ability to monitor islet graft survival and rejection by an endoscopic biopsy will be an immense advantage of islet transplantation into the GSMS. This novel approach could quickly be explored in the clinic.
Author contributions
TT participated in the performance of the research and in the writing of the paper. MF, RB, KM, JL, WL, HI, MW, SB, and CL participated in the performance of the research and in the review of the paper. JDP participated in research design, provided the BMX-001, and reviewed the paper. DL performed statistical analysis and reviewed the paper. KH participated in research design and in review of the paper. DKCC participated in research design and in writing the paper. HH participated in research design, the performance of the research, and writing the paper. HH is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- GSMS
gastric submucosal space
- IBMIR
instant blood-mediated inflammatory reaction
- PBMC
peripheral blood mononuclear cell
- STZ
streptozotocin.
Disclosure of potential conflicts of interest
Jon D Piganelli is a consultant for BioMimetix Pharmaceutical, Inc. No other authors have a conflict of interest.
Acknowledgements
The authors thank Mr. Masahiro Ashizuka (Olympus Corporation of the Americas) for his technical help in performing endoscopic islet transplantation and biopsy.
Funding
This study was supported by NIH grant RO3 (AI096296, HH), by The Uehara Memorial Foundation Postdoctoral Fellowship (TT), by The Mochida Memorial Foundation for Medical and Pharmaceutical Research (TT), by Kawasaki Sukenobu Memorial Fund of Kawasaki Medical School for Research Study (MF), by Kawasaki Medical School Alumni Association Fund for Foreign Study (MF), and by an Ocular Tissue Engineering and Regenerative Ophthalmology Postdoctoral Fellowship (WL).
References
- 1.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, et al.. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012; 35:1436-45; PMID:22723582; http://dx.doi.org/ 10.2337/dc12-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012; 12:1576-83; PMID:22494609; http://dx.doi.org/ 10.1111/j.1600-6143.2011.03977.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 2007; 14:288-97; PMID:17669170; http://dx.doi.org/ 10.1111/j.1399-3089.2007.00419.x [DOI] [PubMed] [Google Scholar]
- 4.Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, van der Windt DJ, Ekser B, Casu A, Houser S, Ezzelarab M, et al.. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant 2009; 9:2485-96; PMID:19775318; http://dx.doi.org/ 10.1111/j.1600-6143.2009.02815.x [DOI] [PubMed] [Google Scholar]
- 5.Fujita M, McGrath KM, Bottino R, Dons EM, Long C, Kumar G, Ekser B, Echeverri GJ, Hata J, Haruma K, et al.. Technique of endoscopic biopsy of islet allografts transplanted into the gastric submucosal space in pigs. Cell Transplant 2013; 22:2335-44; PMID:23336557; http://dx.doi.org/ 10.3727/096368912X662381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toso C, Isse K, Demetris AJ, Dinyari P, Koh A, Imes S, Kin T, Emamaullee J, Senior P, Shapiro AM. Histologic graft assessment after clinical islet transplantation. Transplantation 2009; 88:1286-93; PMID:19996928; http://dx.doi.org/ 10.1097/TP.0b013e3181bc06b0 [DOI] [PubMed] [Google Scholar]
- 7.Bottino R, Balamurugan AN, Smetanka C, Bertera S, He J, Rood PP, Cooper DK, Trucco M. Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation 2007; 14:74-82; PMID:17214707; http://dx.doi.org/ 10.1111/j.1399-3089.2006.00374.x [DOI] [PubMed] [Google Scholar]
- 8.Batinic-Haberle I, Tovmasyan A, Roberts ER, Vujaskovic Z, Leong KW, Spasojevic I. SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid Redox Signal 2014; 20:2372-415; PMID:23875805; http://dx.doi.org/ 10.1089/ars.2012.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara H, Lin YJ, Zhu X, Tai HC, Ezzelarab M, Balamurugan AN, Bottino R, Houser SL, Cooper DK. Safe induction of diabetes by high-dose streptozotocin in pigs. Pancreas 2008; 36:31-8; PMID:18192878; http://dx.doi.org/ 10.1097/mpa.0b013e3181452886 [DOI] [PubMed] [Google Scholar]
- 10.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol 2007; 178:908-17; PMID:17202352; http://dx.doi.org/ 10.4049/jimmunol.178.2.908 [DOI] [PubMed] [Google Scholar]
- 11.Sklavos MM, Tse HM, Piganelli JD. Redox modulation inhibits CD8 T cell effector function. Free Radic Biol Med 2008; 45:1477-86; PMID:18805480; http://dx.doi.org/ 10.1016/j.freeradbiomed.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 12.Hara H, Witt W, Crossley T, Long C, Isse K, Fan L, Phelps CJ, Ayares D, Cooper DK, Dai Y, et al.. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology 2013; 140:39-46; PMID:23566228; http://dx.doi.org/ 10.1111/imm.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, Cooper DK. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int 2008; 21:1163-74; PMID:18764834; http://dx.doi.org/ 10.1111/j.1432-2277.2008.00736.x [DOI] [PubMed] [Google Scholar]
- 14.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996; 20:463-6; PMID:8720919; http://dx.doi.org/ 10.1016/0891-5849(96)02051-5 [DOI] [PubMed] [Google Scholar]
- 15.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997; 46:1733-42; PMID:9356019; http://dx.doi.org/ 10.2337/diab.46.11.1733 [DOI] [PubMed] [Google Scholar]
- 16.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes 2002; 51:2561-7; PMID:12145171; http://dx.doi.org/ 10.2337/diabetes.51.8.2561 [DOI] [PubMed] [Google Scholar]
- 17.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004; 53:2559-68; PMID:15448084; http://dx.doi.org/ 10.2337/diabetes.53.10.2559 [DOI] [PubMed] [Google Scholar]
- 18.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med 2004; 36:233-47; PMID:14744635; http://dx.doi.org/ 10.1016/j.freeradbiomed.2003.10.029 [DOI] [PubMed] [Google Scholar]
- 19.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol 2001; 19:23-45; PMID:11244029; http://dx.doi.org/ 10.1146/annurev.immunol.19.1.23 [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol 2001; 13:675-83; PMID:11312255; http://dx.doi.org/ 10.1093/intimm/13.5.675 [DOI] [PubMed] [Google Scholar]
- 21.Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M, Eizirik DL. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes 2001; 50:2219-24; PMID:11574401; http://dx.doi.org/ 10.2337/diabetes.50.10.2219 [DOI] [PubMed] [Google Scholar]
- 22.Sklavos MM, Bertera S, Tse HM, Bottino R, He J, Beilke JN, Coulombe MG, Gill RG, Crapo JD, Trucco M, et al.. Redox modulation protects islets from transplant-related injury. Diabetes 2010; 59:1731-8; PMID:20413509; http://dx.doi.org/ 10.2337/db09-0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piganelli JD, Flores SC, Cruz C, Koepp J, Batinic-Haberle I, Crapo J, Day B, Kachadourian R, Young R, Bradley B, et al.. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 2002; 51:347-55; PMID:11812741; http://dx.doi.org/ 10.2337/diabetes.51.2.347 [DOI] [PubMed] [Google Scholar]
- 24.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal 2010; 13:877-918; PMID:20095865; http://dx.doi.org/ 10.1089/ars.2009.2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delmastro MM, Piganelli JD. Oxidative stress and redox modulation potential in type 1 diabetes. Clin Dev Immunol 2011; 2011:593863; PMID:21647409; http://dx.doi.org/ 10.1155/2011/593863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delmastro-Greenwood MM, Votyakova T, Goetzman E, Marre ML, Previte DM, Tovmasyan A, Batinic-Haberle I, Trucco MM, Piganelli JD. Mn porphyrin regulation of aerobic glycolysis: implications on the activation of diabetogenic immune cells. Antioxid Redox Signal 2013; 19:1902-15; PMID:23682840; http://dx.doi.org/ 10.1089/ars.2012.5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delmastro-Greenwood MM, Piganelli JD. Changing the energy of an immune response. Am J Clin Exp Immunol 2013; 2:30-54; PMID:23885324 [PMC free article] [PubMed] [Google Scholar]
- 28.Delmastro-Greenwood MM, Tse HM, Piganelli JD. Effects of metalloporphyrins on reducing inflammation and autoimmunity. Antioxid Redox Signal 2014; 20:2465-77; PMID:23472672; http://dx.doi.org/ 10.1089/ars.2013.5257 [DOI] [PubMed] [Google Scholar]
- 29.Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, et al.. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes 2001; 50:1983-91; PMID:11522663; http://dx.doi.org/ 10.2337/diabetes.50.9.1983 [DOI] [PubMed] [Google Scholar]
- 30.Keymeulen B, Korbutt G, De Paepe M, Gorus F, Kloppel G, Pipeleers DG. Long-term metabolic control by rat islet grafts depends on the composition of the implant. Diabetes 1996; 45:1814-21; PMID:8922370; http://dx.doi.org/ 10.2337/diab.45.12.1814 [DOI] [PubMed] [Google Scholar]