Abstract
The short chain fatty acid (SCFA) receptor (free fatty acid receptor-3; FFAR3) is expressed in pancreatic β cells; however, its role in insulin secretion is not clearly defined. Here, we examined the role of FFAR3 in insulin secretion. Using islets from global knockout FFAR3 (Ffar3−/−) mice, we explored the role of FFAR3 and ligand-induced FFAR3 signaling on glucose stimulated insulin secretion. RNA sequencing was also performed to gain greater insight into the impact of FFAR3 deletion on the islet transcriptome. First exploring insulin secretion, it was determined that Ffar3−/− islets secrete more insulin in a glucose-dependent manner as compared to wildtype (WT) islets. Next, exploring its primary endogenous ligand, propionate, and a specific agonist for FFAR3, signaling by FFAR3 inhibited glucose-dependent insulin secretion, which occurred through a Gαi/o pathway. To help understand these results, transcriptome analyses by RNA-sequencing of Ffar3−/− and WT islets observed multiple genes with well-known roles in islet biology to be altered by genetic knockout of FFAR3. Our data shows that FFAR3 signaling mediates glucose stimulated insulin secretion through Gαi/o sensitive pathway. Future studies are needed to more rigorously define the role of FFAR3 by in vivo approaches.
Keywords: FFAR3, FFAR2, insulin secretion, islets
Abbreviations
- BHB
betahydroxybutyrate
- DEG
differentially expressed genes
- FFAR2
free fatty acid receptor 2
- FFAR3
free fatty acid receptor 3
- GPCR
G protein coupled receptor
- GSIS
glucose stimulated insulin secretion
- KRB
Kreb's ringer buffer
- MCPC
1-methylcyclopropane carboxylate
- PTX
pertussis toxin
- WT
wildtype.
Introduction
Short chain fatty acids (SCFAs) are a unique nutrient class as they originate largely from gut microbial fermentation of difficult to digest carbohydrates.1 As the gut microbiota is a novel, recently identified, factor involved in metabolism,1 investigating metabolic effects of SCFAs has emerged as a topical scientific question. Within this nutrient class, each SCFA is distinct and can be classified by the number of carbons in the molecule, which includes the primary SCFAs in the human body; acetate (carbon number is 2, C2), propionate (C3), and butyrate (C4).1 While each of these SCFAs is produced at high concentrations during gut microbial fermentation, in the plasma, acetate is at the highest concentration, followed by propionate and then butyrate.1 Recently, 2 G-protein coupled receptors (GPCRs), FFAR2 and -3 (free fatty acid receptor-2, and -3), that are activated by SCFAs have been described and observed to be expressed in multiple tissue types.2,3 With the identification of these GPCRs, certain biological effects of SCFAs have been attributed to their signaling through these receptors.4,5
Of interest here, it has also been reported that these SCFA receptors, FFAR2 and FFAR3, are expressed in pancreatic β (β) cells,6 where the primary function of β cells is the secretion of insulin to maintain euglycemia. Besides glucose, which is the primary stimulus for insulin secretion, other nutrients can act as insulin secretagogues, such as amino acids and long chain fatty acids, acting either through specific receptors or metabolic pathways.7 As compared to these nutrients, the role of SCFAs in insulin secretion has not been well investigated.1 Considering the existing studies, most of these studies were done over 30 to 40 years ago, and have revealed conflicting results. For acetate, some studies have observed that acetate augments8,9 and other studies that acetate inhibits glucose stimulated insulin secretion (GSIS).10 Compared to acetate, even fewer studies have examined propionate and butyrate in GSIS. One study observed that propionate inhibits GSIS11 and another study observed that butyrate augments GSIS.12 As these receptors are expressed in β cells and GPCRs have a well-described role in insulin secretion,13 investigating if SCFAs mediate GSIS through their cognate receptors is needed. Thus far, one report observed that acetate inhibits GSIS through signaling through these receptors.14
As we begin to examine the role of these SCFA receptors, FFAR2 and FFAR3, in insulin secretion, the pharmacology of these receptors needs to be considered, as each of these GPCRs has unique ligand preferences and potencies for SCFAs.15 For example, propionate is highly selective for FFAR3 as compared to FFAR2.15 Also, these GPCRs signal via unique pathways.3 Specifically, FFAR2 can signal via 2 different Gα pathways (Gαq/11 or Gαi/o) where signaling via each pathway is anticipated to influence GSIS differently (either augment or inhibit, respectively), whereas FFAR3 only signals via one Gα pathway (Gαi/o) which is anticipated to inhibit GSIS.13 While SCFAs can potentially affect GSIS via these receptors, SCFAs also likely can impact GSIS independent of their receptors through anaplerotic pathways as described with other nutrients.16 Considering the above, we, here, investigated the role of FFAR3 in insulin secretion by using islets from FFAR3 ablation mice (Ffar3−/−), and specific endogenous ligands and agonists/antagonists for FFAR3 to dissect specifically how FFAR3 contributes to GSIS.
Materials and Methods
Mice
Ffar3+/− mice (kindly provided by Dr. Yanagisawa, University of Texas Southwestern Medical Center) were maintained on a C57BL/6J background. Heterozygous Ffar3+/− mice were crossed to produce wild type (WT) and knockout (Ffar3−/−) mice and genotyped by PCR as before.17 All animal studies were conducted in accordance with regulations of the Institutional Animal Care and Use Committee at Northwestern University.
Islet isolation
Islets from male mice (age 10–14 weeks) were isolated by a collagenase (Sigma, St. Louis, MO) digestion and separated as before.18 Isolated islets were rested overnight at 37°C in RPMI 1640 supplemented with 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin (with or without 300 ng/ml pertussis toxin, PTX) prior to experiments.
In vitro insulin secretion and insulin content
For the GSIS studies, islets were selected from pooled islet isolations from 2 pancreata to ensure that sufficient islets of similar size could be selected. Next, these islets were preincubated for 30 min in Krebs-Ringer Buffer (KRB; NaCl 130 mM, KCl 4.7 mM, NaH2PO4 0.5 mM, MgSO4 0.5 mM, CaCl2 1.5 mM, HEPES 10 mM, BSA 0.1%, pH 7.4, where the BSA (fraction V, protease-free) was from Roche Pharmaceuticals) and then in KRB plus 2.8 mM glucose for 60 min at 37°C. Further groups of 5 islets were incubated in 1 ml KRB plus different glucose concentrations, or 16.7 mM glucose plus ligands (SCFAs, agonists, or Exendin-4, each from Sigma, St. Louis, MO) for 60 min in a shaking water bath at 37°C. Concentrations of the SCFAs were 100 μM (for propionate and butyrate) and 1 mM (for 1-methylcyclopropane carboxylate, MCPC or β hydroxybutyrate, BHB). After the last incubation period, supernatant was sampled and assayed for insulin by ELISA (ALPCO diagnostics). For islet insulin content, islets were sonicated in acid-ethanol solution and solubilized overnight at 4°C before insulin ELISA.
RNA sequencing and quantitative real time PCR
Total cellular RNA was extracted from isolated islets that were selected from pooled islet isolations from 2 pancreata (total number of islets per sample was 250) using a RNeasy Mini kit (QIAGEN). RNA-sequencing and data analyses were both carried out by the Next Generation Sequencing Core Facility at Northwestern University (n = 3 per genotype; where each sample included islets isolated from separate groups of mice). Alignment and expression analysis were performed using TopHat (v2.0.8b) and Cufflinks (v2.1.1). Differential expression was determined by cuffdiff using an FDR cutoff value of 0.05. After this, the R package, cummeRbund, was used to obtain up- and down-regulated genes. A pathway analysis was performed using GeneCoDis. The generated data is available in Gene Expression Omnibus (GEO) under submission number GSE67991. Quantitative real-time PCR was performed using 1-Step SYBR Green qRT-PCR Kit. The relative gene expression was determined by comparative ΔCt method after normalization to β actin. The primers used are available on request.
Statistical analysis
P values were determined using Student's 2-tailed t-test.
Results
Ffar3−/− islets secrete more insulin
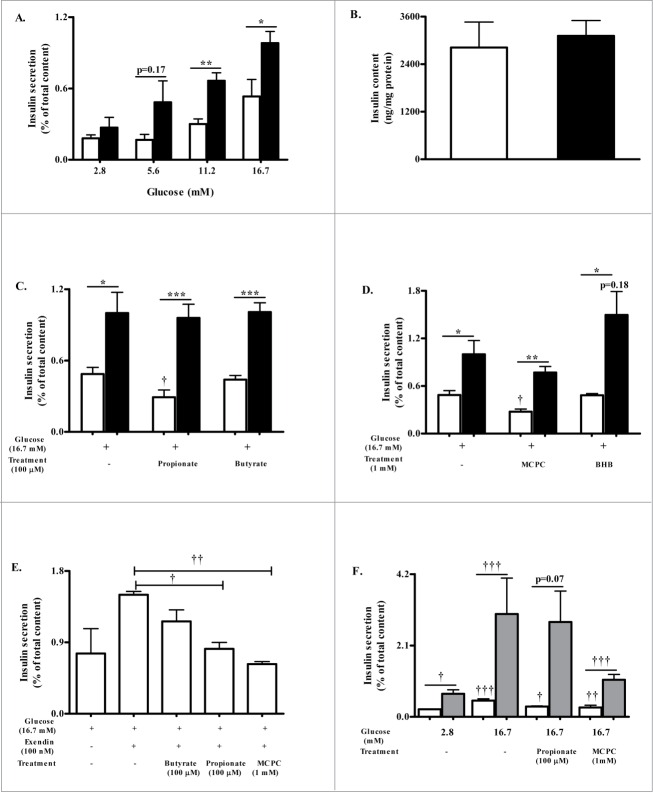
As genetic knockout of FFAR3 may impact insulin secretion, as observed in studies with other GPCR knockout models,19 we first assessed if islets from Ffar3−/− mice have altered insulin secretion to increasing concentrations of glucose. For these studies, we used islets isolated from 10–14 week old Ffar3−/− mice and age matched wildtype (WT) littermates. Both WT and Ffar3−/− islets exhibited glucose concentration dependence in insulin secretion (Fig. 1A). However, WT islets secreted less insulin compared to Ffar3−/− islets at each glucose concentration becoming significant at higher concentrations of glucose (Fig. 1A). This attenuation of GSIS from WT islets was not due to altered insulin content (Fig. 1B). Overall, these data suggest that genetic ablation of Ffar3 increases the insulin secretory capacity to increasing glucose levels.
Figure 1.
FFAR3 contributes to insulin secretion. (A) Insulin secretion in response to increasing glucose concentrations in isolated WT (white bar) and Ffar3−/− (black bar) islets during static insulin secretion assay (n ≥ 3). Insulin secretion is expressed as a percent of total insulin content. (B) Total islet insulin content measured following acid ethanol extraction from 20 islets per replicate. Insulin content was normalized to total protein (n = 3; WT, white bar and Ffar3−/−, black bar). (C) Insulin secretion from WT (white bar) and Ffar3−/− (black bar) islets in response to treatment with high glucose (16.7 mM) alone or in combination with 100 μM propionate or butyrate (n ≥ 3). (D) Insulin secretion from WT (white bar) and Ffar3−/− (black bar) in response to treatment with high glucose (16.7 mM) alone or in combination with 1 mM MCPC or BHB (n ≥ 3). (E) Insulin secretion from WT islets in response to high glucose (16.7 mM) alone or in presence of Exendin-4 (100 nM) plus 100 μM propionate or butyrate or 1 mM MCPC (n≥2 ). (F) Insulin secretion from WT islets that were pretreated overnight with PTX followed by treatment with 16.7 mM glucose or in combination with 100 μM propionate or 1 mM MCPC (n ≥ 3). Gray bars for PTX treated WT islets, white bars for nontreated WT islets. Asterisks represent significance between genotypes; daggers represent significance within a genotype between the indicated treatment condition compared to 16.7 mM glucose alone. *,†P < 0.05; **,††P < 0.01; ***,†††P < 0.001. For A-F, mean ± SEM.
FFAR3 signaling negatively mediates insulin secretion
GSIS can be modulated by nutrients signaling via their cognate GPCRs.7 Considering that islets express specific GPCRs for SCFAs,6 we next assessed if SCFAs impact GSIS through FFAR3, focusing on the most potent and selective SCFAs for FFAR3, propionate and butyrate.3 Using these ligands, we observed with WT islets, GSIS was significantly diminished by propionate by 40%, as compared to high glucose alone (Fig. 1C), but GSIS was not significantly altered by butyrate (Fig. 1C). With the Ffar3−/− islets, GSIS was not altered by propionate, suggesting that propionate inhibits GSIS through a FFAR3-dependent mechanism (Fig. 1C).
As nutrients can often impact GSIS through both receptor-dependent and -independent pathways,7 we wanted to verify that FFAR3 signaling mediates GSIS by using selective modulators for FFAR3. First, we used an agonist and antagonist of FFAR3 to explore GSIS in our model (Fig. 1D). The FFAR3 agonist, 1-methylcyclopropane carboxylate (MCPC), decreased GSIS with WT islets by 43% as compared to 16.7 mM glucose alone (Fig. 1D). This effect was not significantly present in Ffar3−/− islets, suggesting MCPC mediates its action via FFAR3. Next, using an endogenously produced FFAR3 antagonist, β hydroxybutyrate (BHB),4 insulin secretion was not altered with WT islets and actually showed a trend toward increased insulin secretion with Ffar3−/− islets (as compared to untreated Ffar3−/− islets, p = 0.18).
As glucose is not the only insulin secretagogue, we next wanted to assess if FFAR3 signaling would also impact the effect of other insulin secretagogues on GSIS. Therefore, we tested whether these FFAR3 agonists affect insulin secretion in the presence of other secretagogues. Using exendin-4 (glucagon like peptide-1 receptor agonist), a well-known enhancer of GSIS, we tested whether FFAR3 ligands, propionate and MCPC, could counteract exendin-4 actions on insulin secretion in WT islets, observing that propionate and MCPC did inhibit exendin-4 induced GSIS (Fig. 1E).
Ligand signaling via Gαi/o mediates FFAR3 effects on insulin secretion
Determination of how FFAR3 signaling mediates GSIS is needed, where GPCRs primarily contribute to GSIS through signaling through their coupled G-proteins.13 As reported previously, FFAR3 has been observed to couple to Gαi/o signaling pathway.3 To determine if FFAR3 signaling via Gαi/o is the mediator of its action on insulin secretion, islets were pre-treated with pertussis toxin (PTX) to inactivate Gαi/o. GSIS in PTX-treated WT islets in response to propionate or MCPC was observed to not inhibit GSIS (Fig. 1F), indicating that both propionate and the FFAR3 agonist mediate GSIS via FFAR3-Gαi/o signaling. However, for MCPC, there was a slight, non-significant, inhibition (comparing PTX-treated WT islets with and without MCPC, p = 0.1), suggesting MCPC may have FFAR3-independent effects on GSIS.
Effects of FFAR3 ablation on the islet transcriptome
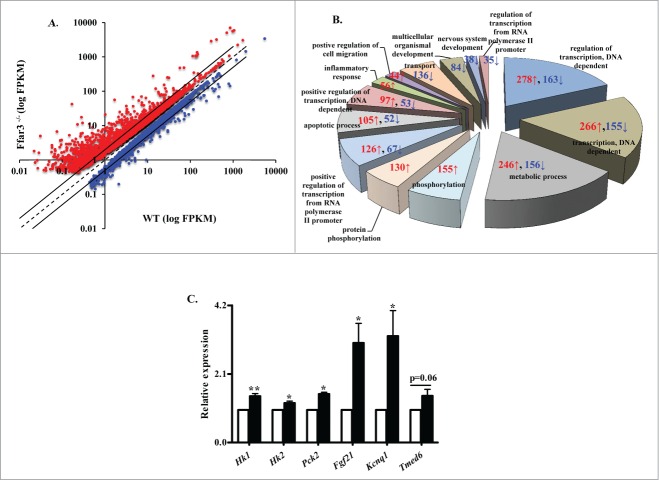
To help us understand the impact of genetic ablation of FFAR3 on GSIS, we assessed the islet transcriptome in Ffar3−/− relative to WT islets. RNA sequencing was performed observing that the expression of 4165 genes was significantly altered (1626 downregulated and 2539 upregulated) in Ffar3−/− as compared to WT islets (Fig. 2A, see also Table 1). The most prominent islet markers, insulin, glucagon, glucokinase, SUR1/Kir6.2 transcripts were abundant but not altered in the Ffar3−/− islets. Interestingly, Ghrl and Sst were downregulated in the Ffar3−/− islets (both significantly) as compared to WT islets. Gene ontology (GO) analysis revealed multiple categories were affected, with metabolic, transcription and transcription regulation processes most profoundly (Fig. 2B). Interestingly, KEGG pathway analyses of differentially regulated genes disclosed key genes implicated in type 2 diabetes (Tnf, Mafa, Mapk10, Pik3r3, Pik3r1, Irs2, each were downregulated) and type 1 diabetes (Gata4, Ptpn22, Btk, each were upregulated).
Figure 2.
Genetic ablation of the FFAR3 dramatically alters the islet transcriptome. (A) Profile plot of the differentially expressed genes in islets isolated from Ffar3−/− mice as compared to WT mice. Red dots: genes upregulated; blue dots: genes downregulated. Transcriptome analysis was performed on RNA collected from islets that were selected from pooled islet isolations from 2 pancreata (total number of islets per sample was 250 per sample; n = 3 per genotype; where each sample was islets isolated from separate groups of mice). (B) Gene ontology chart showing biological processes enrichment obtained from analysis of differentially expressed genes in Ffar3−/− islets. Each pie chart section shows number of genes upregulated (red) and downregulated (blue) in that category. (C) qRT-PCR analysis of select genes from Ffar3−/− and WT islets (n ≥ 3, 2–3 mice per group), white bars for wild type, black bars for Ffar3−/−. All results are expressed as mean ± SEM. *, P < 0.05. FPKM- Fragments per kilobase of transcript per million mapped.
Table 1.
Top 50 genes most significantly upregulated and downregulated in Ffar3−/− islets as compared wildtype islets
| Upregulated | Fold Change | Downregulated | Fold Change |
|---|---|---|---|
| Sh2d1a | Infa | 2200002J24Rik | −3.38 |
| Cd3e | Inf | Egr4 | −3.18 |
| Folr4 | Inf | Nr4a1 | −2.69 |
| Cd3d | Inf | Fosb | −2.54 |
| Cd19 | Inf | Gpr6 | −2.46 |
| Gimap7 | Inf | Dnajb1 | −2.23 |
| Banf2 | Inf | Per1 | −2.18 |
| Duoxa2 | Inf | Atp4a | −2.17 |
| Ms4a1 | Inf | Atf3 | −2.16 |
| Ubd | Inf | Apobec4 | −2.16 |
| Ccr6 | Inf | Adamts18 | −2.13 |
| Glycam1 | Inf | DXBay18,Gm14685 | −2.13 |
| Fam25c | Inf | 1700045I19Rik | −2.12 |
| Expi | Inf | Rbp7 | −2.12 |
| Timd4 | Inf | Egr2 | −2.08 |
| 2310057J18Rik | Inf | Arc | −2.08 |
| Vpreb3 | Inf | Ifnb1 | −2.05 |
| Fcrla | Inf | Aldh1a3 | −2.04 |
| Marco | Inf | Lrrc3b | −2.02 |
| Icos | Inf | C2cd4a | −2.00 |
| Igj | 8.00 | Nr4a2 | −1.99 |
| Prodh2 | 7.37 | Rasd1 | −1.97 |
| Lcn2 | 6.51 | Dnajb4 | −1.97 |
| Blk | 6.40 | Kcna5 | −1.93 |
| 2010001M09Rik | 6.00 | 8430408G22Rik | −1.92 |
| Irf4 | 5.86 | Cldn11 | −1.85 |
| Sprr1a | 5.61 | Hspa1b | −1.80 |
| Faim3 | 5.58 | Fos | −1.78 |
| Cd2 | 5.40 | Zfp184 | −1.73 |
| Ccr7 | 5.36 | Irs2 | −1.73 |
| Ltb | 5.35 | Kcnj3 | −1.72 |
| P2ry10 | 5.33 | 3930402G23Rik | −1.71 |
| Mpzl2 | 5.26 | 4930583H14Rik | −1.71 |
| Il16 | 5.21 | Tnf | −1.71 |
| Cyp1a1 | 5.20 | Hspa1a | −1.70 |
| Gm5771 | 5.09 | Egr3 | −1.69 |
| Ptpn22 | 4.95 | Igsf21 | −1.69 |
| Stat4 | 4.93 | Dpf3 | −1.68 |
| Il2rb | 4.92 | Cbx8 | −1.67 |
| Sept1 | 4.79 | Nap1l5 | −1.67 |
| Tcf7 | 4.79 | Cx3cr1 | −1.67 |
| Lfng | 4.72 | Gem | −1.65 |
| Csf2rb | 4.58 | Fam167b | −1.64 |
| Dnase1l3 | 4.51 | Il1a | −1.64 |
| Cd37 | 4.46 | Kcnj2 | −1.62 |
| B3gnt7 | 4.45 | 5330411J11Rik | −1.57 |
| Ptprcap | 4.44 | Edn2 | −1.56 |
| Prss3 | 4.42 | 2010110P09Rik | −1.55 |
| Krt19 | 4.41 | Agtr1a | −1.55 |
| Hmgcs2 | 4.41 | Pnmal1 | −1.51 |
Inf is abbreviated for infinity.
Examining genes with known roles in islet biology, genetic alterations occurred within the insulin secretion pathway including metabolic enzymes (Fbp2, Hk1, Hk2, Pfkfb2, Pck2, each were upregulated), ion channels and transporters (upregulated: Kcnq1; downregulated: Cacna2d2), exocytosis machinery (upregulated: Sycn; downregulated: Stxbp4), and insulin signaling (upregulated: Foxo1, Akt2, Eif4ebp1; downregulated: Irs2). Also, expression of multiple GPCRs known to influence insulin secretion were altered (downregulated: Ffar1, Ffar2, Glp1r, Gpr119; upregulated: Cckar, Ptger3). Expression of transcription factors essential for β cell function and differentiation were also influenced by Ffar3 ablation (downregulated: Mafa, Pax6, Nkx6–1, Nkx2–2; upregulated: Foxa1, Foxa3). Followup RT-PCR was performed, confirming the observed upregulation of Hk1, Hk2, Pck2, Fgf21 and Kcnq1, a key subset of genes with known roles in islet biology (Fig. 2C). Taken together, FFAR3 ablation results in a dramatic alteration of the islet transcriptome.
Discussion
A recent study by Tang et al.14 showed that FFAR3 and a related SCFA receptor, FFAR2, are both novel β-cell expressed GPCRs contributing to GSIS. In their report,14 the authors show that acetate, one of the 3 major SCFAs, inhibits GSIS by signaling through these receptors, and for both FFAR2 and −3, this effect on GSIS is mediated through a Gαi/o-coupled pathway. Using an approach different from their report (where we used propionate and FFAR3 agonists), our data confirms their findings that FFAR3 activation inhibits GSIS through a Gαi/o-coupled pathway. Thus, in conjunction with this report from Tang et al.,14 it is apparent that FFAR3 is a novel mediator of insulin secretion.
An important difference between these 2 studies is that our data indicates that knockout of FFAR3 leads to increased insulin secretion in response to increasing glucose levels. Multiple reasons could explain this difference. First, Tang et al.14 did not explore GSIS at multiple glucose concentrations, as done here. Another possible explanation is that the genetic approach used to create these FFAR3 knockout mouse models was different, and as a consequence, the downstream genetic changes could be unique between each model. Considering the degree of changes in our transcriptome analyses, this seems plausible. Other possibilities are that the experimental conditions used to assess insulin secretion were not the same and/or the backgrounds of the mouse models were different. Regardless, other GPCR knockout models have shown to have alterations in in vitro GSIS,19 but not all,20 and our particular FFAR3 knockout model has enhanced GSIS with increasing glucose concentrations, as compared to WT islets. Whether or not the change in insulin secretion in response to glucose is the result of FFAR3 deletion itself or other factors is not clear at this time.
A few other notable findings regarding our study warrant discussion. First, activation of FFAR3 resulted in significant inhibition of GSIS by either propionate or the FFAR3 agonist; however, this effect size is modest (see Fig. 1C–D). A possible reason for this modest inhibition of GSIS is that FFAR3 has been suggested to have high ligand independent constitutive activity.15 Thus, activating a constitutively active GPCR would only result in a small change in receptor activity. Unfortunately, for our understanding of this GPCR, this high constitutive active of FFAR3 may be specific to mouse FFAR3 and not human FFAR3.15 Therefore, as future studies explore the role of FFAR3 in human islets, close attention to species differences in receptor pharmacology such as constitutive activity needs to be considered.
Next, propionate, but not butyrate, was observed to inhibit GSIS. In the first published reports on these receptors, it was noted that propionate and butyrate were more specific to FFAR3 (as compared to FFAR2);3 however, these observations were determined using human FFAR2 and FFAR3. Since these original reports, it has been observed that mouse and human orthologs of each receptor have distinct pharmacology for SCFAs. For example, propionate is 12 times more potent at activating mouse FFAR3 than the other endogenous SCFAs,15 whereas butyrate is equally potent at FFAR3 and the other SCFA GPCR, FFAR2. However, it is not clear why butyrate does not inhibit GSIS in our study as FFAR2 signaling has been reported to also inhibit GSIS, as reported by Tang.14 Taken together, our data demonstrates that propionate inhibits GSIS, an observation consistent with previously published findings.11 Future studies need to consider species difference in ligand preference especially when exploring the role of FFAR3 in human versus mouse islets.
Lastly, BHB has been suggested to be a FFAR3 antagonist,4 and therefore, we utilized it here to explore FFAR3 signaling. However, we did not observe an increase in GSIS from BHB, as we hypothesized. Subsequent studies have actually suggested BHB is not an antagonist, but an agonist for FFAR3,21 and we did not observe this possibility either (for example, decreased GSIS). Considering these 2 studies and our findings, it is unclear if BHB signals through FFAR3 in mouse islets. Of interest, most reports suggest that BHB alone has little effect on GSIS,22,23 which is consistent with our data.
The islet transcriptome analyses performed here revealed widespread genetic changes in the Ffar3−/− islets compared to WT islets. Moreover, many genes that specifically contribute to GSIS were either up or downregulated. Whether or not this leads to meaningful changes in GSIS is unclear, but it could possibly explain the increased glucose responsiveness of the Ffar3−/− islets. Two genes, in particular, that were upregulated, Hk1 and Hk2 (which correspond to hexokinase 1 and 2), are hexokinases with low Km values for glucose and if overexpressed would result in more robust insulin secretion, as observed before.24,25 While genetic GPCR knockout models have been observed to impact the expression of other genes and in particular genes important in islet biology,26,27 the impact of these additional genetic changes on functional outcomes such as GSIS needs to be considered.
Taken together, our results, along with recent findings,14 establish FFAR3 as a negative GSIS modulator through its signaling by a Gαi/o pathway. As GPCRs are important diabetes targets,13 consideration of FFAR3 antagonists as a novel mechanism to enhance GSIS and as a viable diabetes treatment approach is needed.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Acknowledgments
We thank Dr. Yanagisawa, University of Texas Southwestern Medical Center, for providing the Ffar3+/− mice. We also thank the Northwestern University Next Generation Sequencing Core Facility for their support. M.P. acquired and analyzed data and wrote the manuscript. B.T.L. conceived and directed the study, interpreted the data, critically revised the manuscript for intellectual content and approved the final version.
Funding
B.T.L. is supported by the Department of Veterans Affairs, Veterans Health Administration, Office for Research and Development, Career Development (grant 1-lK2-BX-001587–01). M.P. is supported by an American Heart Association postdoctoral fellowship grant (15POST22410016).
References
- 1.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL Jr. Short chain fatty acids and their receptors: new metabolic targets. Transl Res 2013; 161(3):131-40; PMID:23146568; http://dx.doi.org/ 10.1016/j.trsl.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 2.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al.. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 2003; 278(28):25481-9; PMID:12711604; http://dx.doi.org/ 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- 3.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al.. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003; 278(13):11312-9; PMID:12496283; http://dx.doi.org/ 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- 4.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 2011; 108(19):8030-5; PMID:21518883; http://dx.doi.org/ 10.1073/pnas.1016088108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al.. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009; 461(7268):1282-6; PMID:19865172; http://dx.doi.org/ 10.1038/nature08530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab 2009; 11 Suppl 4:10-20; PMID:19817784; http://dx.doi.org/ 10.1111/j.1463-1326.2009.01114.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab 2013; 18(2):162-85; PMID:23791483; http://dx.doi.org/ 10.1016/j.cmet.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 8.Patel DG, Singh SP. Effect of ethanol and its metabolites on glucose mediated insulin release from isolated islets of rats. Metabolism 1979; 28(1):85-9; PMID:366333; http://dx.doi.org/ 10.1016/0026-0495(79)90173-2 [DOI] [PubMed] [Google Scholar]
- 9.Shah JH, Wongsurawat N, Aran PP. Effect of ethanol on stimulus-induced insulin secretion and glucose tolerance. A study of mechanisms. Diabetes 1977; 26(4):271-7; PMID:849808; http://dx.doi.org/ 10.2337/diab.26.4.271 [DOI] [PubMed] [Google Scholar]
- 10.Tiengo A, Valerio A, Molinari M, Meneghel A, Lapolla A. Effect of ethanol, acetaldehyde, and acetate on insulin and glucagon secretion in the perfused rat pancreas. Diabetes 1981; 30(9):705-9; PMID:7021270; http://dx.doi.org/ 10.2337/diab.30.9.705 [DOI] [PubMed] [Google Scholar]
- 11.Ximenes HM, Hirata AE, Rocha MS, Curi R, Carpinelli AR. Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell Biochem Funct 2007; 25(2):173-8; PMID:16444779; http://dx.doi.org/ 10.1002/cbf.1297 [DOI] [PubMed] [Google Scholar]
- 12.Montague W, Taylor KW. Regulation of insulin secretion by short chain fatty acids. Nature 1968; 217(5131):853; http://dx.doi.org/ 10.1038/217853a0 [DOI] [PubMed] [Google Scholar]
- 13.Winzell MS, Ahren B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther 2007; 116(3):437-48; PMID:17900700; http://dx.doi.org/ 10.1016/j.pharmthera.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Tang C, Ahmed K, Gille A, Lu S, Gröne HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 2015; 21(2):173-7; PMID:25581519 [DOI] [PubMed] [Google Scholar]
- 15.Hudson BD, Tikhonova IG, Pandey SK, Ulven T, Milligan G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J Biol Chem 2012; 287(49):41195-209; PMID:23066016; http://dx.doi.org/ 10.1074/jbc.M112.396259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 2005; 288(1):E1-15; PMID:15585595; http://dx.doi.org/ 10.1152/ajpendo.00218.2004 [DOI] [PubMed] [Google Scholar]
- 17.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al.. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A 2008. 105(43):16767-72; PMID:18931303; http://dx.doi.org/ 10.1073/pnas.0808567105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, et al.. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest 2011; 121(8):3331-42; PMID:21747171; http://dx.doi.org/ 10.1172/JCI44564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorensen H, Winzell MS, Brand CL, Fosgerau K, Gelling RW, Nishimura E, Ahren B. Glucagon receptor knockout mice display increased insulin sensitivity and impaired beta-cell function. Diabetes 2006; 55(12):3463-9; PMID:17130493; http://dx.doi.org/ 10.2337/db06-0307 [DOI] [PubMed] [Google Scholar]
- 20.Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 2007; 56(4):1087-94; PMID:17395749; http://dx.doi.org/ 10.2337/db06-1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Won YJ, Lu VB, Puhl HL 3rd, Ikeda SR. beta-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J Neurosci 2013; 33(49):19314-25; PMID:24305827; http://dx.doi.org/ 10.1523/JNEUROSCI.3102-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald MJ, Hasan NM, Longacre MJ. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim Biophys Acta 2008; 1780(7–8):966-72; PMID:18439432; http://dx.doi.org/ 10.1016/j.bbagen.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald MJ, Longacre MJ, Stoker SW, Brown LJ, Hasan NM, Kendrick MA. Acetoacetate and beta-hydroxybutyrate in combination with other metabolites release insulin from INS-1 cells and provide clues about pathways in insulin secretion. Am J Physiol Cell Physiol 2008; 294(2):C442-50; PMID:18160486; http://dx.doi.org/ 10.1152/ajpcell.00368.2007 [DOI] [PubMed] [Google Scholar]
- 24.Becker TC, BeltrandelRio H, Noel RJ, Johnson JH, Newgard CB. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J Biol Chem 1994; 269(33):21234-8; PMID:8063745 [PubMed] [Google Scholar]
- 25.Henquin JC, Sempoux C, Marchandise J, Godecharles S, Guiot Y, Nenquin M, Rahier J. Congenital hyperinsulinism caused by hexokinase I expression or glucokinase-activating mutation in a subset of beta-cells. Diabetes 2013; 62(5):1689-96; PMID:23274908; http://dx.doi.org/ 10.2337/db12-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YS, Morinaga H, Kim JJ, Lagakos W, Taylor S, Keshwani M, Perkins G, Dong H, Kayali AG, Sweet IR, et al.. The fractalkine/CX3CR1 system regulates beta cell function and insulin secretion. Cell 2013; 153(2):413-25; PMID:23582329; http://dx.doi.org/ 10.1016/j.cell.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meister J, Le Duc D, Ricken A, Burkhardt R, Thiery J, Pfannkuche H, Polte T, Grosse J, Schöneberg T, Schulz A. The G protein-coupled receptor P2Y14 influences insulin release and smooth muscle function in mice. J Biol Chem 2014; 289(34):23353-66; PMID:24993824; http://dx.doi.org/ 10.1074/jbc.M114.580803 [DOI] [PMC free article] [PubMed] [Google Scholar]